Introduction
Colorectal cancer is frequently encountered in
clinical practice (1). Long-term
surveillance indicates that the majority of colorectal cancers
arise from colorectal polyps (CP) (2).
Polypectomy reduces the risk of fatality from colorectal cancer
(3,4).
Screening using colonoscopy has also been shown to reduce the risk
of colorectal cancer-related fatality (5,6). However,
colonoscopy is not available to all populations or patients, as it
requires a skilled operator and therefore is associated with a
significant cost (7). As colonoscopy
is a limited resource, screening methods are required to select
patients to undergo the procedure.
Fecal occult blood testing is widely available and
reduces mortality from colorectal cancer (8). Fecal occult blood testing is useful for
the diagnosis of advanced colorectal cancer (9); however, such an advanced cancer would not
be amenable to polypectomy and therefore fecal occult blood testing
is not suitable for the detection of patients with CP (10).
It is recommended that laboratory tests are
completed prior to subjecting a patient to a colonoscopy, as this
practice is associated with reduced rates of complications and
lower costs (11). A correlation
between laboratory test results and the presence of CP, however,
has not been reported.
The rate of CP detection is 37% for surveillance
colonoscopy and 25% for screening (12). Kim et al (13), in an analysis of risk factors for CP,
reported that CPs were identified in 47% of patients who underwent
colonoscopy. The authors analyzed the association of CP with total
cholesterol (T-Chol), triglycerides (TG), high-density lipoprotein
(HDL) cholesterol and low-density lipoprotein (LDL) cholesterol
levels. In a similar study, Huang et al (14) analyzed the association between CP and
TG, HDL cholesterol, LDL cholesterol and glycated hemoglobin
(HbA1c). These two studies concluded that the presence of CP is
associated with metabolic risk factors. Therefore, it is expected
that laboratory variables may be correlated with the presence of
CP. The present study investigated whether laboratory variables are
useful for predicting the presence of CP.
Materials and methods
Patients
Patient records for the period between April 2011
and March 2014 were analyzed retrospectively. A total of 1,520
patients underwent colonoscopy during this period. The majority of
colorectal cancers arise from CP, which progress via the
adenoma-carcinoma sequence (15). In
rare cases, de novo colorectal cancers occur (16) and it can sometimes be hard to
distinguish between the adenoma-carcinoma sequence and de
novo carcinogenesis. Patients with colorectal cancer (n=49)
were therefore excluded from the analysis, leaving 1,471 eligible
patients; 731 men (mean age, 68.5±10.8 years) and 740 women (mean
age, 66.7±10.8 years). The study was submitted to the institutional
ethical committee at the National Hospital Organization, Shimoshizu
Hospital (Yotsukaido, Chiba, Japan) and assigned as not a clinical
trial, since it was performed as part of routine clinical practice.
Patient anonymity was preserved.
Colonoscopy
Colonoscopy was performed for patients with
abdominal symptoms, anemia or a positive fecal occult blood test
result. Colonoscopy was also performed for screening. The
colonoscopes used were CF-Q260 and PCF-Q260AI (Olympus, Tokyo,
Japan). The withdrawal time of colonoscopy ranged from 10 to 30
min. The diameter of the smallest polyps detected was 2 mm.
Laboratory variables
The variables analyzed as potential predictors of CP
included white blood cell count, hemoglobin, C-reactive protein,
platelet count, total protein, albumin level, total bilirubin
level, alkaline phosphatase, aspartate aminotransferase, alanine
aminotransferase, γ-glutamyl transpeptidase, lactate dehydrogenase,
uric acid (UA), blood urea nitrogen, creatinine, T-Chol, TG, HDL
cholesterol, LDL cholesterol, blood glucose, HbA1c, body mass
index, carcinoembryonic antigen and carbohydrate antigen 19-9.
Blood was collected in the fasting period from the majority of
patients.
