Introduction
In conventional tumor therapies, chemotherapy,
radiotherapy and surgical treatment all have limitations and side
effects that affect normal cells and endanger to the immunity
system (1,2). Patients even succumb of serious side
effects, such as myelotoxicity and myocardial injury. To search for
novel effective antitumor agents with less toxic effects, natural
products have been focused on previously (3,4). In the
1960s, the antitumor function of fungal polysaccharides was
discovered and became a popular area of the research field
(5). Since then, it has been widely
studied owing to the immunostimulating and low toxicity side
effects in the host. Fungal polysaccharides, such as lentinan and
Ganoderma lucidum polysaccharide have previously been used
in clinical cancer therapies (6).
Natural killer (NK) cells are innate lymphocytes
that are capable of eliminating tumor cells and are therefore used
for cancer therapy (7).
Lymphokine-activated killer (LAK) cells are activated NK cells that
are traditionally prepared from isolated T cells cultured with
interleukin-2 (IL-2) and are capable of recognizing cancer cells in
a non-major histocompatibility complex-restricted manner (8). LAK cells have been utilized in
vivo in animals and in humans to treat cancer and can kill
NK-non-sensitive tumor cells (9).
Phellinus pullus is a wood rot fungi widely
distributed in China that has not been used as a traditional herbal
medicine. To the best of our knowledge, there are no
pharmacological studies on Phellinus pullus. The present
study isolated a crude polysaccharide (APS-3) from Phellinus
pullus. Its antitumor activity was evaluated in vitro
and in vivo. In addition, the immunomodulatory effects of
APS-3 were also assessed to analyze the underlying mechanisms of
its antitumor activity.
Materials and methods
Ethical statement
The present study was carried out in strict
accordance with the recommendations of the Ethical Review Committee
of Affiliate Jinan Central Hospital Affiliated to Shandong
University (Shandong, China).
Preparation of polysaccharoses
The polysaccharose APS-3 was obtained from the
fruiting bodies of wildly grown Phellinus pullus in the
Shandong province of China. The dry fruit body was cleaned, crushed
and extracted with boiling NaOH (1.5%) for 30 min three times. The
extract was combined and precipitated with 80% ethanol. The
precipitates were collected and dissolved with distilled water. The
Savage method (10) and activated
carbon were used to remove protein and pigment from the extract,
and subsequently the polysaccharide APS-3 was obtained. The APS-3
content was measured by the phenol-sulfate acid method and was
prepared as a 2-mg/ml stock solution. The stock solution was
sterilized using a 0.45-µm filter (Pall Corporation, Port
Washington, NY, USA), and further diluted with cell culture medium
to the defined concentrations as indicated.
Cell culture and growth inhibition
test
Mouse sarcoma 180 (S180) tumor cells (Cell Bank of
Type Culture Collection of Chinese Academy of Sciences, Shanghai,
China) were cultured and harvested from ascites of the infected
mouse. In vitro culture was performed in RPMI-1640 medium
(Gibco Life Technologies, Thermo Fisher Scientific, Inc., Carlsbad,
CA, USA) supplemented with 10% fetal bovine serum (Zhejiang
Tianhang Biological Technology Co., Ltd., Huzhou, China), and 300
mg/l L-glutamine (Gibco Life Technologies, Thermo Fisher
Scientific, Inc.), and antibiotics in a humidified 5%
CO2 at 37°C. A total of 1×105 cells/ml of
exponentially growing cells were cultured in the medium with 0.1,
0.2, 0.4, 0.8 and 1.6 mg/ml APS-3 for 24 h, and subsequently, cell
growth inhibition was tested by the MTT method.
APS-3 treatment in vivo
Animal treatment and experimental design
A total of 25 female Swiss mice (18–22 g) were
provided by the Laboratory Animal Center of Shandong University
[animal license number: SCXK (Lu) 20090001]. The animals were
allowed free access to a standard diet and sterile water, and were
maintained in a sterile and ventilated room under controlled
environmental conditions (25±1°C, 50±10% humidity and 12-h
light/12-h dark cycle).
