Introduction
Tat-protein transduction domain (PTD) [the PTD
domain of human immunodeficiency virus (HIV)-tat] is a peptide of
9–11 amino acids in length and is predominantly comprised of basic
amino acids, such as arginine and/or lysine (1–3).
Accumulating evidence indicates that PTD has the capability to
carry proteins, peptides, nucleic acid and viral particles into
cells. Although the detailed mechanisms for transduction remain to
be elucidated, negatively charged heparan sulphate on the cell
surface could have an important role in this process. Due to its
unique characteristics, PTD has numerous applications, including
delivery of functional proteins, viral particles and enhancing the
DNA (RNA) transfection efficiency. A previous study has also
suggested that PTD combined with certain antigens can enhance
immunogenicity (4).
Currently, the cervical cancer prophylactic vaccines
based on human papillomavirus (HPV) L1 virus-like particles (VLPs),
including Gardasil (HPV6/11/16/18 quatrivalent vaccine developed by
Merck, Kenilworth, NJ, USA) and Cervarix (HPV16/18 bivalent vaccine
developed by GlaxoSmithKline, Brentford, UK) have been approved in
a number of developed countries, and can induce satisfactory
protective effects (5,6). However, this protection is type-specific
and there is weak or no protection against other HPV types. The
discovery of cross-neutralizing responses induced by L2 derived
from rabbit, bovine and human papillomavirus underline the
potential application as a promising broad-spectrum vaccine.
Further studies have identified highly conserved common epitopes
located within the first 200 amino acids of the N-terminal of L2
(7–9),
which are essential for inducing cross-neutralizing antibodies.
However, immunogenicity of polypeptide of L2 is much lower compared
to L1 VLP, which has impeded its further application. Extensive
efforts have been made to increase the immunogenicity of L2
peptides. Strategies include increasing the epitope number by
constructing polypeptides with repeat cross-response epitopes from
one serotype or tandem epitopes from several serotypes, and the
utilization of Trx (recombinant tobacco mosaic virus) or
bacteriophage VLP as vectors to display L2 epitopes (10–12). These
strategies alone, or combined, improved the immunogenicity of L2
peptides to a certain extent.
In the present study, two HPV16L2 peptides, L2-N88
and L2-N200 (the first 88 or 200 amino acids of the N-terminal of
L2, respectively) were chosen as model antigens and a series of L2
peptide vaccines were constructed by fusing or mixing with PTD, and
evaluated their specific humoral responses and cross-protection by
enzyme-linked immunosorbent assay (ELISA) and pseudovirion
neutralization assay, respectively.
Materials and methods
Expression and purification PTD-L2
polypeptides
L2 vaccines were constructed by fusing PTD with
HPV16L2 peptides, L2-N88 and L2-N200, designated PTD-L2-N88 and
PTD-L2-N200, respectively. First, the sequence-verified polymerase
chain reaction amplification products encoding L2-N88, L2-N200,
PTD-L2-N88 and PTD-L2-N200 were cloned into pET-22 (Novagen,
Madison, WI, USA) plasmids using XhoI and NdeI
restriction sites. The constructs were confirmed by sequencing, and
were transformed into Escherichia coli Rosetta (DE3) cells.
Transformed cells were grown at 37°C until they reached an optical
density (OD) at 600 nm value of 0.8. Protein expression was induced
with 1 mM isopropyl β-D-1-thiogalactopyranoside for 4 h. Cell
pellets were lysed and resuspended by binding buffer, 20 mM
Tris-HCl, 500 mM NaCl, 20 mM imidazole and 8 M urea (pH 8.0),
followed by centrifugation at 5,000 × g for 25 min at 4°C. The
clear supernatant was applied to a HisTrap FF column (GE
Healthcare, Beijing, China) according to the manufacturer's
protocol. The peak fraction was collected and extensively dialyzed
into phosphate-buffered saline (PBS) buffer (pH 7.4) for 12–14 h at
4°C. The dialyzed fractions were centrifuged for 10 min, and the
clear supernatants collected. Protein concentration was determined
by the bicinchoninic acid acid method (Bio-Rad, Hercules, CA,
USA).