Statistical analysis
One-way analysis of variance (ANOVA) was performed
to analyze the association between each variable and the presence
of CP. The mean UA level was analyzed, according to age group, with
one-way ANOVA. A χ2 test was performed to analyze the association
between age group and CP prevalence. The χ2 test was
also applied to analyze the correlation between the percentage of
patients with UA >7 mg/dl. Logistic regression analysis was
performed to establish a regression equation that could predict the
presence of CP. Receiver-operator characteristic analysis was
applied to investigate the performance of the regression equation.
P<0.05 was considered to indicate a statistically significant
difference. JMP 10.0.2 (SAS Institute Inc., Cary, NC, USA) was used
for statistical analysis.
Results
Associations between laboratory
variables and presence of CP
The associations between each laboratory test
variable and the presence of CP is presented in Table I. Not all the patients were subjected
to each laboratory test. Patients with CP were of a more advanced
age compared to those without CP (P<0.0001). Serum UA levels
were higher in patients with CP, compared with those without CP
(P=0.0007). These results suggest that age and UA level were
strongly associated with the presence of CP; these variables were
chosen for further analysis.
 | Table I.Descriptive statistics for age and
laboratory variables, according to colorectal polyp (CP)
status. |
Table I.
Descriptive statistics for age and
laboratory variables, according to colorectal polyp (CP)
status.
|
|
| Colonoscopy negative
for CP | Colonoscopy positive
for CP |
|
|---|
|
|
|
|
|
|
|---|
| Variables | Patients, no. | Patients, no. | Mean ± SD | Patients, no. | Mean ± SD | P-value |
|---|
| Age, years | 1,471 | 775 |
66.4±11.9 | 696 |
68.9±9.4 | <0.0001 |
| WBC,
102/µl | 698 | 381 |
6961±2087 | 317 |
6134±1884 | 0.2559 |
| Hb, g/dl | 697 | 381 |
13.0±2.1 | 316 |
13.1±2.1 | 0.3069 |
| CRP, mg/dl | 376 | 209 |