The exponentially growing S180 cells were washed
with Hank's balanced salt solution and adjusted to a suspension
containing 1×107 cells/ml in serum-free RPMI-1640
medium. A total of 20 mice were implanted with 200 µl of the cell
suspension by subcutaneous injection to the fore right subaxillary.
Seven days after tumor cell inoculation, the tumor-bearing mice
were randomly divided into 4 groups (5 mice in each group): Mice
received APS-3 intragastrically at the respective doses of 1.5, 3
and 6 g/kg/day (the 3 treated groups, respectively); and the
control mice received the same volume of 0.9% normal saline (NS)
intragastrically (the model control, MC). A total of 5 normal mice
treated with only NS served as the normal control (NC). All
treatments were administered once daily for 18 consecutive
days.
Determination of survival time
After 18 days of treatment, the number of surviving
mice in each group was recorded and the percentage of life
prolongation was calculated as follows: Life prolongation rate (%)
= (survival time of the treated group - survival time of the
MC)/survival time of the model control × 100.
Tumor inhibition rate
Tumor size was measured daily and the tumor volume
was calculated using the following formula: V=0.5ab2, where a is
the largest and b is the smallest perpendicular diameter. After 18
days of treatment, all the mice were euthanized, and the tumors
were removed and weighed. The tumor inhibition rate was calculated
as follows: Inhibition rate (%) = (mean tumor weight in the model
control mice - mean tumor weight in the treated mice)/mean tumor
weight in the model control mice × 100.
Histopathology
Tumor tissues were fixed in formalin,
paraffin-embedded and subsequently separated into sections. The
difference in the tumor tissues between the model control mice and
the treated group mice was assessed by histopathological analysis
following staining with hematoxylin and eosin (H&E).
Immune-stimulant function of
APS-3
Evaluation of NK cytotoxicity
Spleens of the mice were removed under sterile
conditions, disaggregated in D-hanks and filtered through a
200-mesh stainless-steel sieve to obtain a single-cell suspension.
Lymphocytes were collected and suspended in RPMI-1640 medium at a
concentration of 1×106 cells/ml following treatment with
red blood cell lysis buffer. The activity of NK cells was tested
using the cytotoxicity assay on YAC-1 cells (Cell Bank of Type
Culture Collection of Chinese Academy of Sciences). A total of 100
µl NK and YAC-1 cells were added to a 96-well plate in triplicate
to obtain an effector/target (E/T) ratio of 20:1 and co-incubated
for 12 h. The amount of released lactate dehydrogenase (LDH) in
culture supernatants was determined using the LDH Cytotoxicity
assay kit (Biovision, Inc., Milpitas, CA, USA) according to the
manufacturer's protocol. The optical density (OD) was read at 490
nm with a microplate reader. The percentage of NK cell cytotoxicity
was calculated with the formula: Cytotoxicity (%) = (experimental
release - spontaneous release)/(maximum release - spontaneous
release) × 100. Spontaneous release was spontaneous LDH release
from target cells incubated with medium alone, and maximum release
was obtained from target cells lysed with NP-40.
Evaluation of LAK cytotoxicity
Lymphocytes were collected at a concentration of
1×106 cells/ml, as described for the NK cells, and were
cultured in the medium with 1,000 U/ml of IL-2. The cells collected
by centrifuge 72 h later were LAK cells. The concentration of the
LAK cells was adjusted to 2×106 cells/ml. Mouse mast
cell tumor P815 cells (Cell Bank of Type Culture Collection of
Chinese Academy of Sciences) were used as target cells and the
concentration was 1×106 cells/ml. LAK cells and P815
cells were co-incubated for 24 h with an E/T ratio of 20:1. A
single effector cell and single target cell were used as controls.
Each well was measured with the MTT method. The percentage of LAK
cell cytotoxicity was calculated with the formula: Cytotoxicity (%)
= [1- (ODco-incubated effector cell and target cell -
ODeffector cell)/ODtarget cell] × 100.
