Introduction
Lung neoplasm is the leading cause of cancer-related
mortalities worldwide. Approximately 80–85% of cases are
non-small-cell lung cancer. The 5-year survival rate for lung
cancer is 18% (1). Even for those
patients with early-stage disease who undergo surgical resection,
the postoperative recurrence rate is higher than that of other
types of cancer (2). Lung neoplasm has
the highest mortality rate in the USA, accounting for almost 86,220
mortalities in 2010, with an estimated 116,000 new cases and 86,930
mortalities in 2014 (1,3). It is well known that early diagnosis and
treatment lead to better outcome. However, our understanding of
possible factors connected with early diagnosis is limited. Thus,
it is essential to identify novel and useful biological tumor
markers that may more accurately establish the diagnosis of lung
neoplasm. In recent years, studies have suggested that preoperative
osteopontin (OPN) is positively correlated with lung neoplasm
progression, which may be a clinically useful biomarker for
predicting lung cancer (4–6).
OPN is a 44-kDa, secreted, highly acidic, and
adhesive protein that is detected in whole body liquids (7). Accepted as the primary phosphorylated
glycoprotein of the bone, OPN is simultaneously secreted in a large
number of tissues and cells, including blood vessels, skin tissues,
as well as immune cells (8). In
clinical practice, OPN is regarded as a chemotactic factor as well
as a matrix factor and a cytokine, possessing pleiotropic functions
(9). OPN is involved in many
physiological processes, bone remodeling, macrophage migration,
angiogenesis, and neutrophil migration (8,10).
Nevertheless, previous findings showed that OPN is highly involved
in the development of cardiovascular diseases and other types of
cancer, including colorectal cancer, gastric cancer, breast cancer,
and ovarian neoplasm (11–15). In addition, OPN has been found to be
associated with the presence and progression of lung neoplasm,
demonstrating that plasma OPN concentrations were higher in lung
neoplasm patients when compared with healthy individuals (5). Previous findings have demonstrated that
OPN increased the invasion of lung cancer cells by triggering ROCK
signaling mediated by the FAK/PI3K/AKT pathway, which in turn
induces lamellipodia formation by inactivating cofilin (16). Furthermore, increasing OPN serum levels
are positively associated with advanced disease states and smoking
history (6). A higher expression of
OPN in NSCLC tumors was associated with poor patient outcome
(17–19). Therefore, OPN may be considered a new
diagnostic marker for predicting, detecting, and evaluating lung
neoplasm. Additionally, OPN is a promising biomarker for lung
neoplasm prediction, diagnosis, prognosis and even metastasis
(5,20,21).
However, other studies have presented inconsistent results
(22). Due to these conflicting
results, we conducted a meta-analysis to evaluate the potential
usefulness of serum OPN levels for the prediction and diagnosis of
lung neoplasm.
Materials and methods
Search strategy
Related articles were identified by searching the
PubMed, Web of Science, Google Scholar, China National Knowledge
Infrastructure, and Wang Fang databases comprehensively for
published articles that assessed the correlations between OPN serum
levels and lung neoplasm for the period up to March 31, 2015, using
the keywords ‘Lung Neoplasms’ or ‘lung neoplasm’ or ‘lung cancers’
or ‘lung cancer’ or ‘cancer of lung’ or ‘lung carcinoma’ or ‘lung
adenocarcinoma’ or ‘lung tumor’, ‘Osteopontin’ or ‘Sialoprotein’ or
‘Secreted Phosphoprotein 1’ or ‘Bone Sialoprotein 1’ or ‘Uropontin’
or ‘OPN’. The search was performed without language restriction.
Additional potential relevant articles were retrieved through a
manual search of references from original reports.
Selection criteria
Any randomized intervention case-control studies
that involved the association of OPN serum levels with lung
neoplasm as a primary outcome were initially taken into
consideration. Aside from studies involving healthy participants as
controls, studies that had patients diagnosed with lung neoplasm
confirmed by histopathologic examinations and associated with OPN
serum levels were also included for the initial review of the
articles. Studies that did not provide the number of lung neoplasm
cases or sufficient information regarding serum OPN expression
levels were not included. A relatively low number of participants
in a study was not considered sufficient grounds for exclusion, but
all the included studies had ≥20 participants. Any studies that
were duplicates, lacked complete data, or had unavailable data were
excluded. If the same participants were involved in more than one
study, only the most recent or most complete study was included
after careful re-examination.
