Introduction
A migraine is more than just a headache; it is a
complex neurological disorder that involves altered sensory
perception and processing, affecting ~15% of the population, and
the mechanisms underlying migraine have not been completely
elucidated (1). The trigeminovascular
pathway and neuropeptide calcitonin gene-related peptide (CGRP)
have important roles in migraines (2–5).
Trigeminovascular doctrine combines nerves, blood vessels and
neurotransmitters to explain certain animal experiments results and
clinical manifestations of migraines. Electrical stimulation of the
trigeminal ganglion (ESTG) in humans leads to increased
extracerebral blood flow and local release of CGRP and substance P
(6). This phenomenon causes
extravasation of plasma proteins and neurogenic inflammation, which
can stimulate spreading of the trigeminal nerve vascular fiber and
nerve impulses in the brainstem, hypothalamus and cortex,
eventually leading to migraine.
The pathogenesis of a migraine remains to be
elucidated, and a variety of theories offer different targets for
migraine treatment. Serotonin receptor agonists and nonsteroidal
anti-inflammatory drugs have a clear therapeutic effect on
migraines. Other targets, such as glutamate receptors, nitric oxide
synthase and adenosine Al receptor (A1R), have been recently found
to treat migraine, but they have not been clinically used (7,8). The
physiological effects of adenosine, an important neurotransmitter,
are mediated by adenosine receptor. Four adenosine receptor
subtypes have been discovered, which are A1R, adenosine A2a
receptor (A2aR), A2bR and A3R (9). A1R
and A2aR are high-affinity receptors, which have an important role
in pain information transmission and regulation. In addition to the
aforementioned drugs, several Chinese medicines, including Tianshu
capsule (TSC), also have an important role in migraine treatment.
In China, a number of neurologists apply TSC as an acute and
prophylactic drug treatment for migraines (10).
The present study aimed to determine whether CGRP,
A2aR and A1R are involved in migraine pain information transmission
in the ESTG migraine rat model. The possible mechanisms of TSC for
migraine treatment were also explored.
Materials and methods
Animals
A total of 40 male Sprague-Dawley rats weighing
280–320 g (Vital River Laboratory Animal Technology Co., Ltd.,
Beijing, China) were used. All the rats were maintained under
standard laboratory housing conditions with a 12 h-light-dark cycle
and had free access to food and water. All the experimental
protocols were approved by the Ethics Committee for the Use of
Experimental Animals at Binzhou Medical University (Binzhou,
China). All the procedures were performed with utmost caution to
minimize animal suffering. All the rats were randomly divided into
four groups: Blank (n=10), ESTG (n=10), sham-operated (SO; n=10)
and TSC groups (n=10).
Experimental protocols
ESTG
The rats in the ESTG model group were anesthetized
with 10% chloral hydrate [4 ml/kg, intraperitoneal injection
(i.p.)] and placed in a stereotaxic frame (ZH-B; Zhenghua
Biological Instrument Co., Ltd., Huaibei, China). The calvarium was
exposed through a midline incision. A hole was made with a cranial
drill 3.2–3.4 mm posteriorly to and 2.8–3.2 mm laterally from the
bregma. A disposable concentric needle electrode (DCN37; Alpine
Biomed Corp., Fountain Valley, CA, USA) was lowered into the right
TG (at a depth of ~9.2 mm from the dura mater). TG was electrically
stimulated for 30 min with square pulses at 10 Hz and 0.5 mA, with
a pulse duration of 5 msec. Correct electrode placement of the
electrode needle was confirmed through ipsilateral contraction of
the masseter muscle during stimulation.
The rats in the SO group underwent a surgical
procedure similar to that performed in the rats of the ESTG group.
However, the concentric bipolar electrode was only lowered into the
right TG and was maintained for only 30 min. The TG was not
electrically stimulated.
Drug administration
The rats in the TSC group received intragastric
administration of TSC (Jiangsu Kangyuan Pharmaceutical, Jiangsu,
China), which was dissolved in saline at a dose of 3.5 mg/kg/day
for 7 days. Subsequently, the rats were anesthetized with 10%
chloral hydrate (4 ml/kg; i.p.) and subjected to ESTG 30 min after
the last drug administration.
All the rats were sacrificed after 30 min of
stimulation. The trigeminal nucleus caudalis (TNC) and ipsilateral
TG were immediately removed and stored at −80°C for western blot
analysis or reverse transcription-quantitative polymerase chain
reaction (RT-qPCR).