0.77±1.99 | 167 |
0.88±3.45 | 0.6958 |
| Plt,
104/µl | 690 | 378 |
21.9±7.1 | 312 |
22.2±6.9 | 0.5895 |
| TP, g/dl | 452 | 254 |
6.9±0.8 | 198 |
6.9±0.7 | 0.4954 |
| Alb, g/dl | 334 | 181 |
4.1±0.5 | 153 |
4.0±0.6 | 0.2336 |
| T-Bil, mg/dl | 462 | 260 |
0.77±0.46 | 202 |
0.74±0.32 | 0.4288 |
| ALP, IU/l | 244 | 130 |
233.1±81.7 | 114 |
229.0±84.5 | 0.6964 |
| AST, IU/l | 634 | 335 |
25.1±25.3 | 279 |
25.4±11.9 | 0.8642 |
| ALT, IU/l | 668 | 366 |
22.6±28.0 | 302 |
23.1±0.8 | 0.7863 |
| GGT, IU/l | 279 | 147 |
56.3±274.7 | 132 |
44.9±57.5 | 0.6401 |
| LDH, IU/l | 363 | 192 |
201.3±60.7 | 171 |
205.9±124.8 | 0.6508 |
| UA, mg/dl | 272 | 138 |
5.0±1.4 | 134 |
5.5±1.4 | 0.0007 |
| BUN, mg/dl | 434 | 234 |
14.9±4.8 | 200 |
16.5±14.5 | 0.1109 |
| Cr, mg/dl | 670 | 367 |
0.84±0.44 | 303 |
0.86±0.25 | 0.3734 |
| T-Chol, mg/dl |
273 | 164 |
204.9±39.9 | 109 |
196.4±33.6 | 0.0703 |
| TG, mg/dl |
253 | 117 |
124.7±81.9 | 136 |
138.2±79.8 | 0.1867 |
| HDL, mg/dl |
192 | 103 |
61.3±17.6 | 89 |
56.4±16.3 | 0.0512 |
| LDL, mg/dl |
264 | 129 |
118.4±29.7 | 135 |
117.5±25.4 | 0.7762 |
| BG, mg/dl |
350 | 184 |
116.6±39.0 | 166 |
122±46.8 | 0.1892 |
| HbA1c, % |
172 | 83 |
6.2±1.0 | 89 |
6.2±1.1 | 0.7721 |
| BMI,
kg/m2 |
252 | 124 |
22.5±3.9 | 128 |
22.5±3.6 | 0.8825 |
| CEA, ng/ml |
183 | 99 |
12.0±63.3 | 84 |
49.1±391.5 | 0.3550 |
| CA19-9, U/ml |
182 | 98 |
14.7±12.4 | 84 |
38.8±221.7 | 0.2836 |
Association between age and presence
of CP
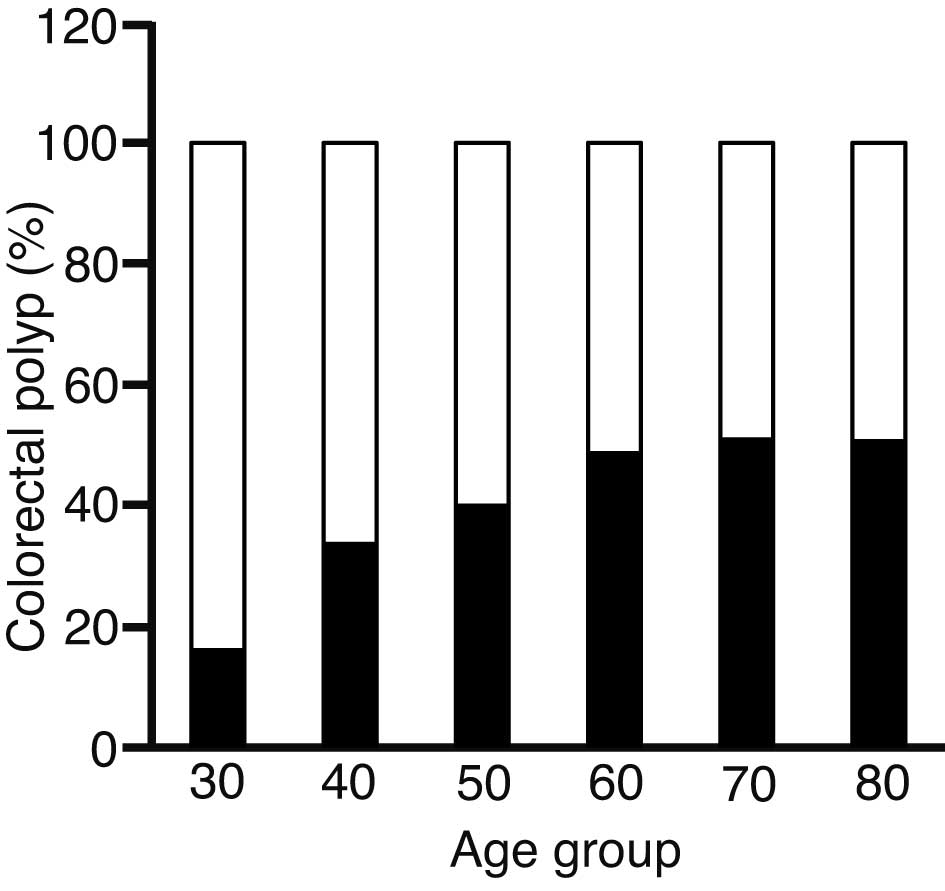
The association between the percentage of patients
with CP and age is illustrated in Fig.
1; the presence of CP increased with age. This association was
statistically significant (P=0.0001) (Table II). The number of patients in their
20s and 90s were 3 and 5, respectively. As these ages were
significantly fewer in number compared with the other age groups,
these patients were omitted from further analysis.
 | Table II.Association between age and
prevalence of colorectal polyps (CP). |
Table II.