Data extraction
To minimize the bias and improve reliability, two
investigators extracted information according to the selection
criteria independently and reached a consensus on all the items
through discussion and re-examination. The following relevant data
were extracted from eligible studies prospectively: surname of
first author, year of publication, study type, study design, sample
size, source of controls, age, gender, ethnicity and country of
origin, and detection method of OPN serum levels. Due to subjects
from different ethnicities, information was extracted separately
and classified into Asians and Caucasians.
Statistical analysis
The current statistical meta-analysis was conducted
utilizing a random effects model (DerSimonian and Laird method) or
a fixed effects model (Mantel-Haenszel method) of individual study
results when data from independent studies could be combined. The
random effects model was applied when heterogeneity existed among
studies, while the fixed effects model was applied when there was
no statistical heterogeneity. The summary standardized mean
difference (SMD) with 95% confidence intervals (CI) was calculated
for the case versus control category of OPN serum levels with the
Z-test. The subgroup meta-analyses were also conducted by ethnicity
and source of controls to determine the potential effect
modification. Heterogeneity across the enrolled studies was
evaluated using Cochran's Q-statistic. P<0.05 was considered to
indicate statistical significance (23).
As a result of the low statistical power of
Cochran's Q-statistic, the I2 test was also measured to
reflect the possibility of heterogeneity between studies (24). Sensitivity analysis was performed to
evaluate whether the results were potentially affected
significantly by deleting single studies individually to determine