RT-qPCR
The TG and TNC of mice were rapidly isolated for
total RNA preparation as described in the previous section. Total
RNA (1 µg) was reverse transcribed in a 25-µl reaction volume using
Premix Ex Tap II (Takara Bio, Shiga, Japan). PCR amplifications and
fluorescence detections were performed using the CFX96™ RT-PCR
detection system C1000 according to the manufacturer's protocol.
The PCR conditions were as follows: 95°C for 30 sec, followed by 40
cycles of 95°C for 5 sec and 60°C for 30 sec. A relative quantity
of each sample was calculated using the 2−ΔΔCt method.
Nucleotide sequences of the specific primers for the selected genes
were as follows: β-actin (forward, AGAGCTATGAGCTGCCTGACG and
reverse, CTTCTGCATCCTGTCAGCGAATGC); CGRP (forward,
TCCTGGTTGTCAGCATCTTG and reverse, CTCAGCCTCCTGTTCCTCCT); A1R
(forward, GGCCACAGACCTACTTCCAC and reverse, ACCGGAGAGGGATCTTGACT);
and A2aR (forward, GTCCTCACGCAGAGTTCCAT and reverse, CAC CTG TCA
CCA AGC CAT T).
Western blot analysis
The tissues were lyzed in lysis buffer (Beyotime
Biotechnology, Shanghai, China) that contained freshly added
protease inhibitor. Equivalent amounts of protein lysates and
loading buffer were loaded on 7.5/12.5% polyacrylamide gels,
separated by sodium dodecyl sulfate-polyacrylamide gel
electrophoresis, and subsequently electrophoretically transferred
onto PVDF membranes. These membranes were blocked with 5% non-fat
milk in Tris-buffered saline + 0.1% Tween-20 buffer at room
temperature for 1 h and incubated overnight at 4°C with the
following primary antibodies: Rabbit polyclonal anti-CGRP (1:500;
cat. no. ab47027), rabbit polyclonal anti-adenosine receptor A2a
(1:1,000; cat. no. ab3461), rabbit polyclonal anti-adenosine
receptor A1 (1:500; cat. no. ab82477) (all from Abcam, Shanghai,
China) and β-actin (1:1,000; cat. no. AA128; Beyotime
Biotechnology). The membranes were incubated with the corresponding
horseradish peroxidase-conjugated secondary antibody at room
temperature for 1 h, and the proteins were visualized using an
electrochemiluminescence kit (1:5,000; cat. no. A0208; both from
Beyotime Biotechnology).
Statistical analysis
All the values are presented as the mean ± standard
deviation. Independent Student's t-test was used to compare the
data from two groups. One-way analysis of variance followed by
Tukey's post-hoc test was applied when more than two groups of data
were compared. P<0.05 was considered to indicate a statistically
significant difference. SPSS 16.0 software (SPSS, Inc., Chicago,
IL, USA) was used for statistical analysis.
Results
Effect of electrical stimulation on
CGRP, A1R and A2aR expression in the TG and TNC
According to the western blot analysis and RT-qPCR
results, electrical stimulation significantly increased CGRP and
A2aR expression in the TNC and ipsilateral TG compared with the
blank groups and SO groups (P<0.05) (Figs. 1 and 2).
Electrical stimulation also significantly decreased A1R expression
in the TNC and ipsilateral TG compared with the blank groups and SO
groups (P<0.05) (Figs. 1 and
2), in which no differences were
detected.
 | Figure 1.Results of RT-qPCR analysis. A
relative quantity of each sample was calculated using the
2−ΔΔCt method. (A) RT-qPCR analysis of CGRP, A2aR and
A1R mRNA expression levels in ipsilateral TG. No significant
differences in the three genes expression were observed between the
blank and SO groups, while electrical stimulation significantly
increased CGRP and A2aR mRNA expression in ipsilateral TG compared
with the blank and SO groups. Electrical stimulation also
significantly decreased A1R mRNA expression in ipsilateral TG
compared with the blank and SO groups. (B) RT-qPCR analysis of
CGRP, A2aR and A1R mRNA expression in the TNC. No significant
differences in the three gene expressions were observed between the
blank and SO groups, while electrical stimulation significantly
increased CGRP and A2aR mRNA expression in the TNC compared with
the blank and SO groups. Electrical stimulation also significantly
decreased A1R mRNA expression in the TNC compared with the blank
and SO groups. *P<0.05 compared with the blank and SO group.