Association between age and
prevalence of colorectal polyps (CP).
|
|
| CP (−) | CP (+) |
|---|
|
|
|
|
|
|---|
| Age group,
years | Patients, no. | % | χ2
test | % | χ2
test |
|---|
| 30 | 31 | 83.9 | 5.7225 | 16.1 | 6.3720 |
| 40 | 92 | 66.3 | 3.2389 | 33.7 | 3.6065 |
| 50 | 120 | 60.0 | 1.2187 | 40.0 | 1.3570 |
| 60 | 497 | 51.3 | 0.1790 | 48.7 | 0.1993 |
| 70 | 577 | 49.1 | 1.4498 | 50.9 | 1.6144 |
| 80 | 146 | 49.3 | 0.3148 | 50.7 | 0.3505 |
Serum UA level and the presence of
CP
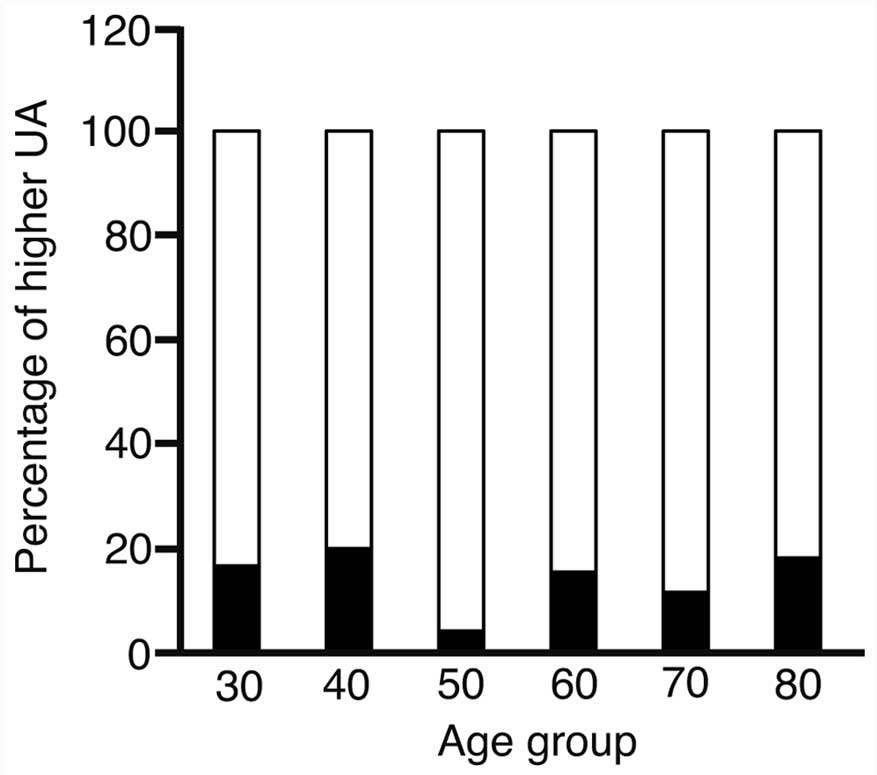
The serum UA level was correlated with the presence
of CP; however, there was a possibility that this association was
confounded by an association of UA level with age. Fig. 2 indicates that there was no association
between a higher UA level and age.
The χ2 test confirmed an absence of
correlation between UA level and age (P=0.6279).
The above data indicated that the presence of CP
correlated with aging and UA. To investigate the possibility that
age and UA level could predict the presence of CP, logistic
regression analysis was performed (P=0.0008) (Table III). The regression equation was as
follows: ln(p/1 − p) = 2.79015 − 0.01836 × age −
0.28542 × UA level (mg/dl), where p represents the presence
of CP.
 | Table III.Logistic regression analysis for the
association between age and serum uric acid level. |
Table III.
Logistic regression analysis for the
association between age and serum uric acid level.
|
Characteristics | χ2
test | Odds | Likelihood test
(P-value) |
|---|
| Age |
2.53 | 0.981808 | 0.1083 |
| Uric acid | 10.03 | 0.751701 | 0.0011 |
The likelihood ratio χ2 test showed a
P-value for age and UA level of 0.1083 and 0.0011, respectively,
indicating a strong correlation between UA level and the presence
of CP.
Receiver-operator characteristic
analysis
To investigate how well the regression equation
predicted the presence of CP, receiver-operator characteristic
analysis was applied (Fig. 3). The
area under the curve was 0.62092. The threshold value of P was
0.4370, and the sensitivity and specificity of the threshold value
were 77.6 and 44.2%, respectively.
