Introduction
Lung carcinoma is a tumor characterized by extremely
invasion and metastatic behavior, properties that require
interaction of the lung cancer cells with the growth factors.
Insulin-like growth factors (IGFs) are one of the prominent
families of growth factors observed to be closely involved in the
adjustment of cell growth and transformation. IGF-1, a
multifunctional protein peptide, has a significant role in cellular
growth, proliferation, differentiation and transformation in
numerous malignancies, including lung cancer (1–9). In
vitro studies show that IGF-1 increments lung cell growth and
invasive potential, proposing a role for the IGF-1 pathway in the
etiology of lung cancer (1–9). Although numerous studies have observed
that IGF-1 has an important role in lung cancer, the clinical
survival associated with circulating IGF-1 levels is not clear. The
effect of serum IGF-1 on disease relapse and survival in lung
cancer patients are investigated as unsatisfactory (1–9). Thus, the
clinical significance of serum IGF-1 concentrations in lung cancer
patients remains to be elucidated.
The IGF binding protein (IGFBP) family consists of
six structurally related proteins; all members are expressed in the
normal lung tissue (1–9). Their role is to bind and regulate their
effects. IGFBP-3 is the prominent binding protein of IGF-1 and it
regulates the mitogenic and antiapoptotic actions of the IGFs. In
the majority of studies, IGFBP-3 has been observed to correlate
with circulating IGF-1 concentrations. Furthermore, in particularly
IGFBP-3 has direct IGF-independent effects on apoptosis and
cellular growth. The studies show that IGFBP-3 is correlated with
the lung cancer prognosis (1–9). IGFBP-3 may use an inhibitory influence on
the growth of lung cancer. Serum concentrations of IGFBP-3 are
significantly diminished in lung cancer. Additionally, IGFBP-3
overexpression by lung cancer cells with aggressive biological
characteristics has been a paradox.
The importance of the serological IGF-1 and IGFBP-3
concentrations in lung cancer patients remains to be elucidated.
Due to conflicting results reported in recent epidemiological and
clinical studies investigating the IGF axis and lung cancer, the
present study was conducted to investigate whether circulating
IGF-1 and IGFBP-3 concentrations have diagnostic, predictive and
prognostic values in lung cancer patients.
Materials and methods
Patients
A total number of 80 patients with histologically or
cytologically confirmed non-small cell lung cancer (NSCLC) and SCLC
treated and followed-up in the Institute of Oncology (Istanbul
University, Istanbul, Turkey) were enrolled in the study. The
patients had bidimensionally measurable disease without a history
of chemo/radiotherapy in the last 6 months. The metastatic diseases
were staged with various imaging modalities, such as computed
tomography (CT), magnetic resonance imaging and positron emission
tomography/CT scan. The pathological diagnosis of lung cancer was
established according to the revised World Health Organization
classification of lung tumors and staged relying on the revised
tumor-node-metastasis staging for lung cancer (10,11).
The clinical history, physical examination, series
of biochemistry tests and complete blood cell counts were used as
the pretreatment evaluation. Those with Eastern Cooperative
Oncology Group performance status ≤2 and appropriate blood
chemistry tests received a platinum-based chemotherapy with/without
radiotherapy depending on the stage of disease. The response to
chemotherapy was evaluated radiologically after 2–3 cycles of
chemotherapy, according to the revised Response Evaluation Criteria
in Solid Tumors criteria (12). The
non-responders to chemotherapy and patients with recurrent diseases
were treated with second-line chemotherapy, provided when they had
a good performance status. Chemotherapy was discontinued when
disease progression or unacceptable toxicity occurred.
A total of 30 age- and gender-matched healthy
subjects were included in the study. The study was approved by the
Ethics Committee of Istanbul University. Written informed consent
was obtained from all the patients.
Measurement of serum IGF-1 and IGFBP-3
levels
Serum samples were drawn from patients and healthy
controls by venipuncture and clotted at room temperature on first
admission prior to the treatment. The sera were collected following
centrifugation and frozen immediately at −20°C until analysis.
Serum IGF-1 and IGFBP-3 levels were determined by
the Immulite 2000 systems (all from Siemens Healthcare Diagnostics
Products Ltd., Sudbury, UK). The Immulite 2000 method is an
automated solid-phase, enzyme-labeled chemiluminescent immunometric
assay method. Sample pretreatment was accomplished in an onboard
dilution step.