RT-qPCT, reverse transcription-quantitative polymerase chain
reaction; CGRP, calcitonin gene-related peptide; A2aR, adenosine
A2a receptor; A1R, adenosine A1 receptor; TG, trigeminal ganglion;
TNC, trigeminal nucleus caudalis; SO, sham-operated. |
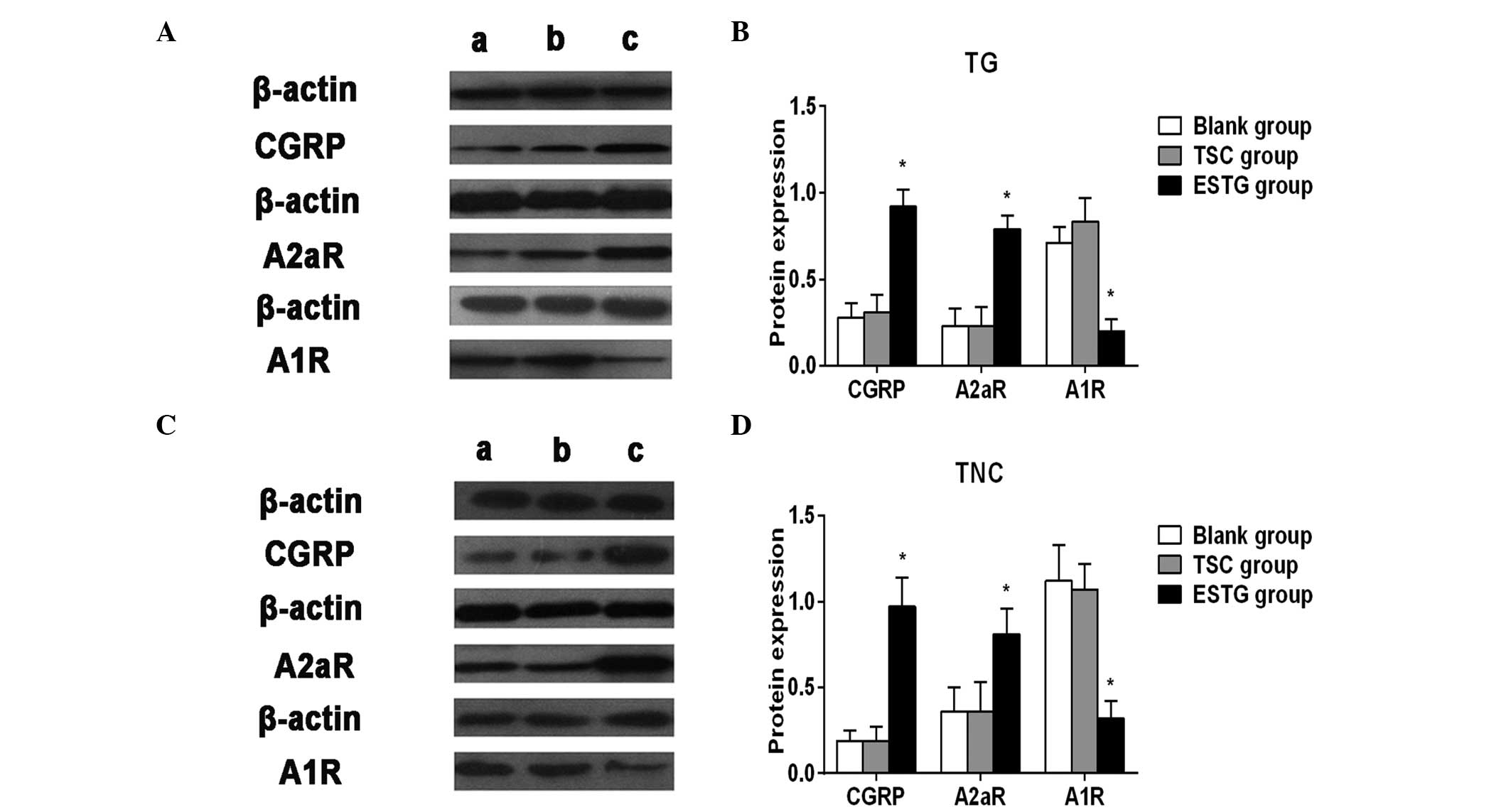 | Figure 2.(A) Representative images of CGRP,
A2aR and A1R expression in ipsilateral TG. (B) Western blot
analysis of the expression of CGRP, A2aR and A1R in ipsilateral TG.