Immunization of mice
For immunization, purified L2-N88, PTD-L2-N88 and
L2-N200, PTD-L2-N200 were diluted to proper concentration with PBS
and sterilized using 0.22 µM filters. The mix-type L2 vaccines,
termed PTD + L2-N88 and PTD + L2-N200 were prepared by mixing
purified L2-N88 or L2-N200 (100 µg each) with PTD (Scilight
Biotechnology LLC, Beijing, China) according to a molar ratio of
1:1. Female BALB/c mice (4–6-week old) were randomly divided into 8
groups, with 8 animals for each vaccination group, and 5 mice for
the control groups. The mice were immunized subcutaneously 3 times.
The priming injection at day 0 used vaccines formulated in complete
Freund's adjuvant, and the subsequent 2 boost injections used
vaccines prepared in incomplete Freund's adjuvant at days 14 and
28. Blood samples were collected 7 days after the last boost. All
the animals were purchased from Vital River Laboratories (Beijing,
China), and maintained under pathogen-free conditions at the animal
facilities of Peking University First Hospital (Beijing, China).
All the animal experimental procedures in this study were approved
by the Animal Ethics Committee of Peking University First
Hospital.
Detection of anti-L2 antibodies
Antibodies against HPV16L2 in immunized mice were
measured from serum by ELISA. Microtiter plates were coated
overnight at 4°C with 100 µl of coating buffer containing 1 µg of
full-length HPV16L2 protein, washed twice using PBS with 0.2%
Tween-20 (PBST), blocked with 100% fetal bovine serum at 37°C for 2
h, followed by washing twice again with PBST. Mouse serum (50 µl)
was serially diluted in 2-fold steps starting at 1:100,
subsequently added to the ELISA plate and incubated for 1 h at
37°C. Plates were washed and incubated for 1 h at 37°C with 50 µl
horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G
(IgG) (1:500 dilution) (CW0102; CWBio, Beijing, China). Subsequent
to washing again with PBST, 100 ml of the chromogenic substrate
3,3′,5,5′-tetramethylbenzidine was added to each well and the
absorbance at 450 nm was measured after 10–20 min with an automated
plate reader (Bio-Rad). An OD value 4× over that of the control
sera was taken as a positive result.
Pseudovirion neutralization assay
HEK293FT cells were seeded 24 h prior to infection
at a density of 1×104 cells/well in 96-well microtiter
plates. The pseudovirions were diluted 1:2,000 in Dulbecco's
modified Eagle's medium without phenol red and mixed with anti-sera
at different dilutions. Following gentle agitation at 4°C for 1 h,
the culture medium was replaced by 100 µl of the pseudovirion-sera
mixture and cultured for a further 72 h. Finally, secreted
embryonic alkaline phosphatase (SEAP) activity in cell culture
supernatants was determined using a chemical chromatic assay.
Briefly, 20 µl 0.05% CHAPS and 200 µl substrate solution (2 M
diethanolamine with 1 mM MgCl2, 0.5 mM ZnCl2
and 3 mM pNPP) were added to 40 µl of culture supernatant and
incubated for 2 h without light. The OD values at 405 nm were
determined using a Bio-Rad Spectrophotometer. The neutralizing
titer was expressed as the reciprocal of the maximum dilution of
serum that caused ≥50% activity reduction of SEAP.
Statistical analysis
The neutralization titers were analyzed using
parametric tests (one-way analysis of variance followed by
Bonferroni comparisons). P<0.05 was considered to indicate a
statistically significant difference. All statistical analysis was
carried out with the GraphPad Prism 6.0 software (GraphPad Software
Inc., La Jolla, CA, USA).