Statistical analysis
Median values were used to classify variables and
the Mann-Whitney U test was used to compare clinical and laboratory
parameters. Survival was calculated from the first admission date
to the date of mortality from any cause, or to the last contact
with the patient or any family member. Kaplan-Meier test was used
to estimate the survival and the differences in survival were
evaluated by the log-rank statistics. P≤0.05 was considered to
indicate a statistically significant difference. Statistical
analysis was carried out using SPSS 16.0 software (SPSS, Inc.,
Chicago, IL, USA).
Results
Patient characteristics
A total of 80 pathologically confirmed lung cancer
patients were enrolled in the study. Baseline histopathological and
demographic data of patients are listed in Table I. The median age of patients was 58.5
years old, with a range of 36–80 years, where males constituted the
majority of the group (n=72, 90%). The predominance of the patients
had NSCLC (n=68, 85%) and metastatic disease (n=45, 56%).
 | Table I.Patient and disease features. |
Table I.
Patient and disease features.
| Parameter | Patients, n |
|---|
| Total patients | 80 |
| Age, years |
|
| ≥60 | 37 |
|
<60 | 43 |
| Gender |
|
| Male | 72 |
|
Female | 8 |
| Histology |
|
|
NSCLC | 68 |
|
Adenocarcinoma | 33 |
|
Squamous cell | 27 |
|
Undifferentiated | 8 |
| SCLC | 12 |
| Stage |
|
| II | 4 |
| III | 30 |
| IV | 34 |
|
Limited | 1 |
|
Extended | 11 |
| Response to
chemotherapy |
|
| Yes | 41 |
| No | 30 |
Serum concentrations
No significant difference was determined in the
serum IGF-1 concentration between lung cancer patients and healthy
individuals (median values, 145.5 vs. 160.5 ng/ml; P=0.403)
(Table II, Fig. 1A). However, baseline serum IGFBP-3
concentrations of the lung cancer patients were significantly lower
compared to the control group (median values, 3.175 vs. 4.235
µg/ml; P<0.001) (Table II,
Fig. 1B).
 | Table II.Serum values of IGF-1 and IGFBP-3 in
lung cancer patients and healthy subjects. |
Table II.
Serum values of IGF-1 and IGFBP-3 in
lung cancer patients and healthy subjects.
|
| Median serum levels
(range) |
|
|---|
|
|
|
|
|---|
| Serum assay | Patients (n=80) | Controls (n=30) | P-value |
|---|
| IGF-1, ng/ml | 145.5 (15–345) | 160.5 (58.7–293) |
0.403 |
| IGFBP-3, µg/ml | 3.175
(0.62–5.65) | 4.235 (2.6–5.95) | <0.001 |
Table III
demonstrates the correlations between the serum levels of IGF-1 and
IGFBP-3 and known clinicopathological factors. The male patients
had elevated serum IGF-1 concentrations compared to female patients
(P=0.025). Furthermore, patients with NSCLC histology and
metastatic stage in NSCLC had elevated serum IGF-1 (P=0.022 and
P=0.005, respectively) and IGFBP-3 (P=0.039 and P=0.043,
respectively) levels compared with those with SCLC histology and
non-metastatic stage in NSCLC. However, no other clinical features,
including age of patient, tumor histology and chemotherapy
responsiveness, were correlated with serum assays of IGF-1 and
IGFBP-3 (P>0.05).
 | Table III.Comparisons between the IGF-1/IGFBP-3
assays and clinical parameters. |
Table III.
Comparisons between the IGF-1/IGFBP-3
assays and clinical parameters.