No significant differences in the the three proteins expression
were observed between the (a) blank groups and the (b) TSC groups,
while pretreatment with TSC significantly decreased CGRP and A2aR
expression in ipsilateral TG compared with the (c) ESTG groups. TSC
significantly increased A1R expression compared with the ESTG
groups. (C) Representative images of CGRP, A2aR and A1R expression
in the TNC. (D) Western blot analysis of the expression of CGRP,
A2aR and A1R in the TNC. No significant differences in the three
proteins expression levels were observed between the blank and the
TSC groups, while pretreatment with TSC significantly decreased
CGRP and A2aR expression in the TNC compared with the ESTG groups.
TSC significantly increased A1R expression compared with the ESTG
groups. *P<0.05 compared with the blank and TSC groups. CGRP,
calcitonin gene-related peptide; A2aR, adenosine A2a receptor; A1R,
adenosine A1 receptor; TG, trigeminal ganglion; TNC, trigeminal
nucleus caudalis; TSC, Tianshu capsule. |
Effect of pretreatment with TSC on CGRP, A1R and
A2aR expression in the TG and TNC. According to the western blot
results, TSC pretreatment with TSC significantly decreased CGRP and
A2aR expression in the TNC and ipsilateral TG compared with the
ESTG group (P<0.05) (Fig. 2). TSC
significantly increased A1R expression compared with the ESTG group
(P<0.05) (Fig. 2). No differences
were detected between the blank and TSC groups.
Discussion
Trigeminovascular theory is currently dominating the
pathogenesis of migraine. This theory suggests that the activation
of the trigeminovascular system (TVS) results in increased CGRP
release that causes neurogenic inflammation, which is characterized
by meningeal vascular expansion, plasma protein leakage and mast
cell degranulation (11). Neurogenic
inflammation is an important part of the pathogenesis of migraine.
CGRP is essential vasoactive compound that cause neurogenic
inflammation. Studies have identified that the CGRP expression
levels in the external jugular vein, cubital vein and cerebrospinal
fluid were significantly higher in patients with migraines compared
to those in the healthy controls, and CGRP content is greater in
the external jugular vein than that in the cubital vein (12). These results suggest that trigeminal
nerve endings, which dominate the cerebravl blood wall, can release
CGRP. Further research has found that CGRP nerve fibers dominating
cerebrovascular are mainly from the TG (13). The present study showed that the
expression levels of CGRP protein and CGRP mRNA in TG were higher
in the migraine model rats compared to the in normal rats, further
suggesting that the elevated CGRP levels during migraine attacks
may be the result of CGRP release from the TG neuron. The results
also showed that the ESTG model rats can stimulate TVS activation
during a migraine attack.
TNC includes secondary neurons that are responsible
for integrating the nociceptive information from the TG neurons.
TNC and TG activities reflect the activation of trigeminal nerve
nociceptive pathway in the central nervous system. TNC is a key
component of pain transmission process. CGRP may be involved in the
neuron activity adjustment (14). The
present study identified that the CGRP protein and mRNA levels in
TNC were higher in the ESTG model rats than those in normal rats.
This finding suggests that CGRP is important in migraine
transmission.
Adenosine, a strong vasoactive compound, and its
receptors have an important role in pain transmission and
information regulation. Adenosine injection in the vein, spine and
intraventricular area has an analgesic effect (15–17).
Numerous animal experiments (18–20) showed
that A1 receptor activation can have a role in pathological
neuralgia, inflammation and pain in animal models. Other studies
showed that selective A1R agonists can inhibit neurogenic
vasodilation and CGRP release, thereby inhibiting migraine
generation and transfer (21).
Combined with the experimental results, the A1R expression in TNC
and TG in migraine model rats was lower than that in normal rats.
This result suggests that decreased expression of A1R has an
important role in the pathogenesis of migraine, whereas activation
or increased expression of adenosine A1R may suppress the
occurrence of migraines.
A2aR is widely distributed in the central nervous
system and mediates the pathophysiology of numerous diseases, such
as neuronal protection, stroke, pain and neurodegenerative disease,
through interaction with other important receptors (22–24).
Adenosine can directly stimulate the nociceptive nerve through A2aR
and A2aR-knockout mice have a high pain threshold (25). The present study found that the A2aR
expression in TG and TNC of the ESTG group was higher than that of
the blank control group, which was similar with CGRP expression.