Results
Construction, expression and
purification of L2 peptide vaccines
Previous studies reported that the antibodies
induced by 1–88 and 1–200 peptides derived from the N-terminal
region of HPV-16 L2 could neutralize HPV-16 pseudovirions and
cross-neutralize heterologous pseudovirions. The peptide of 1–200
exhibited a higher immunogenicity compared to the 1–88 peptide. In
order to study the effect of the transduction domain on the
immunogenicity of L2 peptide, the L2 peptide vaccines were
constructed with PTD mixed peptide (PTD: HPV16L2-88 and PTD:
HPV16L2-200) and PTD fusion peptide (PTD-HPV16L2-88 and
PTD-HPV16L2-200), respectively. The four proteins, HPV16L2-88,
PTD-HPV16L2-88, HPV16L2-200 and PTD-HPV16L2-200, were expressed in
the Escherichia coli expression system, and were purified
under denaturing conditions using the HisTrap FF column (GE
Healthcare). This was followed by dialysis for 12–14 h with PBS
buffer (pH 7.4) at 4°C to harvest related purified L2 peptide
vaccines. The purity of the four recombinant proteins obtained were
99.1, 98.9, 95.4 and 93.7%, respectively. The total protein
concentrations were 1,626.1, 1,709.2, 1,660.6 and 1,570.3 µg/ml,
respectively.
PTD-L2 peptide vaccines generate
potent anti-HPV16 humoral immune responses
Inducing a high humoral immune response is the
primary function of the preventive vaccine. In order to detect the
effect of the humoral immune response induced by HPV16L2 peptide
vaccines, the full-length HPV16L2 protein expressed by prokaryotic
expression system was used as a coating antigen to detect the serum
anti-L2 antibody (IgG) level by indirect ELISA results (Fig. 1) showed that each HPV16L2 vaccine could
induce specific L2 antibodies with different titers and two results
were summarized. First, total anti-L2 antibody induced by peptide
vaccines of HPV16L2 N-88 (which included HPV16L2-N88,
PTD-HPV16L2-N88 and PTD: HPV16L2-N88) was significantly lower than
that of HPV16L2-N200 regardless of the involvement of PTD, and PTD
participation mode was the mixture or fusion. The results are