the influence of individual data sets on the pooled SMD. The funnel
plot was constructed to assess the effect of publication bias on
the validity of the estimates. The symmetry of the funnel plot was
subsequently evaluated using Egger's linear regression test
(25). Tests were two-sided, and
P<0.05 was considered to indicate a statistically significant
difference. STATA software, version 12.0 (Stata Corporation,
College Station, TX, USA) was used for statistical analysis.
Results
Baseline characteristics of included
studies
The original search yielded a total of 31 papers
related to the searched keywords. The flow chart of the study
selection process is provided in Fig.
1. Ten studies (22,26–34) were
found to meet the inclusion criteria, with the publication year
ranging from 2001 to 2014. All the articles identified were
case-control studies that assessed the association of OPN serum
levels and lung neoplasm in Asian populations (22,26–28,31–33) (7 studies) and Caucasian populations
(29,30,34) (3
studies). The detection method applied in the present meta-analysis
was enzyme-linked immunosorbent assay. Table I shows the characteristics of the
enrolled studies.
 | Table I.Characteristics of included studies
focused on protein expression of OPN. |
Table I.
Characteristics of included studies
focused on protein expression of OPN.
| Author (year) | Ethnicity | Source of
controls | Sample size
(case/control) | Method | (Refs.) |
|---|
| Song (2014) | Asians | PB | 32/35 | ELISA | (26) |
| Qiu and Fu
(2012) | Asians | PB | 56/40 | ELISA | (27) |
| Xu et al
(2012) | Asians | PB | 102/30 | ELISA | (28) |
|
|
| HB | 102/42 | ELISA |
| Karadag et al
(2011) | Caucasians | PB | 63/25 | ELISA | (29) |
| Zhou et al
(2011) | Asians | HB | 56/45 | ELISA | (22) |
|
|
|