| Parameter | Median IGF-1, ng/ml
(range) | Median IGFBP-3, µg/ml
(range) |
|---|
| Age, P-value | 0.596 | 0.707 |
| ≥60
years | 147 (30.90–330) | 3.23 (0.74–5.38) |
| <60
years | 145 (12–345) | 3.16 (0.62–5.65) |
| Gender, P-value | 0.025 | 0.070 |
| Male | 154 (12–345) | 3.26 (0.32–5.65) |
|
Female | 111 (15–166) | 2.76 (0.82–4.34) |
| Histology,
P-value | 0.022 | 0.039 |
|
NSCLC | 162 (12–345) | 3.29 (0.74–5.65) |
| SCLC | 114 (39.7–199) | 2.84 (0.62–3.71) |
| Histology in NSCLC,
P-value | 0.667 | 0.427 |
|
Adeno | 167 (12–304) | 3.32 (1.14–5.54) |
|
Epidermoid | 146 (30.9–330) | 3.23 (0.74–5.65) |
| Stage in NSCLC,
P-value | 0.005 | 0.043 |
|
Non-metastatic (I–III) | 141 (12–260) | 3.17 (0.74–5.58) |
|
Metastatic (IV) | 212 (15–330) | 3.72 (1.14–5.65) |
| Response to
chemotherapy, P-value | 0.514 | 0.710 |
| Yes | 142 (30.9–273) | 3.28 (0.74–5.58) |
| No | 154 (41.9–345) | 3.17 (0.62–5.27) |
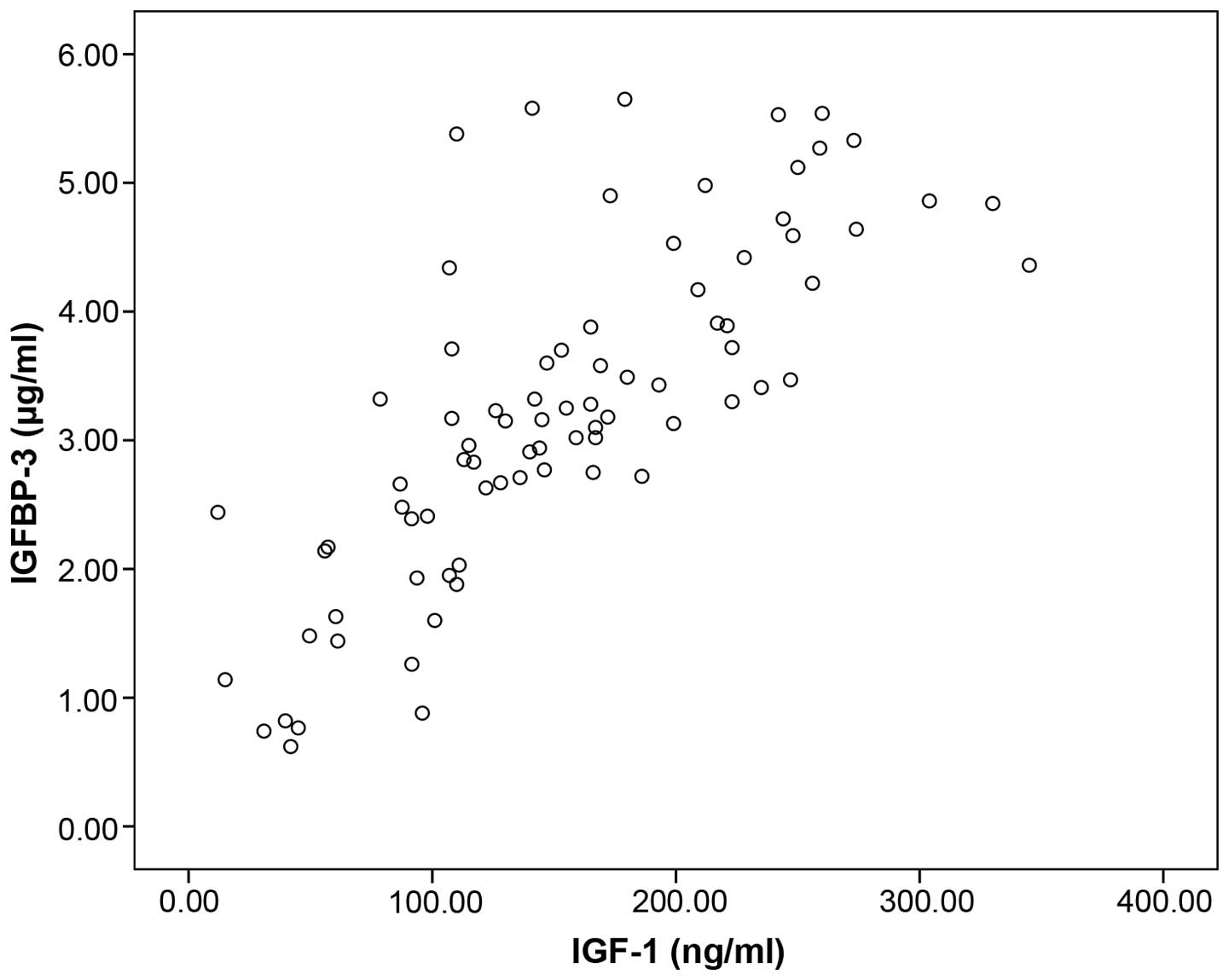
There was a significant association between the
serum concentrations of IGF-1 and IGFBP-3 in patients with lung
cancer (rs=0.804, n=80, P<0.001, Spearman's correlation)
(Fig. 2).