Discussion
Previous investigations into the correlation between
laboratory test results and the presence of CP have focused on
components of metabolic syndrome (17), and the literature regarding the
association between UA level and the presence of CP is limited.
Orannapalai et al (18)
analyzed the correlation between laboratory test results and the
presence of CP. Patients were divided into 2 groups, based on UA
level; >7 and ≤7 mg/dl. The presence of CP was higher in the
group with a UA level of >7 mg/dl. In the present study, the
average level of UA was higher in patients with CP compared with
patients without CP, which is consistent with the results of the
previous report. The underlying reason for this association is
unknown. Notably, Karaman et al (19) found that the average UA level was
higher in patients with neoplastic CP, as compared to those with
non-neoplastic CP. Patients with a higher UA level are also prone
to cancer of the colon, liver and lung (20). These results suggest that a raised
serum UA level may be involved in tumorigenesis (21).
There is limited information available on the CP
predictors. Eisner et al (22)
performed urinary metabolomics in search of such a predictor and
reported that nicotinate and nicotinamide metabolites and the
degradation of ketone bodies are associated with the presence of
CP. They proposed a tool involving the use of urinary metabolomics
to select patients at risk of CP, who would undergo further
investigation with colonoscopy. The performance of this tool is
more efficient than that of fecal occult blood testing. In the
present study, age and UA level were associated with the presence
of CP. It has previously been reported that advanced age is
associated with the presence of CP (23). UA levels are also higher in patients
with CP, as discussed above. The present data are therefore
consistent with previous reports. Fecal occult blood testing is
intended to select patients with colorectal cancer, rather than
pre-cancerous CP (24). Eisner et
al (22) analyzed fecal occult
blood testing as a tool for the detection of CP. Fecal occult blood
testing has been shown to have a sensitivity of 2.6–15.1% and a
specificity of 94.5–99.4%. In terms of the detection of CP using UA
level, the present regression equation showed a greater
sensitivity, but a poorer specificity.
In conclusion, advanced age and higher serum UA
levels are associated with the presence of CP. Logistic regression
analysis obtained a regression equation with a greater sensitivity
and poorer specificity for the detection of CP, compared with fecal
occult blood testing.
References
|
1
|
Brenner H, Kloor M and Pox CP: Colorectal
cancer. Lancet. 383:1490–1502. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stoian M, State N, Rusu E, Stoica V,
Gavril RS, Gherasim A and Radulian G: Malignancy and mortality of
colorectal polyps. Rev Med Chir Soc Med Nat Iasi. 118:399–406.
2014.PubMed/NCBI
|
|
3
|
Zauber AG, Winawer SJ, O'Brien MJ,
Lansdorp-Vogelaar I, van Ballegooijen M, Hankey BF, Shi W, Bond JH,
Schapiro M, Panish JF, et al: Colonoscopic polypectomy and
long-term prevention of colorectal-cancer deaths. N Engl J Med.
366:687–696. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Anderloni A, Jovani M, Hassan C and Repici
A: Advances, problems and complications of polypectomy. Clin Exp
Gastroenterol. 7:285–296. 2014.PubMed/NCBI
|
|
5
|
Manser CN, Bachmann LM, Brunner J, Hunold
F, Bauerfeind P and Marbet UA: Colonoscopy screening markedly
reduces the occurrence of colon carcinomas and carcinoma-related
death: A closed cohort study. Gastrointest Endosc. 76:110–117.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rosa I, Fidalgo P, Soares J, Vinga S,
Oliveira C, Silva JP, Ferro SM, Chaves P, Oliveira AG and Leitão
CN: Adenoma incidence decreases under the effect of polypectomy.
World J Gastroenterol. 18:1243–1248. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wallace MB and Kiesslich R: Advances in
endoscopic imaging of colorectal neoplasia. Gastroenterology.