This result suggests that CGRP and A2aR may cause migraine and pain
transmission when combined. In addition, a previous study showed
that CGRP cannot affect synaptic transmission in the brain neurons
but can enhance excitatory synapse potential depending on A2aR
activation and A1R inhibition (26).
Another study revealed that A2aR activation can inhibit A1R,
suggesting that transmission of migraine information requires
multiple neurotransmitter interactions (27).
TSC is a modern Chinese medicine that consists of
Chuanxiong and Tianma. TSC can promote blood circulation to remove
blood stasis and activate meridians to alleviate pain. This Chinese
medicine is mainly used for migraine treatment and has a beneficial
effect; however, the underlying mechanism remains to be elucidated.
The experiments showed that the CGRP, A1R and A2aR expression in
the TSC intervention group did not significantly differ with that
in the blank group, whereas no significant differences were found
between the ESTG and TSC groups, suggesting that TSC can improve
the levels of the three proteins involved in the pathogenesis of
migraine for relief. Other studies showed that TSC can adjust the
expression of vasoactive compounds, such as nitric oxide, CGRP and
neurotransmitters, including serotonin, during migraine attacks,
thereby relieving migraine symptoms (28,29). In
conclusion, TSC can adjust to a variety of vasoactive compounds and
neurotransmitters involved in the pathogenesis of migraines,
thereby affecting the onset and progression of migraine.
The CGRP, A2aR and A1R expression in TG and TNC
significantly changed the migraine rats more than the normal rats.
CGRP not only had an important role in neurogenic inflammation, but
may also have mediated migraine pain information with adenosine.
TSC can adjust the expression of these three proteins, thereby
providing a new experimental basis and targets for the pathogenesis
and treatment of migraines. However, the interaction mechanisms of
CGRP, A1R and A2aR in the pathogenesis of migraines require further
study.
Acknowledgements
The present study was supported by the Science and
Technology Project in Shandong Province (project no. J12LL63).
References
|
1
|
Goadsby PJ: Recent advances in
understanding migraine mechanisms, molecules and therapeutics.
Trends Mol Med. 13:39–44. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Noseda R and Burstein R: Migraine
pathophysiology: Anatomy of the trigeminovascular pathway and
associated neurological symptoms, cortical spreading depression,
sensitization, and modulation of pain. Pain. 154(Suppl 1): S44–S53.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ho TW, Edvinsson L and Goadsby PJ: CGRP
and its receptors provide new insights into migraine
pathophysiology. Nat Rev Neurol. 6:573–582. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Russo AF: Calcitonin gene-related peptide
(CGRP): A new target for migraine. Annu Rev Pharmacol Toxicol.
55:533–552. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Villalón CM and Olesen J: The role of CGRP
in the pathophysiology of migraine and efficacy of CGRP receptor
antagonists as acute antimigraine drugs. Pharmacol Ther.
124:309–323. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Goadsby PJ, Edvinsson L and Ekman R:
Release of vasoactive peptides in the extracerebral circulation of
humans and the cat during activation of the trigeminovascular
system. Ann Neurol. 23:193–196. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Goadsby PJ: New targets in the acute
treatment of headache. Curr Opin Neurol. 18:283–288. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Goadsby PJ: Post-triptan era for the
treatment of acute migraine. Curr Pain Headache Rep. 8:393–398.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fredholm BB, IJzerman AP, Jacobson KA,
Klotz KN and Linden J: International Union of Pharmacology. XXV.
Nomenclature and classification of adenosine receptors. Pharmacol
Rev. 53:527–552. 2001.PubMed/NCBI
|
|
10
|
Gao H, Yu Z, Ma K and Han G: Tianshu
capsule for migraine: A systemic review. Chin Tradit Pat Med.
36:1156–1160. 2014.
|
|
11
|
Messlinger K: Migraine: Where and how does
the pain originate? Exp Brain Res. 196:179–193. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Moreno MJ, Abounader R, Hébert E, Doods H
and Hamel E: Efficacy of the non-peptide CGRP receptor antagonist
BIBN4096BS in blocking CGRP-induced dilations in human and bovine
cerebral arteries: Potential implications in acute migraine
treatment. Neuropharmacology. 42:568–576. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gibson SJ, Polak JM, Bloom SR, Sabate IM,
Mulderry PM, Ghatei MA, McGregor GP, Morrison JF, Kelly JS, Evans
RM, et al: Calcitonin gene-related peptide immunoreactivity in the
spinal cord of man and of eight other species. J Neurosci.