Statistical analysis
Each experiment was performed at least in
triplicate. All the results are expressed as the mean ± standard
deviation. The data were analyzed by the Student's unpaired t-test
and P<0.05 was considered to indicate a statistically
significant difference.
Results
Growth inhibition of S180 cell by
APS-3 in vitro
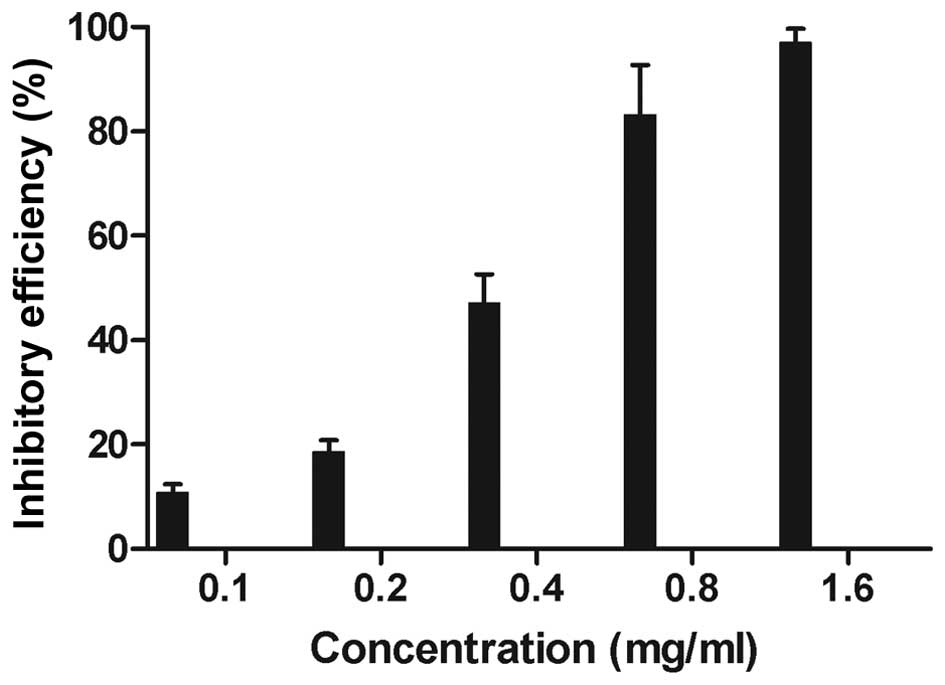
The proliferation of the S180 cells was evidently
inhibited after 24-h treatment by APS-3 (P<0.05) (Fig. 1). The results also indicated that there
was a marked dose-dependent inhibition of cell viability.
Antitumor effect in vivo
Determination of survival time
On day 19 of the treatment, the model control mice
were ill or had died. However, the majority of the mice in the
treated group survived. All doses of APS-3 significantly prolonged
survival time of the mice, showing life prolongation ratios of 36,
80 and 85% at APS-3 doses of 1.5, 3 and 6 g/kg/day, respectively.
However, as all the mice were sacrificed on day 19 of the
treatment, the life prolongation ratios do not signify the final
result.
Tumor inhibition rate
Seven days after S180 cell injection, tumors of ~500
mm3 formed in 20 mice. As shown in Fig. 2, APS-3 inhibited tumor growth in a
time-dependent manner. The tumor volume decreased after 3 days of
APS-3 treatment, but a significant decrease was observed after 9
days of treatment. On day 10 of treatment, the tumor in 1 treated
mouse completely disappeared. The tumor volume in the model control
mice increased to 1,902 mm3 at day 16, while the tumors
in the APS-3-treated mice were only 995.7, 476.4 and 348.0
mm3, respectively. After APS-3 treatment for 18 days,
all mice were sacrificed and the tumors were removed. The tumor
weight in the model control mice was 1.79 g, while that in the
APS-3-treated mice was only 1.09, 0.35 and 0.26 g at the dose of
1.5, 3 and 6 g/kg/day, and the tumor inhibition ratio was 39.11,
80.45 and 85.47%, respectively.