138:2140–2150. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shaukat A, Mongin SJ, Geisser MS, Lederle
FA, Bond JH, Mandel JS and Church TR: Long-term mortality after
screening for colorectal cancer. N Engl J Med. 369:1106–1114. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Viana Freitas BR, Kibune Nagasako C, Pavan
CR, Silva Lorena SL, Guerrazzi F, Saddy Rodrigues Coy C, Ayrizono
ML and Mesquita MA: Immunochemical fecal occult blood test for
detection of advanced colonic adenomas and colorectal cancer:
Comparison with colonoscopy results. Gastroenterol Res Pract.
2013:384–561. 2013.
|
|
10
|
Bretagne JF, Manfredi S, Piette C, Hamonic
S, Durand G and Riou F: Yield of high-grade dysplasia based on
polyp size detected at colonoscopy: A series of 2295 examinations
following a positive fecal occult blood test in a population-based
study. Dis Colon Rectum. 53:339–345. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sonnenberg A: Test sequence in the
management of gastrointestinal bleeding. Endoscopy. 44:43–47. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Anderson JC, Butterly LF, Goodrich M,
Robinson CM and Weiss JE: Differences in detection rates of
adenomas and serrated polyps in screening versus surveillance
colonoscopies, based on the new hampshire colonoscopy registry.
Clin Gastroenterol Hepatol. 11:1308–1312. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim YJ, Lee KJ, Park SY, Han JH, Kwon KY
and Kim JH: Association between Dyslipidemia and the Prevalence of
Colon Polyps Based on a Health Evaluation of Subjects at a
Hospital. Korean J Fam Med. 35:143–151. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang HE, Yang YC, Wu JS, Wang RH, Lu FH
and Chang CJ: The relationship between different glycemic statuses
and colon polyps in a Taiwanese population. J Gastroenterol.
49:1145–1151. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Al-Sohaily S, Biankin A, Leong R,
Kohonen-Corish M and Warusavitarne J: Molecular pathways in
colorectal cancer. J Gastroenterol Hepatol. 27:1423–1431. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tanaka T: Colorectal carcinogenesis:
Review of human and experimental animal studies. J Carcinog.
8:52009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim BC, Shin A, Hong CW, Sohn DK, Han KS,
Ryu KH, Park BJ, Nam JH, Park JW, Chang HJ, et al: Association of
colorectal adenoma with components of metabolic syndrome. Cancer
Causes Control. 23:727–735. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Orannapalai N, Attawettayanon W, Kanngern
S, Boonpipattanapong T and Sangkhathat S: Predicting the occurrence
of cancer-associated colorectal polyp using a metabolic risk score.
Mol Clin Oncol. 2:124–128. 2014.PubMed/NCBI
|
|
19
|
Karaman H, Karaman A, Erden A, Poyrazoglu
OK, Karakukcu C and Tasdemir A: Relationship between colonic polyp
type and the neutrophil/lymphocyte ratio as a biomarker. Asian Pac
J Cancer Prev. 14:3159–3161. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Boffetta P, Nordenvall C, Nyrén O and Ye
W: A prospective study of gout and cancer. Eur J Cancer Prev.
18:127–132. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wilson FP and Berns JS: Tumor lysis
syndrome: New challenges and recent advances. Adv Chronic Kidney
Dis. 21:18–26. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Eisner R, Greiner R, Tso V, Wang H and
Fedorak RN: A machine-learned predictor of colonic polyps based on
urinary metabolomics. Biomed Res Int. 2013:3039822013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Clipp EC, Carver EH, Pollak KI, Puleo E,
Emmons KM, Onken J, Farraye FA and McBride CM: Age-related
vulnerabilities of older adults with colon adenomas: Evidence from
Project Prevent. Cancer. 100:1085–1094. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Launois R, Le Moine JG, Uzzan B, Fiestas
Navarrete LI and Benamouzig R: Systematic review and
bivariate/HSROC random-effect meta-analysis of immunochemical and
guaiac-based fecal occult blood tests for colorectal cancer
screening. Eur J Gastroenterol Hepatol. 26:978–989. 2014.
View Article : Google Scholar : PubMed/NCBI
|