Follow-up
The median follow-up time was 58 weeks, with a range
of 3.7–149.3 weeks. The median survival was 94.4 weeks (95%
confidence interval, 73.1–115.7). The 1- and 2-year overall
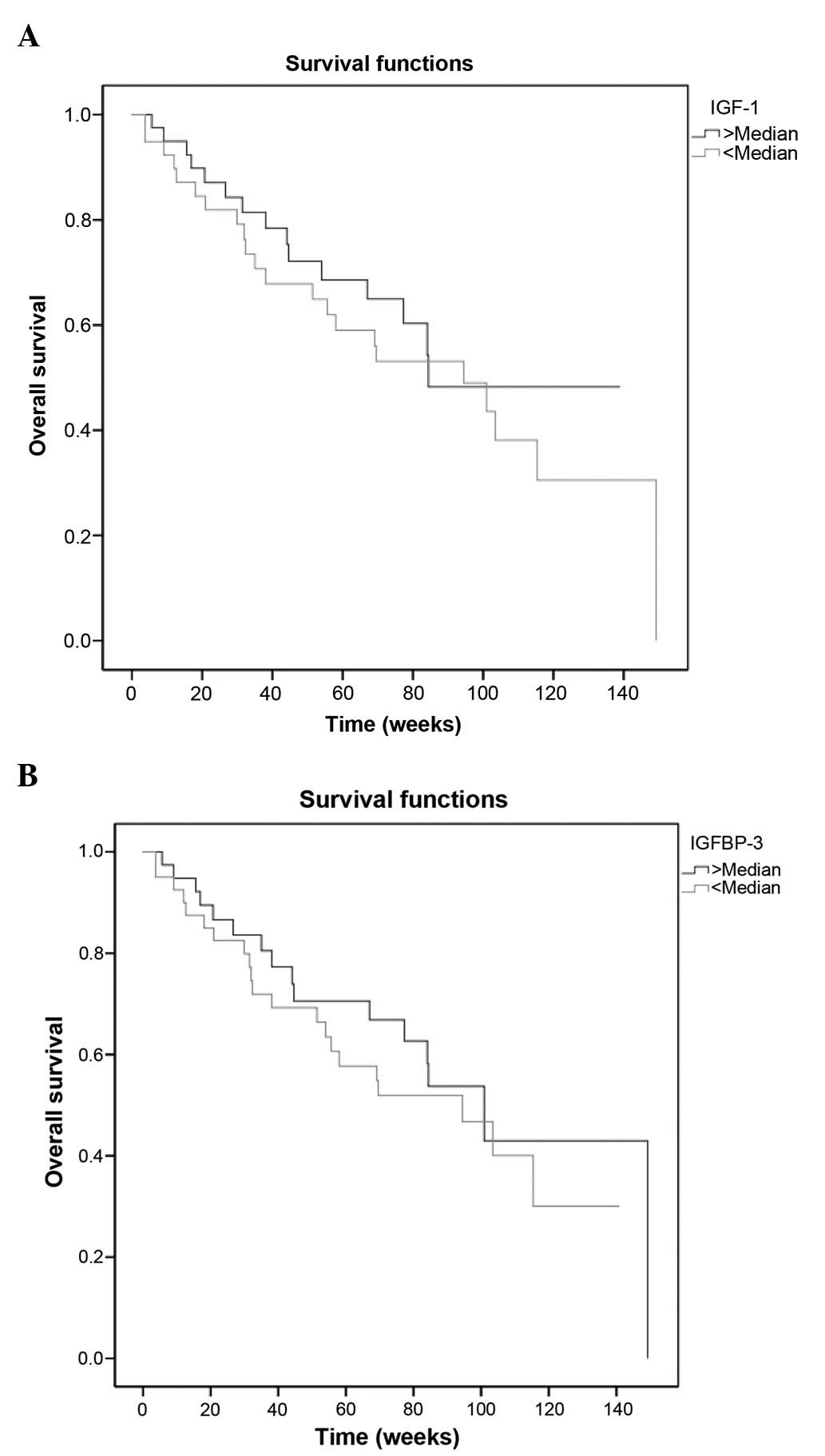
survival rates were 68.5 and 40.6%, respectively. As expected,
histology (P=0.004), metastasis (P=0.005) and response to
chemotherapy (P=0.009) had prognostic factors on survival (Table IV). However, serum IGF-1 and IGFBP-3
concentrations were not associated with outcome (P=0.552 and
P=0.471, respectively) (Table IV,
Fig. 3A and B).