Histopathology
Fig. 3 shows that in
the paraffin sections of the tumor tissues stained with H&E,
regularly arranged tumor cells were observed in the model control
mice, and a few necrotic areas and no inflammatory cell
infiltrations were observed. However, in the APS-3-treated mice,
tumor cells disappeared, and a large range of structureless
necrotic areas were observed. Furthermore, inflammatory cell
infiltration and granulation tissues were observed in these areas.
These findings correlated with the tumor volume and weight in the
model control mice and APS-3-treated mice.
Evaluation of NK cytotoxicity
Following treatment of the tumor-bearing mice with
APS-3, NK cell cytotoxicity was tested using the LDH Cytotoxicity
assay kit, with YAC-1 cells as the target cells. Splenic
lymphocytes of APS-3 treated mice showed more cytotoxicity compared
to the splenocytes from the normal mice and model mice (P<0.01)
(Fig. 4). These results indicate that
APS-3 increased NK cell cytotoxicity of the tumor-bearing mice.
Evaluation of LAK cytotoxicity
Cytotoxicity of the LAK cells to P815 cells was
detected in all the tumor-bearing mice and is shown in Fig. 5. LAK cell cytotoxicity of APS-3-treated
mice was not only higher than model control mice, but also higher
than NC mice.
Discussion
Growth of cancer cells can destroy the surrounding
environment and release a danger signal, and the immune system of
the body can cause the immune response to this danger signal
(11). These signals can lead to
inflammation, activate the antitumor effector cells and the
antigen-presenting cells, triggering the immune response of T cells
and B cells (12,13). Immunity of the tumor-burdened body is
extremely low, although it is possible to recognize and present the
tumor antigen, and produce an immune response (14). Therefore, increasing the immune system
of the body to recognize a dangerous signal or activate the
effector cells with antitumor activity, such as NK cells and LAK
cells, is the main target of tumor immunotherapy (15). Polysaccharide drugs are novel antitumor
substances, which exhibit a pharmacology role through numerous
channels, and for multi-targets, immune regulation is the main
pathway (16–18). For example, the antitumor function of
lentinan was by way of generation of activated NK cells (19,20).
The polysaccharide APS-3 was obtained through a
decoction extraction using 1.5% NaOH and alcohol precipitation of
the fruiting bodies of the fungus Phellinus pullus. APS-3
can inhibit the proliferation of S180 cells in vitro. In
vivo studies further confirmed its antitumor effect on
S180-transplanted mice tumors, and the highest antitumor rate was
85.47%. Furthermore, the antitumor effect of APS-3 was dose- and
time-dependent. It is possible that if the treatment had been
extended, certain mice may have recovered entirely. However, it can
significantly improve the activity of NK cells and LAK cells in
S180 sarcoma mice, demonstrating that immune enhancement is the
main mechanism of the antitumor function of APS-3. In view of the
poly-target of fungal polysaccharides, the antitumor function of
APS-3 compounds is via numerous links, and a number of targets
exhibit its antitumor and immune regulation. Other antitumor
mechanisms of APS-3 require further research.
In conclusion, the present study verified the
antitumor activity of APS-3, suggesting that APS-3 can be used as a
possible candidate for tumor prevention or treatment, and by
contrast, APS-3 can be used as an immune-enhancement agent in
chemotherapy or surgical treatment.
Acknowledgements
The authors would like to acknowledge the financial
support of the Medical and Health Technology Development Plan of
Shandong province (grant no. 2014WS0002) and the China Postdoctoral
Science Foundation (grant no. 2014M551913).
References
|
1
|
Sagar SM, Yance D and Wong RK: Natural
health products that inhibit angiogenesis: A potential source for
investigational new agents to treat cancer-Part 1. Curr Oncol.