| 103/45 | ELISA |
| Blasberg et al
(2010) | Caucasians | HB | 60/56 | ELISA | (30) |
| Yin and Zhang
(2009) | Asians | PB | 80/40 | ELISA | (31) |
|
|
| HB | 80/40 | ELISA |
| Dong et al
(2008) | Asians | PB | 120/49 | ELISA | (32) |
|
|
| HB | 120/23 | ELISA |
| Weng and Fu
(2007) | Asians | PB | 48/40 | ELISA | (33) |
|
|
| HB | 48/45 | ELISA |
| Fedarko et
al (2001) | Caucasians | PB | 20/77 | ELISA | (34) |
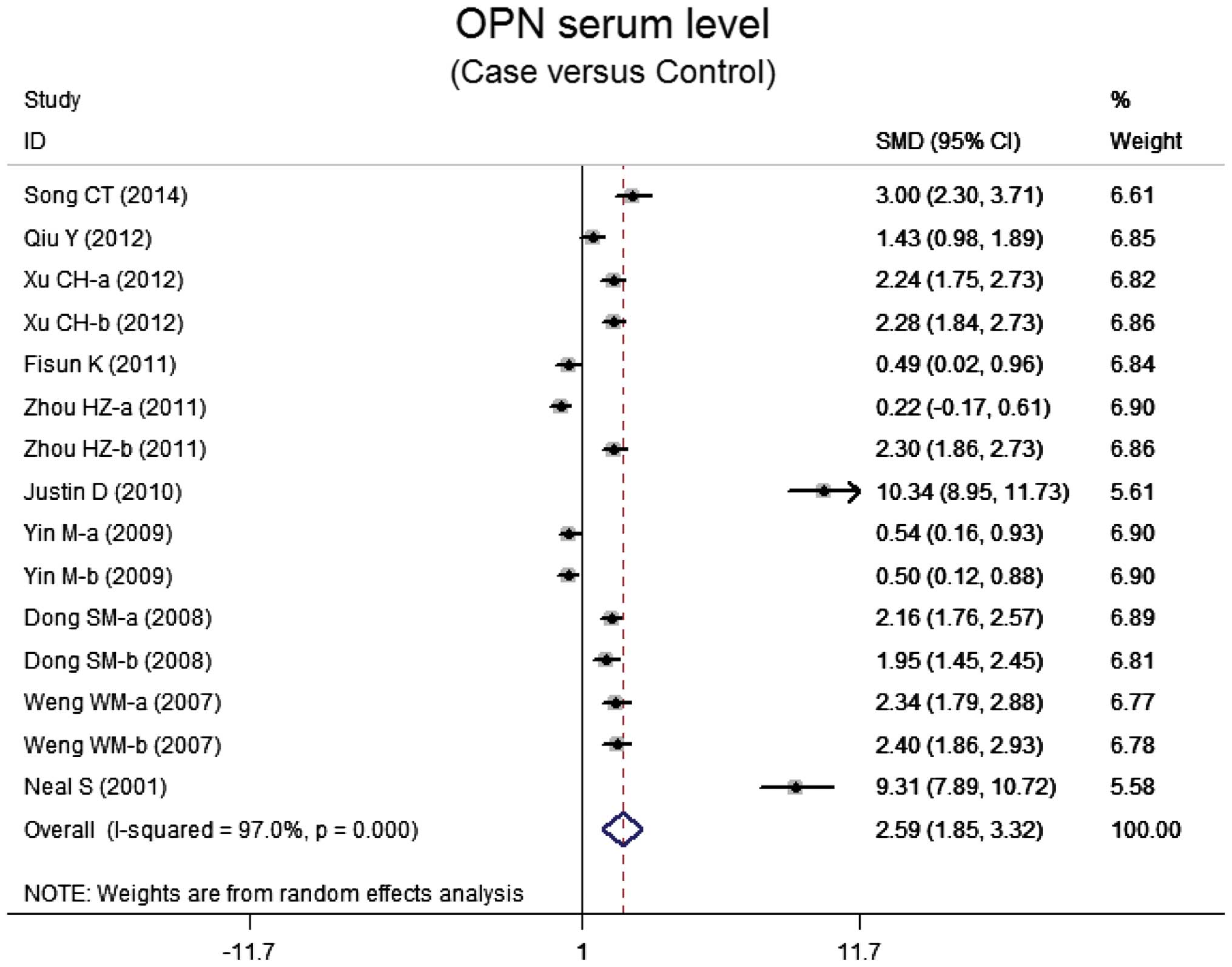
OPN serum levels in lung neoplasm
Ten case-control studies referred to the OPN serum
levels in lung neoplasm. The results of the correlation between the
levels of OPN and lung neoplasm are shown in Fig. 2. Using the random effects model, we
determined that there was heterogeneity (P<0.001). The
meta-analysis results identified a positive association between OPN
serum levels and lung neoplasm (SMD=5.59, 95% CI: 1.85–3.32,
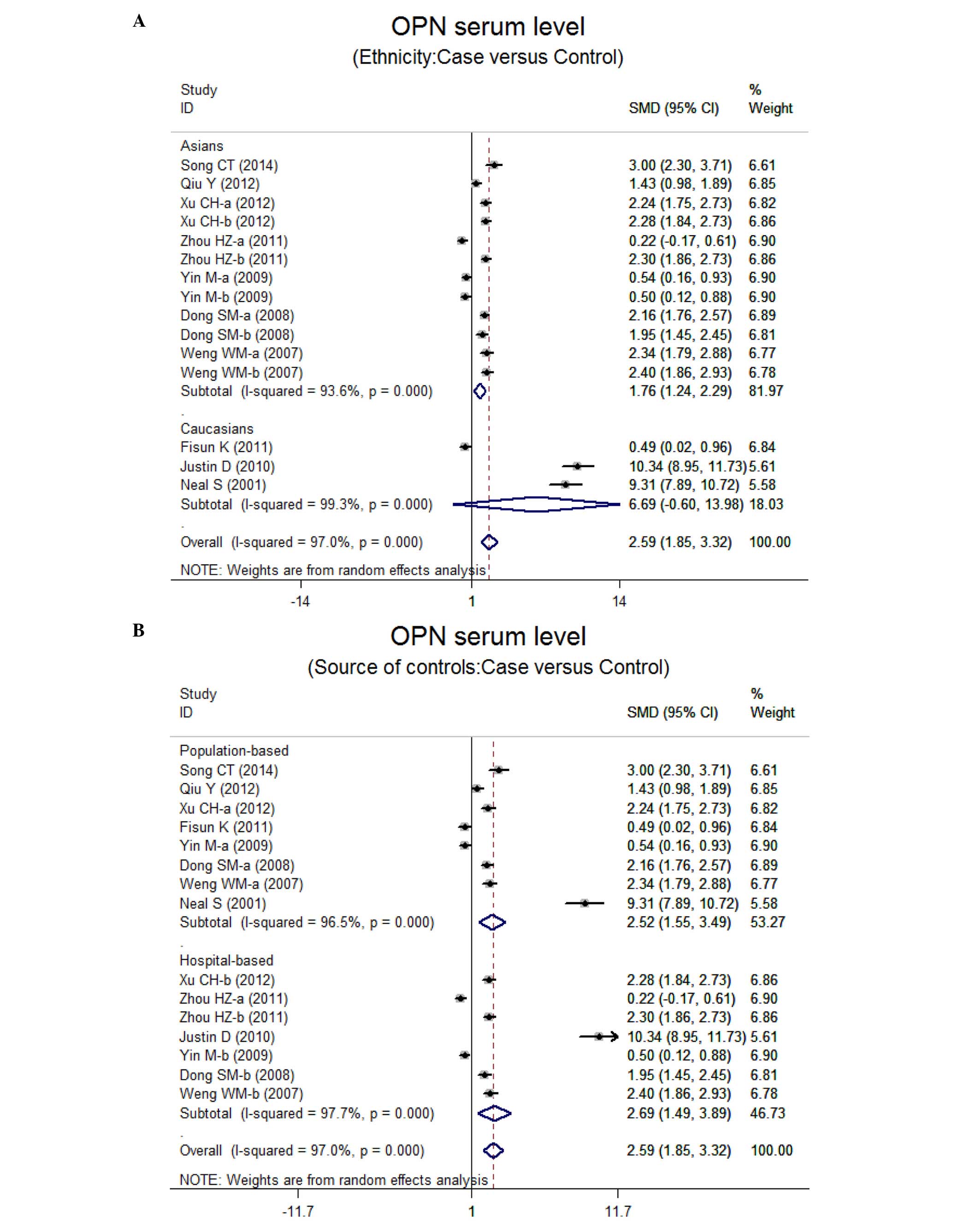
P<0.001). The subgroup analysis based on ethnicity suggested
that a high level of serum OPN was the main risk factor for lung
neoplasm in Asian populations (SMD=1.76, 95% CI: 1.24–2.29,
P<0.001), but not in Caucasian populations (P=0.072) (Fig. 3). Further subgroup analyses by source
of controls showed an obvious association between the levels of OPN
and lung neoplasm in the population-based (PB) and hospital-based
(HB) subgroups (all P<0.001) (Fig.
3).
Sensitivity analysis and publication
bias
Sensitivity analysis showed that the statistical
indications changed slightly when the study of Zhou et al
(22) or Yin and Zhang (31) was omitted. Therefore, the current
meta-analysis data were inconsistent. The graphical funnel plots of
the 10 studies are a little asymmetrical, and Egger's test shows a
publication bias in the current meta-analysis (t=6.38, P<0.001)
(data not shown).