consistent with the view that the polypeptide size affects its
immunogenicity. Second, in the HPV16L2-N88 groups, the use of PTD
(fusion or mixture) did not result in a significant increase in the
total antibodies produced by the vaccine, and the level of specific
antibody titer was low in all groups. By contrast, in the
HPV16L2-N200 groups, the antibody titer of the vaccine to
PTD-HPV16L2-N200 (PTD fused with HPV16L2-N200) was twice that of
HPV16L2-N200, which was 6–7 times the N88 groups. However, mixed
use of PTD did not improve the total antibody titer.
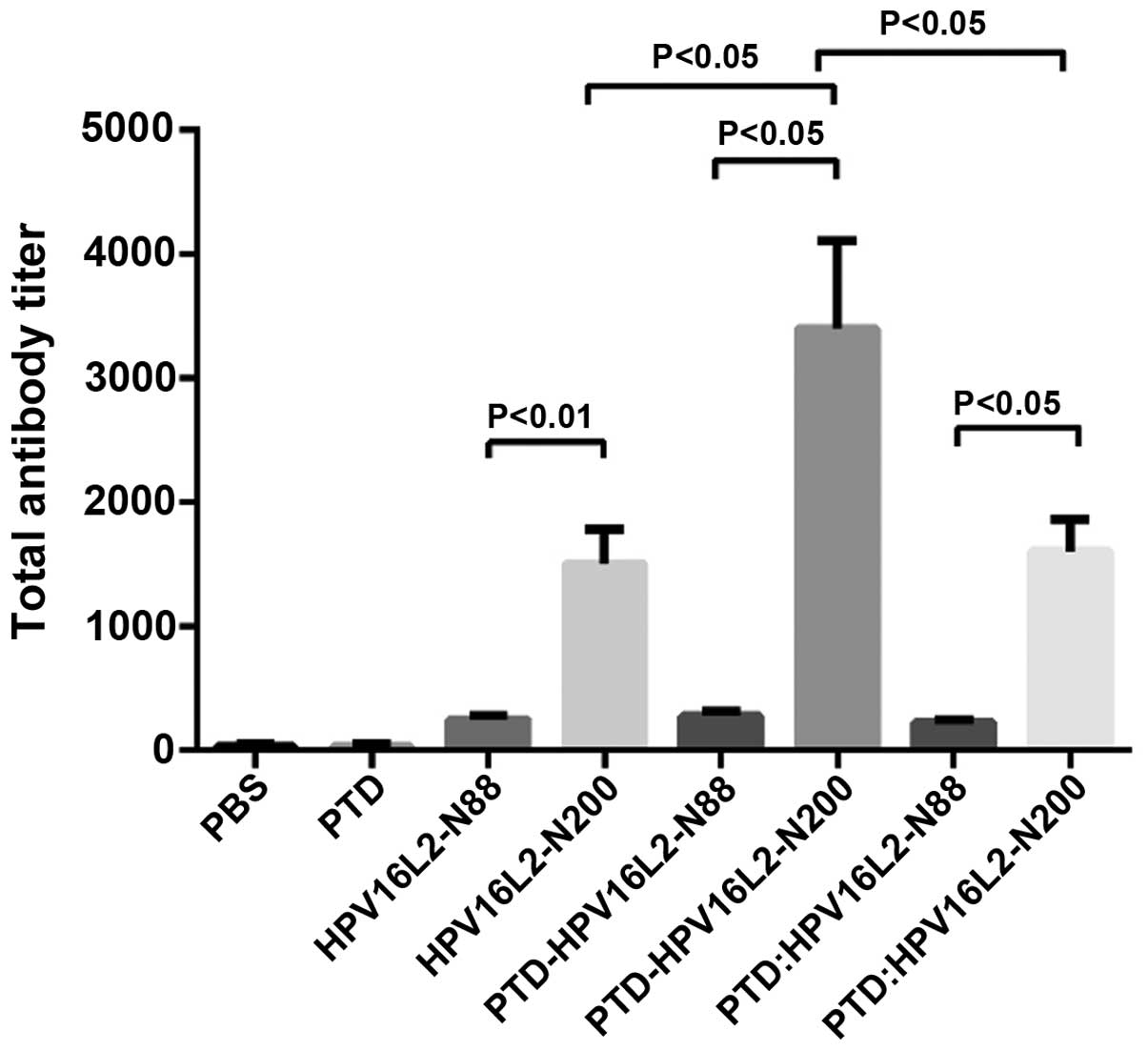 | Figure 1.Antibody titer of mice vaccinated with
HPVL2 relative proteins. Animals were immunized with HPV16L2-N88,
HPV16L2-N200, PTD-HPV16L2-N88, PTD-HPV16L2-N200 and PTD:
HPV16L2-N88 (mol ratio, 1:1) and PDT: HPV16L2-N200 (mol ratio,
1:1), the effective amount of protein was 100 µg/100 µl/animal.
Protein was mixed with Freunds complete adjuvant, and the animals
were immunized at weeks 0, 2 and 4. One week after the completion
of the immunization, the serum was collected for indirect ELISA. No
significant difference was identified between the total antibody
levels in HPV16L2-N88-related groups. In the HPV16L2-N200-related
groups, the total antibody titers in the PTD-HPV16L2-N200 groups
were higher than those of the HPV16L2-N200 groups (P<0.05) and
PTD: HPV16L2-N200 groups (P<0.05). No significant difference was
identified between the HPV16L2-N200 and PTD: HPV16L2-N200 groups.
In all conditions, the total antibodies in HPV16L2-N200-related
groups were higher than those in the HPV16L2-N88 groups. HPV, human
papillomavirus; PTD, protein transduction domain; ELISA,
enzyme-linked immunosorbent assay. |
A summary of the existing data shows that for the
HPV N-end peptide vaccines, the involvement of PTD in the induction
of HPV16L2-specific antibodies did not significantly increase the
level of total antibody. Although, the combination of PTD and N200
doubled the antibody titer, which is still not a satisfactory
consequences.