13:14–26. 2006.PubMed/NCBI
|
|
2
|
Wang S, Wu X, Tan M, Gong J, Tan W, Bian
B, Chen M and Wang Y: Fighting fire with fire: Poisonous Chinese
herbal medicine for cancer therapy. J Ethnopharmacol. 140:33–45.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nobili S, Lippi D, Witort E, Donnini M,
Bausi L, Mini E and Capaccioli S: Natural compounds for cancer
treatment and prevention. Pharmacol Res. 59:365–378. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bao X, Yuan H, Wang C, Liu J and Lan M:
Antitumor and immunomodulatory activities of a polysaccharide from
Artemisia argyi. Carbohydr Polym. 98:1236–1243. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kamasuka T, Momoki Y and Sakai S:
Antitumor activity of polysaccharide fractions prepared from some
strains of Basidiomycetes. Gan. 59:443–445. 1968.PubMed/NCBI
|
|
6
|
Yin X, Ying J, Li L, Zhang H and Wang H: A
meta-analysis of lentinan injection combined with chemotherapy in
the treatment of nonsmall cell lung cancer. Indian J Cancer.
52:E29–E31. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lim O, Jung MY, Hwang YK and Shin EC:
Present and future of allogeneic natural killer cell therapy. Front
Immunol. 6:2862015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Helfand SC, Soergel SA, Modiano JF, Hank
JA and Sondel PM: Induction of lymphokine-activated killer (LAK)
activity in canine lymphocytes with low dose human recombinant
interleukin-2 in vitro. Cancer Biother. 9:237–244. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li Y, Meng FD, Tian X, Sui CG, Liu YP and
Jiang YH: Impact of IL-2 and IL-2R SNPs on proliferation and tumor-
killing activity of lymphokine-activated killer cells from healthy
chinese blood donors. Asian Pac J Cancer Prev. 15:7965–7970. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang WJ: Biochemistry technology of
polysaccharide compound (11-12th). Hangzhou: Zhejiang University
Press. 12–21. 1999.
|
|
11
|
Sounni NE and Noel A: Targeting the tumor
microenvironment for cancer therapy. Clin Chem. 59:85–93. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Trinchieri G: Cancer Immunity: Lessons
From Infectious Diseases. J Infect Dis. 212(Suppl 1): S67–S73.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guo H, Callaway JB and Ting JP:
Inflammasomes: mechanism of action, role in disease, and
therapeutics. Nat Med. 21:677–687. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu R, Xiong S, Zhang L and Chu Y:
Enhancement of antitumor immunity by low-dose total body
irradiationis associated with selectively decreasing the proportion
and number of T regulatory cells. Cell Mol Immunol. 7:157–162.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
West EJ, Scott KJ, Jennings VA and Melcher
AA: Immune activation by combination human lymphokine-activated
killer and dendritic cell therapy. Br J Cancer. 105:787–795. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lin CY, Chuang TF, Liao KW, Huang YJ, Pai
CC and Chu RM: Combined immunogene therapy of IL-6 and IL-15
enhances anti-tumor activity through augmented NK cytotoxicity.
Cancer Lett. 272:285–295. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huyan T, Li Q, Yang H, Jin ML, Zhang MJ,
Ye LJ, Li J, Huang QS and Yin DC: Protective effect of
polysaccharides on simulated microgravity-induced functional
inhibition of human NK cells. Carbohydr Polym. 101:819–827. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cheng KC, Huang HC, Chen JH, Hsu JW, Cheng
HC, Ou CH, Yang WB, Chen ST, Wong CH and Juan HF: Ganoderma
lucidum polysaccharides in human monocytic leukemia cells: From
gene expression to network construction. BMC Genomics. 8:411–428.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ng ML and Yap AT: Inhibition of human
colon carcinoma development by lentinan from shiitake mushrooms
(Lentinus edodes). J Altern Complement Med. 8:581–589. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ren M, Ye L, Hao X, Ren Z, Ren S, Xu K and
Li J: Polysaccharides from Tricholoma matsutake and Lentinus edodes
enhance 5-fluorouracil-mediated H22 cell growth inhibition. J
Tradit Chin Med. 34:309–316. 2014. View Article : Google Scholar : PubMed/NCBI
|