 | Table IV.Univariate survival analyses. |
Table IV.
Univariate survival analyses.
| Parameter | Median survival time
week ± SE | 1-year survival rate
± SD, % | P-value |
|---|
| Age of patients,
years |
|
| 0.43 |
| ≥60 |
69.6±21.8 |
61.0±8.6 |
|
|
<60 |
100.9±14.9 |
71.8±7.3 |
| Gender |
|
| 0.75 |
| Male |
100.9±10.3 |
68.6±5.7 |
|
|
Female |
69.1±35.7 |
64.3±21.0 |
| Histology |
|
| 0.004 |
|
NSCLC |
100.9±9.6 |
72.4±5.7 |
|
| SCLC |
51.4±27.1 |
45.8±15.0 |
| Histology in
NSCLC |
|
| 0.811 |
|
Adeno |
100.9±13.1 |
75.7±8.1 |
|
|
Epidermoid |
101.3±13.9 |
71.1±9.4 |
| Stage in NSCLC |
|
| 0.005 |
|
Non-metastatic (II–III) |
149.3±0.0 |
81.9±6.7 |
|
Metastatic (IV) |
67.0±18.3 |
56.3±9.7 |
| Response to
chemotherapy |
|
| 0.009 |
| Yes |
149.3±0.0 |
77.8±6.5 |
|
| No |
69.6±18.9 |
56.7±10.1 |
| Median serum IGF-1
level |
|
| 0.552 |
|
Normal |
94.4±22.1 |
64.9±7.9 |
|
|
Elevated |
84.4±0.0 |
68.6±8.0 |
|
| Median serum IGFBP-3
level |
|
| 0.471 |
|
Normal |
94.4±23.0 |
66.4±7.6 |
|
|
Elevated |
100.9±13.6 |
70.6±7.9 |
|
Discussion
There is a hypothesis that circulating
concentrations of IGF-1 and IGFBP-3 may be significant in
determining lung cancer risk. In a case-control study consisting of
208 lung cancer patients, an elevated plasma level of IGF-1 and
decreased plasma IGFBP-3 concentration was associated with an
increased risk of lung cancer (1).
However, in other case-control studies, no correlation was found
between serum IGF-1 concentration and lung cancer risk; only high
serum concentrations of IGFBP-3 were associated with a reduced lung
cancer risk (2–4).
In literature, only a limited number of trials have
studied serum IGF-1 and IGFBP-3 levels in human lung cancer
(5–9).