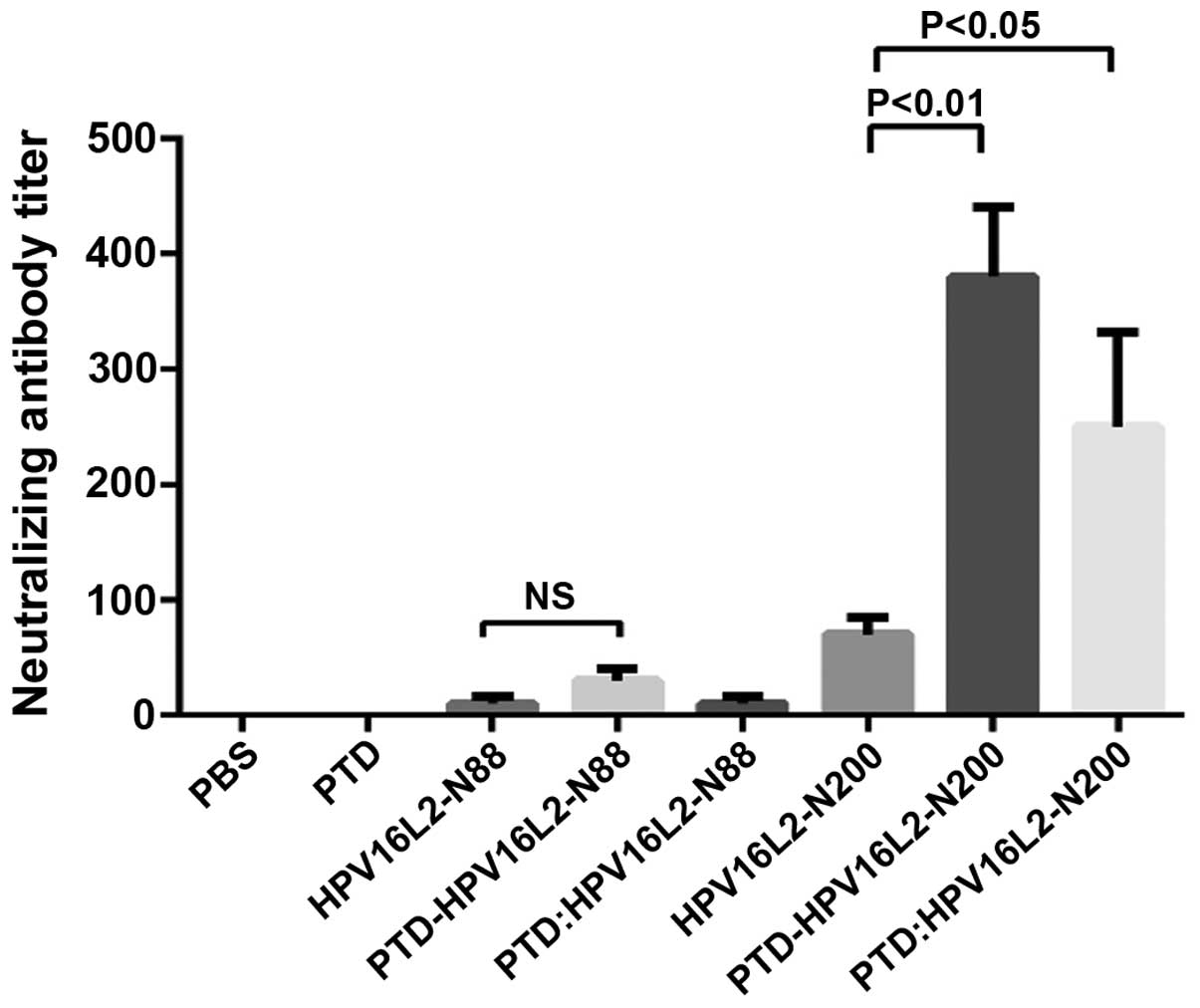
Serum-neutralizing antibodies can neutralize the
related viral particles to prevent them from infecting host cells,
which is key in protective efficacy of the vaccine-induced
immunity. To determine whether the anti-L2 serum antibodies
stimulated by L2 peptide vaccines with mixed or fusion form of PTD
own the ability to neutralize the virus particles, the pseudovirion
neutralization experiments were carried out to detect the serum
levels of anti-HPV16 neutralizing antibody titers in each mouse
group (Fig. 2). The result which was
consistent with the total antibody test was that the neutralizing
antibody titer of all the HPV16L2-N88 relative groups were still
extremely low. It was demonstrated that PTD was not effective in
improving the humoral immune response to the HPV-N88 vaccine.