4:3101–3111. 1984.PubMed/NCBI
|
|
14
|
Fischer MJ, Koulchitsky S and Messlinger
K: The nonpeptide calcitonin gene-related peptide receptor
antagonist BIBN4096BS lowers the activity of neurons with meningeal
input in the rat spinal trigeminal nucleus. J Neurosci.
25:5877–5883. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sjölund KF, Belfrage M, Karlsten R,
Segerdahl M, Arnér S, Gordh T and Solevi A: Systemic adenosine
infusion reduces the area of tactile allodynia in neuropathic pain
following peripheral nerve injury: A multi-centre,
placebo-controlled study. Eur J Pain. 5:199–207. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Poon A and Sawynok J: Antinociception by
adenosine analogs and inhibitors of adenosine metabolism in an
inflammatory thermal hyperalgesia model in the rat. Pain.
74:235–245. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ma HC, Wang YF, Feng CS, Zhao H and Dohi
S: Effects of adenosine agonist R-phenylisopropyl-adenosine on
halothane anesthesia and antinociception in rats. Acta Pharmacol
Sin. 26:181–185. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lavand'homme PM and Eisenach JC: Exogenous
and endogenous adenosine enhance the spinal antiallodynic effects
of morphine in a rat model of neuropathic pain. Pain. 80:31–36.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Maione S, de Novellis V, Cappellacci L,
Palazzo E, Vita D, Luongo L, Stella L, Franchetti P, Marabese I,
Rossi F, et al: The antinociceptive effect of
2-chloro-2-C-methyl-N6-cyclopentyladenosine (2-Me-CCPA), a highly
selective adenosine A1 receptor agonist, in the rat. Pain.
131:281–292. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sjölund KF, Sollevi A, Segerdahl M,
Hansson P and Lundeberg T: Intrathecal and systemic
R-phenylisopropyl-adenosine reduces scratching behaviour in a rat
mononeuropathy model. Neuroreport. 7:1856–1860. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Goadsby PJ, Hoskin KL, Storer RJ,
Edvinsson L and Connor HE: Adenosine A1 receptor agonists inhibit
trigeminovascular nociceptive transmission. Brain. 125:1392–1401.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen JF, Sonsalla PK, Pedata F, Melani A,
Domenici MR, Popoli P, Geiger J, Lopes LV and de Mendonça A:
Adenosine A2A receptors and brain injury: Broad spectrum of
neuroprotection, multifaceted actions and ‘fine tuning’ modulation.
Prog Neurobiol. 83:310–331. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Takahashi RN, Pamplona FA and Prediger RD:
Adenosine receptor antagonists for cognitive dysfunction: A review
of animal studies. Front Biosci. 13:2614–2632. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Moro S, Gao ZG, Jacobson KA and Spalluto
G: Progress in the pursuit of therapeutic adenosine receptor
antagonists. Med Res Rev. 26:131–159. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ledent C, Vaugeois JM, Schiffmann SN,
Pedrazzini T, El Yacoubi M, Vanderhaeghen JJ, Costentin J, Heath
JK, Vassart G and Parmentier M: Aggressiveness, hypoalgesia and
high blood pressure in mice lacking the adenosine A2a receptor.
Nature. 388:674–678. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sebastião AM, Macedo MP and Ribeiro JA:
Tonic activation of A(2A) adenosine receptors unmasks, and of A(1)
receptors prevents, a facilitatory action of calcitonin
gene-related peptide in the rat hippocampus. Br J Pharmacol.
129:374–380. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cunha-Reis D, Ribeiro JA and Sebastião AM:
A1 and A2A receptor activation by endogenous adenosine is required
for VIP enhancement of K+-evoked [3H]-GABA
release from rat hippocampal nerve terminals. Neurosci Lett.
430:207–212. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang Q, Yang T, Zhou M and Zhou D:
Effects of Tianshu capsule on plasma NO, NOS, CGRP contents and
hemodynamic of migraine animal models. J Clin Neurol. 21:279–282.
2008.
|
|
29
|
Tianhua YZ, Zhou M, Liu Y and Zhou D:
Effect of Tianshu capsule on the plasma levels of β-endorphin and
5-Hydroxytryptamine and c-fos expression of brain tissue in
migraine rats. J Clin Neurol. 21:368–370. 2008.
|
