Firstly, Lee et al (5) measured
the serum levels of IGF-1 and IGFBP-3 in 41 lung cancer patients
(5). The serum IGF-1 levels in the
lung cancer patients was significantly lower compared to the
controls (P<0.01) and NSCLC patients showed significantly
reduced serum concentrations of IGF-1 compared with SCLC patients
(P<0.01). Similarly, they found that squamous cell carcinoma
patients tended to exhibit reduced IGF-1 serum concentrations
compared to those with adenocarcinoma histology. Similar to IGF-1,
the concentration of IGFBP-3 was significantly lower in lung cancer
patients compared with healthy subjects (P<0.05). However, no
significant difference was identified between NSCLC and SCLC
groups. These results indicated that serum concentrations of IGF-1
and IGFBP-3 may be useful tumor markers for diagnosing and
identifying tumor types of lung cancer. In another study, Unsal
et al (6) found that serum
IGF-1 and IGFBP-3 concentrations were reduced in 24 lung carcinoma
patients compared with normal subjects, but no significant
difference was identified between the groups (P=0.07 and P=0.06,
respectively). However, the serum IGF-1/IGFBP-3 ratio was
significantly reduced in the distant and nodal metastatic patients,
and stage of tumor was inversely correlated with this ratio
(P=0.04). Additionally, in a novel study, the expression and
clinical significance of IGF-1 and IGFBP-3 were investigated in the
serum and cancer tissues from 57 patients with NSCLC (7). Expression levels of IGF-1 in lung cancer
tissues and serum from lung cancer patients were significantly
increased compared to the control group (P<0.05 and P=0.034,
respectively). Conversely, expression levels of IGFBP-3 in cancer
tissues and serum in patients were significantly lower compared to
the healthy subjects (P<0.05 and P=0.042, respectively). No
significant differences were observed for serum IGF-1 and IGFBP-3
levels and the location of tumor, tumor size or pathological grade,
but they were significantly associated with lymph node involvement
levels, local invasion, distant metastasis and disease stage. The
serum levels of IGF-1 and IGFBP-3 in NSCLC patients had a
significant inverse correlation (P=0.023). However, no significant
correlation was observed between the expression intensity of IGF-1
and IGFBP-3 (P=0.062). Serum IGF-1 levels were significantly
different with a positive association at different levels of IGF-1
expression intensity. However, serum IGFBP-3 levels showed no
significant differences with a small positive correlation. These
findings suggest that IGF-1 upregulation and downregulation of
IGFBP-3 may be potential diagnostic biomarkers for NSLCL
patients.
Although several studies have assessed the
association of circulation concentrations of IGF system with lung
carcinoma risk over the last years, little is known regarding the
prognostic role of the IGF system in patients with lung cancer. Han
et al (8) investigated whether
pretreatment IGF-1 and IGFBP-3 levels would predict the prognosis
in patients with 77 advanced NSCLC enrolled in a phase II trial of
cisplatin plus irinotecan chemotherapy. Serum levels of IGFBP-3
were significantly elevated in female patients (P=0.017),
non-squamous cell cancer (P=0.013) and no smokers (P=0.003).
However, no statistically significant differences were observed for
serum IGF-1 concentrations according to gender, performance status,
tumor stage, pathology and smoking behavior. In a univariate
analysis, elevated concentrations of IGF-1 and IGFBP-3 were
predictive markers of favorable progression-free (P=0.001 and
P=0.007, respectively) and overall survival (P=0.025 and P=0.001,
respectively). Furthermore, multivariate analysis also revealed
that serum IGF-1 and IGFBP-3 were independent prognostic factors
for progression-free (P<0.0001 and P=0.001, respectively) and
overall survival (P=0.004 and P=0.043, respectively). Increased
plasma IGF-1 and IGFBP-3 levels were concluded to be associated
with a favorable prognosis in advanced NSCLC patients. In another
study, 68 advanced non-squamous NSCLC patients treated with
gefitinib were investigated for the prediction to chemotherapy
responsiveness and prognostic impacts (9). IGF-1-positive serum predicts a negative
response to gefitinib therapy (P=0.0003). Similarly, IGF-1 positive
was also independent of a poor prognostic factor (P=0.04). Contrary
to IGF-1, the serum level of IGFBP-3 did not have a prognostic
factor nor serve as a surrogate predictive marker for the effect of
therapy.
In the present study, the serum concentrations of
IGF-1 and IGFBP-3 were investigated in 80 patients with different
histology and tumor stages of lung cancer who were enrolled in the
study. No significant differences were identified in serum IGF-1
concentrations between lung cancer patients and healthy controls
(P=0.403). However, baseline serum IGFBP-3 levels of the lung
cancer patients were significantly lower compared to the control
group (P<0.001). The male patients had elevated serum IGF-1
concentrations compared to females (P=0.025). Furthermore, patients
with NSCLC histology and metastatic stage in NSCLC had elevated
serum IGF-1 (P=0.022 and P=0.005, respectively) and IGFBP-3
(P=0.039 and P=0.043, respectively) levels compared with those with
SCLC histology and non-metastatic stage in NSCLC. However, no other
clinical parameters, such as age of patient, tumor histology and
chemotherapy responsiveness, were correlated with serum assays of
IGF-1 and IGFBP-3 (P>0.05). There was a significant association
between IGF-1 and IGFBP-3 serum levels in patients with lung cancer
(P<0.001). Neither serum IGF-1 nor IGFBP-3 concentrations were
associated with outcome (P=0.552 and P=0.471, respectively).