Discussion
In this meta-analysis, we investigated the possible
relationship between the serum levels of OPN and the development of
lung neoplasm. Our results suggest that lung neoplasm patients have
higher OPN serum levels, demonstrating that OPN serum levels may be
important in the development of lung neoplasm. Previous findings
have shown that there was a significant reverse correlation between
OPN and overall and disease-free survival (20). Generally, OPN, an integrin-binding
protein, is involved in numerous signaling pathways, such as cancer
cell growth, adhesion, apoptosis, metastasis, proliferation,
migration, invasion, and angiogenesis (7,35,36). OPN is capable of inducing cell invasion
by markedly increasing the expression of Rho-associated kinase 1
(ROCK1), an upstream effector of LIMK/cofilin mediated by the
FAK/PI3K/AKT pathway. ROCK1 inactivated cofilin by phosphorylating
LIMK and cofilin which block lamellipodia formation. Thus, the
invasion of lung neoplasm cells can be increased by OPN under
stress conditions via the FAK/PI3K/AKT pathway and ROCK1 expression
induction (16). Furthermore, OPN is
involved in lung neoplasm angiogenesis because OPN expression in
cancer tissue was positively correlated with microvascular density
(37). In addition, the OPN level was
correlated with αv integrin expression and the decreased apoptotic
activity of lung adenocarcinomas cells (38). OPN also led to an obvious promotion of
in vitro invasion and in vivo lung metastasis by
increasing the levels of matrix metalloproteinase-2 and urokinase
plasminogen activator (39). Thus,
higher levels of OPN promotes tumor progression through invasion,
angiogenesis, metastasis and inhibition of apoptosis. In
concordance with our results, Zhang et al (40) identified that higher levels of OPN were
positively associated with stage, lymph node metastasis, tumor size
and pathology of lung neoplasms because of their anti-apoptotic or
proliferative effects on lung neoplasm. Thus, OPN is a possible
biomarker for lung neoplasm detection, prognosis, and even their
apeutic intervention.
Given the fact that other factors may influence the
relationship between serum OPN level and lung neoplasm, we
conducted a stratified analysis based on ethnicity and source of
controls. The subgroup analysis by ethnicity showed that higher
serum levels of OPN were evident in Asians, while no similar result
was observed among Caucasian populations. One possible explanation
may be the different genetics and environments of the two
ethnicities. Additionally, there was no obvious effect of source of
controls on the relationship between OPN and ovarian neoplasm.
Thus, higher serum levels of OPN remain strongly connected with the
progression of ovarian neoplasm. This lends further credence to the
creditability of the analysis as it indicates that the samples are
random and well selected. Therefore, our results were partially
consistent with the hypothesis that higher serum levels of OPN may
have a strong connection with lung neoplasm, suggesting that the
serum OPN level is an optimal marker for lung neoplasm
identification and prognosis.
Limitations of this meta-analysis should be
considered. First, possible selection biases were identified, there
was a highly significant heterogeneity among the 10 evaluable
articles. Additionally, the Egger's test showed a publication bias
in our meta-analysis. Second, is that certain unpublished articles
and abstracts were not taken into account as their data were not
available, leading to potential publication bias in the present
study. Language may also introduce a bias. Specifically, English or
Chinese studies were selected while excluding qualified studies in
other languages. A third potential limitation is that our
meta-analysis was weakened by an inability to extract the original
data from the included studies. Despite the above limitations, this
is the first systematic review on the association of serum OPN
levels with the risk of lung neoplasm. Application of a statistical
approach to combine the results from multiple studies in this
meta-analysis and to achieve strong results led to the research
methods being carried out using strict inclusion and exclusion
criteria, indicating the validity and significance of our
conclusion.
In summary, the present meta-analysis indicates that
increased serum levels of OPN may contribute to an aggressive
progression of lung neoplasm. Based on the data obtained, OPN is a
potentially useful marker for diagnosing lung neoplasms. Hoverer,
in light of the limited sample size included and the potential for
bias in the current study, additional investigations on serum OPN
levels and lung neoplasms are needed.