However, in the HPV16L2-N200 groups, the specific-neutralizing
antibody titer induced by PTD-HPV16L2-N200 was 5 times that of the
PTD group, and the PTD mixed involvement also increased the
neutralizing antibody by ~2-fold. The neutralizing antibody titer
of PTD-HPV16L-N200 was significantly higher than that of PTD:
HPV16L-N200 (P<0.05).
Humoral immune response results demonstrated that no
change of humoral immune response occurred in the short peptide
vaccine HPV16L2-N88 with or without PTD. However, the humoral
immune response of the longer peptide vaccine (HPV16L2-N200) fused
with PTD was improved significantly. This suggested that the fusion
form appeared to have a stronger immune-enhancing effect.
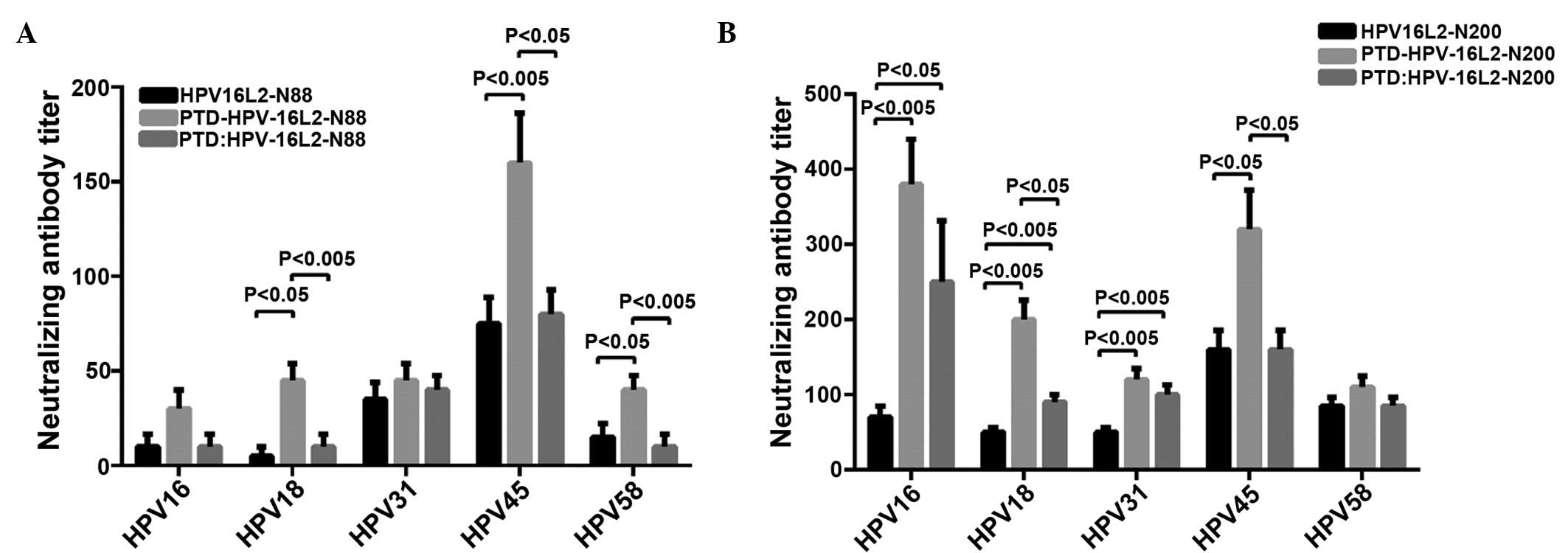
HPV16L2 peptide vaccines induce
cross-neutralizing activity
The N-terminal of HPV16 L2 contains linear
cross-neutralizing epitopes, which cannot only neutralize the
homotype of HPV virus particles, but also has a cross-neutralizing
activity for allotype of HPV particles. To determine whether PTD
can enhance the ability of L2 peptide vaccines in inducing
cross-neutralizing antibodies, HPV18, HPV31, HPV45 and HPV58
pseudovariola were diluted to a suitable titer for the detection of
serum levels of cross-protective neutralizing antibodies for
corresponding HPV type in each group. The results showed that
immunizing with HPV16L2-N88 alone cannot produce effective
cross-neutralizing antibodies in the animals. Whereas HPV16L2-N200
alone resulted in the generation of cross-protective neutralizing
antibodies for all types of pseudovirus (HPV18, HPV31, HPV45 and
HPV58) in immunized mice of all the experimental groups.