In conclusion, although little is known, findings
suggest that the IGF family may have a significant role in the
etiology and progression of lung carcinoma in the last years.
However, there are numerous controversial findings regarding the
patterns of expression of these gene products in the literature;
thus, the definitive functional compatibility of these alterations
remains to be elucidated. Parallel arguments were true for patterns
of quantifying of the circulating serum IGF levels. In the present
study, serum concentrations of IGFBP-3 may be a diagnostic marker
in lung cancer patients compared to serum IGF-1 concentrations.
However, predictive and prognostic values of the two serum assays
were not identified. Although the small sample size and the short
follow-up time are limitations and may have influenced the results,
this study still contributes a significant deal to the literature
in that it was carried out with the serum instead of tissue, and it
contained all the stages of the disease. Larger-scale studies in
larger patient populations are required to determine the exact role
of serum IGF-1 and IGFBP-3 levels in lung cancer.
References
|
1
|
Yu H, Spitz MR, Mistry J, Gu J, Hong WK
and Wu X: Plasma levels of insulin-like growth factor-I and lung
cancer risk: A case-control analysis. J Natl Cancer Inst.
91:151–156. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
London SJ, Yuan JM, Travlos GS, Gao YT,
Wilson RE, Ross RK and Yu MC: Insulin-like growth factor I,
IGF-binding protein 3, and lung cancer risk in a prospective study
of men in China. J Natl Cancer Inst. 94:749–754. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen B, Liu S, Xu W, Wang X, Zhao W and Wu
J: IGF-I and IGFBP-3 and the risk of lung cancer: A meta-analysis
based on nested case-control studies. J Exp Clin Cancer Res.
28:892009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cao H, Wang G, Meng L, Shen H, Feng Z, Liu
Q and Du J: Association between circulating levels of IGF-1 and
IGFBP-3 and lung cancer risk: A meta-analysis. PLoS One.
7:e498842012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee DY, Kim SJ and Lee YC: Serum
insulin-like growth factor (IGF)-I and IGF-binding proteins in lung
cancer patients. J Korean Med Sci. 14:401–404. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Unsal E, Köksal D, Yurdakul AS, Atikcan S
and Cinaz P: Analysis of insulin like growth factor 1 and insulin
like growth factor binding protein 3 levels in bronchoalveolar
lavage fluid and serum of patients with lung cancer. Respir Med.
99:559–565. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang Z, Wang Z, Liang Z, Liu J, Shi W, Bai
P, Lin X, Magaye R and Zhao J: Expression and clinical significance
of IGF-1, IGFBP-3, and IGFBP-7 in serum and lung cancer tissues
from patients with non-small cell lung cancer. Onco Targets Ther.
6:1437–1444. 2013.PubMed/NCBI
|
|
8
|
Han JY, Choi BG, Choi JY, Lee SY and Ju
SY: The prognostic significance of pretreatment plasma levels of
insulin-like growth factor (IGF)-1, IGF-2, and IGF binding
protein-3 in patients with advanced non-small cell lung cancer.
Lung Cancer. 54:227–234. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Masago K, Fujita S, Togashi Y, Kim YH,
Hatachi Y, Fukuhara A, Nagai H, Irisa K, Sakamori Y, Mio T, et al:
Clinical significance of epidermal growth factor receptor mutations
and insulin-like growth factor 1 and its binding protein 3 in
advanced non-squamous non-small cell lung cancer. Oncol Rep.
26:795–803. 2011.PubMed/NCBI
|
|
10
|
Brambilla E, Travis WD, Colby TV, Corrin B
and Shimosato Y: The new World Health Organization classification
of lung tumours. Eur Respir J. 18:1059–1068. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Detterbeck FC, Boffa DJ and Tanoue LT: The
new lung cancer staging system. Chest. 136:260–271. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D and
Verweij J: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|