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chansky K, Sculier JP, Crowley JJ, Giroux
D, Van Meerbeeck J and Goldstraw P: International Staging Committee
and Participating Institutions: The International Association for
the Study of Lung Cancer Staging Project: Prognostic factors and
pathologic TNM stage in surgically managed non-small cell lung
cancer. J Thorac Oncol. 4:792–801. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hu Z, Lin D, Yuan J, Xiao T, Zhang H, Sun
W, Han N, Ma Y, Di X, Gao M, et al: Overexpression of osteopontin
is associated with more aggressive phenotypes in human non-small
cell lung cancer. Clin Cancer Res. 11:4646–4652. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Han SS, Lee SJ, Kim WJ, Ryu DR, Won JY,
Park S and Cheon MJ: Plasma osteopontin is a useful diagnostic
biomarker for advanced non-small cell lung cancer. Tuberc Respir
Dis (Seoul). 75:104–110. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chang YS, Kim HJ, Chang J, Ahn CM and Kim
SK and Kim SK: Elevated circulating level of osteopontin is
associated with advanced disease state of non-small cell lung
cancer. Lung Cancer. 57:373–380. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Moszynski R, Szubert S, Szpurek D,
Michalak S and Sajdak S: Role of osteopontin in differential
diagnosis of ovarian tumors. J Obstet Gynaecol Res. 39:1518–1525.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vetrone SA, Montecino-Rodriguez E,
Kudryashova E, Kramerova I, Hoffman EP, Liu SD, Miceli MC and
Spencer MJ: Osteopontin promotes fibrosis in dystrophic mouse
muscle by modulating immune cell subsets and intramuscular
TGF-beta. J Clin Invest. 119:1583–1594. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cho HJ, Cho HJ and Kim HS: Osteopontin: A
multifunctional protein at the crossroads of inflammation,
atherosclerosis, and vascular calcification. Curr Atheroscler Rep.
11:206–213. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lund SA, Giachelli CM and Scatena M: The
role of osteopontin in inflammatory processes. J Cell Commun
Signal. 3:311–322. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Okyay K, Tavil Y, Sahinarslan A, Tacoy G,
Turfan M, Sen N, Gurbahar O, Boyaci B, Yalcin R, Demirkan D, et al:
Plasma osteopontin levels in prediction of prognosis in acute
myocardial infarction. Acta Cardiol. 66:197–202. 2011.PubMed/NCBI
|
|
12
|
Song G, Ouyang G, Mao Y, Ming Y, Bao S and
Hu T: Osteopontin promotes gastric cancer metastasis by augmenting
cell survival and invasion through Akt-mediated HIF-1alpha
up-regulation and MMP9 activation. J Cell Mol Med. 13(8B):
1706–1718. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu YY, Zhang YY, Lu WF, Mi YJ and Chen YQ:
Prognostic value of osteopontin expression in breast cancer: A
meta-analysis. Mol Clin Oncol. 3:357–362. 2015.PubMed/NCBI
|
|
14
|
Yuan SM, Wang J, Huang HR and Jing H:
Osteopontin expression and its possible functions in the aortic
disorders and coronary artery disease. Rev Bras Cir Cardiovasc.
26:173–182. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang YD, Chen H, Liu HQ and Hao M:
Correlation between ovarian neoplasm and serum levels of
osteopontin: A meta-analysis. Tumour Biol. 35:11799–11808. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kang CG, Han HJ, Lee HJ, Kim SH and Lee
EO: Rho-associated kinase signaling is required for
osteopontin-induced cell invasion through inactivating cofilin in
human non-small cell lung cancer cell lines. Bioorg Med Chem Lett.
25:1956–1960. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rud AK, Boye K, Oijordsbakken M,
Lund-Iversen M, Halvorsen AR, Solberg SK, Berge G, Helland A,
Brustugun OT and Mælandsmo GM: Osteopontin is a prognostic
biomarker in non-small cell lung cancer. BMC Cancer. 13:5402013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sun BS, Li Y, Zhang ZF, You J and Wang CL:
Osteopontin combined with CD44v6, a novel prognostic biomarker in
non-small cell lung cancer undergoing curative resection. Ann
Thorac Surg. 96:1943–1951. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Peng B, Wang YH, Huang Z, Feng SJ and Wang
YS: Prognostic significance of osteopontin in patients with lung
cancer: A meta-analysis. Int J Clin Exp Med. 7:4616–4626.