HPV16L2-N88-related vaccines induced cross-protective antibody, as
shown in Fig. 3A, while Fig. 3B showed the result of HPV16L2-N200.
HPV16L2-N88 alone resulted in an extremely low titer
of cross-protective neutralizing antibodies in immunized animals,
and the PTD fusion vaccine significantly increased the antibody
titers in HPV18, 45 and 58 pseudovirions (P<0.05, P<0.005 and
P<0.05, respectively). The PTD peptide mixed with HPV16L2-N88
also increased the antibody titers of partial types. The
cross-protective neutralizing antibody induced by HPV16L2-N200
alone was higher than that of HPV16L2-N88, and that was also
significantly increased by fusion of HPV16L2-N200 and PTD (Fig. 3B). When animals were immunized with the
mixture of PTD and HPV16L2-N88 or HPV16L2-N200 (molar ratio of
1:1), the ability to produce cross-protective neutralizing
antibodies was improved, but the effect was significantly worse
than that of fusion forms. In conclusion, the pseudovirus
neutralization experiments showed that the cross-neutralizing
antibody levels in the HPVL2-N200 group (HPVL2-N200, PTD-HPVL2-N200
and PTD + HPVL2-N200) were significantly higher than those in the
L2-N88 group (HPVL2-N88, PTD-HPVL2-N88 and PTD + HPVL2-N88).
Whether fused or mixed with PTD, each L2-N200 peptide vaccine had a
higher ability to induce cross-neutralizing antibody to different
degrees, and the fusion of PTD had a better immune-enhancing effect
compared to the mixed from PTD.
Discussion
High-risk HPV is an important virological cause of
cervical cancer. In recent years, epidemiological studies have
shown that HPV is strongly associated with head and neck cancer,
and esophageal cancer. The present preventive HPV vaccine in the
market is the L1 VLP-based subunit vaccine, and it can induce the
body to generate a high titer of serum-neutralizing antibodies.
However, as the serum-neutralizing antibodies are type-specific,
there is little cross efficacy between different types of
neutralizing antibody (13).
Increasing the number of vaccines to extend the scope of protection
will not only increase vaccine production costs further, but also
increase the risk of side effects from the vaccine. Studies have
found that L2 had cross-neutralization epitopes, and can induce a
broad spectrum of cross-neutralizing antibodies. Further studies
showed that the N-terminal of HPVL2 also had linear
cross-neutralizing epitopes. The present studies on the L2 protein
for HPV16 showed that the main neutralizing antibody epitopes
present in the 11–200 region of the N-terminal can generate a
cross-protective effect on 16, 18, 31, 45, 52 and 58 pseudovirion
particles (14). However, the
limitation of the HPVL2 peptide vaccine applications is low
immunogenicity, and low titers of induced protection neutralizing
antibody. Improving the immunogenicity has attracted increasing
attention in research.