2014.PubMed/NCBI
|
|
20
|
Yan CH, Lv M, Li H, Song X, Yan F, Cao S
and Ren X: Osteopontin is a novel prognostic biomarker in
early-stage non-small cell lung cancer after surgical resection. J
Cancer Res Clin Oncol. 141:1371–1378. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Karapanagiotou EM, Terpos E, Dilana KD,
Alamara C, Gkiozos I, Polyzos A and Syrigos KN: Serum bone turnover
markers may be involved in the metastatic potential of lung cancer
patients. Med Oncol. 27:332–338. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhou H, Wu T and Zhu Y: The clinical
significance of serum osteopontin in diagnosis of lung cancer. J
Radioimmunol. 24:458–459. 2011.(In Chinese).
|
|
23
|
Jackson D, White IR and Riley RD:
Quantifying the impact of between-study heterogeneity in
multivariate meta-analyses. Stat Med. 31:3805–3820. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Peters JL, Sutton AJ, Jones DR, Abrams KR
and Rushton L: Comparison of two methods to detect publication bias
in meta-analysis. JAMA. 295:676–680. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zintzaras E and Ioannidis JP: HEGESMA:
Genome search meta-analysis and heterogeneity testing.
Bioinformatics. 21:3672–3673. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Song C: Clinical significance of
determination of changes CEA, SA, OPN, and VEGF levels after
operative in patients with lung cancer. Frontier Med. 15:75–76.
2014.
|
|
27
|
Qiu Y and Fu Z: Clinical significance of
determination on serum levels of vascular endothelial growth factor
per platelet count and osteopontin patients with locally advanced
non-small cell lung cancer (unpublished PhD thesis). Hebei Medical
University. Shijiazhuang: R734.2:2012.
|
|
28
|
Xu C, Yu K and Wang Q: The levels and
clinical significance of osteopontin in serum of patients with lung
cancer. J Chin Physician. 14:179–181. 2012.
|
|
29
|
Karadag F, Gulen ST, Karul AB, Kilicarslan
N, Ceylan E, Kuman NK and Cildag O: Osteopontin as a marker of
weight loss in lung cancer. Scand J Clin Lab Invest. 71:690–694.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Blasberg JD, Pass HI, Goparaju CM, Flores
RM, Lee S and Donington JS: Reduction of elevated plasma
osteopontin levels with resection of non-small-cell lung cancer. J
Clin Oncol. 28:936–941. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yin M and Zhang J: Study on OPN levels of
serum in non-small cell lung cancer. Ningxia Med J. 31:679–680.
2009.
|
|
32
|
Dong S, Shao Z, Pan Y, Dai J and Chen G:
The clinical significance of detection of serum osteopontin in
patient with lung cancer. Mod J Integr Tradit Chin West Med.
17:3740–3741. 2008.
|
|
33
|
Weng W and Fu C: Value of serum
osteopontin determination in patient with lung carcinoma. Lab Med
Clin. 4:1025–1026. 2007.
|
|
34
|
Fedarko NS, Jain A, Karadag A, Van Eman MR
and Fisher LW: Elevated serum bone sialoprotein and osteopontin in
colon, breast, prostate, and lung cancer. Clin Cancer Res.
7:4060–4066. 2001.PubMed/NCBI
|
|
35
|
Chambers AF and Vanderhyden BC: Ovarian
cancer biomarkers in urine. Clin Cancer Res. 12:323–327. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lin CK, Chao TK, Lai HC and Lee HS: LMX1A
as a prognostic marker in ovarian mucinous cystadenocarcinoma. Am J
Clin Pathol. 137:971–977. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yu TT, Han ZG, Shan L, Tao J, Zhang T,
Yuan SF and Shen HL: Expression of osteopontin in non-small cell
lung cancer and correlative relation with microvascular density.
Asian Pac J Cancer Prev. 15:29–32. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Štemberger C, Matušan-Ilijaš K, Avirović
M, Bulat-Kardum L, Ivančić A, Jonjić N and Lučin K: Osteopontin is
associated with decreased apoptosis and αv integrin expression in
lung adenocarcinoma. Acta Histochem. 116:222–229. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sun BS, You J, Li Y, Zhang ZF and Wang CL:
Osteopontin knockdown suppresses non-small cell lung cancer cell
invasion and metastasis. Chin Med J (Engl). 126:1683–1688.
2013.PubMed/NCBI
|
|
40
|
Zhang T, Zhang DM, Zhao D, Hou XM, Liu XJ,
Ling XL and Ma SC: The prognostic value of osteopontin expression
in non-small cell lung cancer: A meta-analysis. J Mol Histol.
45:533–540. 2014. View Article : Google Scholar : PubMed/NCBI
|