The Tat-PTD, a basic amino acid-rich polypeptide
fragment of 9–11 amino acids in length, can cross the cell membrane
in a receptor-independent mechanism (15). This polypeptide could cross the lipid
bilayer of cells either alone or as a fused form of certain
polypeptides or nucleotides (16,17) and
deliver the carried Tat/PTD-fused proteins/peptides to the relevant
cells (18). However so far, the
mechanism of how Tat-PTD mediates the associated substances
entering the cell membrane and having a relevant role remains to be
elucidated. In certain studies it has been shown that the HIV-1 tat
protein basic domain rapidly translocates through the plasma
membrane and accumulates in the cell nucleus. PTD can enhance the
transduction potential of heterologous peptides and proteins in
vitro and in vivo (1,3), improve
immunogenicity of certain antigens and enhance the immune response
results. Following the addition of PTD, the fusion type of
PTD-L2-N200 can significantly improve the titers of anti-HPV16L2
total antibodies, as well as those of neutralizing antibody for
HPV16 and other high-risk HPV types (HPV16, 18, 31, 45 and 58). By
contrast, neither its fusion nor the mixed form of PTD in the
L2-N88 group was able to improve the level of total antibodies and
cross-neutralizing antibodies clearly. This suggested that the
mechanism for immunogenicity of the L2 polypeptide enhanced by PTD
is different from other carrier protein and antigen hapten. In the
present study, the neutralizing antibody titer/total antibody titer
was used to assess the quality of antibodies. Fused PTD
significantly increased the quality of L2-N200 group-induced
antibodies. However, for the full-length HPV16L2, the ratio was
significantly lower than that of the PTD fusion polypeptide vaccine
(data not shown), although it can induce higher titers of total
antibody and neutralizing antibodies. We speculate that the PTD
peptide can penetrate the lipid bilayer of the cell membrane, and
PTD can cause certain channels to open so that certain sections of
the peptide vaccine can enter the reaction site or get closer to
the effect site. When the PTD and peptide vaccine were fused
together, the peptide vaccine was incorporated into the membrane
structure, and all the components of the vaccine can be used in the
reaction of PTD, which make PTD-HPV16L2-N200 more effective in
comparison to the mixed.
References
|
1
|
Vivès E, Brodin P and Lebleu B: A
truncated HIV-1 Tat protein basic domain rapidly translocates
through the plasma membrane and accumulates in the cell nucleus. J
Biol Chem. 272:16010–16017. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Frankel AD and Pabo CO: Cellular uptake of
the tat protein from human immunodeficiency virus. Cell.
55:1189–1193. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ho A, Schwarze SR, Mermelstein SJ, Waksman
G and Dowdy SF: Synthetic protein transduction domains: Enhanced
transduction potential in vitro and in vivo. Cancer Res.
61:474–477. 2001.PubMed/NCBI
|
|
4
|
Chen X, Lai J, Pan Q, Tang Z, Yu Y and
Zang G: The delivery of HBcAg via Tat-PTD enhances specific immune
response and inhibits Hepatitis B virus replication in transgenic
mice. Vaccine. 28:3913–3919. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Su JH, Wu A, Scotney E, Ma B, Monie A,
Hung CF and Wu TC: Immunotherapy for cervical cancer: Research
status and clinical potential. BioDrugs. 24:109–129. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Segal L, Wilby OK, Willoughby CR, Veenstra
S and Deschamps M: Evaluation of the intramuscular administration
of Cervarix™ vaccine on fertility, pre- and post-natal development
in rats. Reprod Toxicol. 31:111–120. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kawana K, Yoshikawa H, Taketani Y,
Yoshiike K and Kanda T: Common neutralization epitope in minor
capsid protein L2 of human papillomavirus types 16 and 6. J Virol.
73:6188–6190. 1999.PubMed/NCBI
|
|
8
|
Kawana K, Matsumoto K, Yoshikawa H,
Taketani Y, Kawana T, Yoshiike K and Kanda T: A surface
immunodeterminant of human papillomavirus type 16 minor capsid
protein L2. Virology. 245:353–359. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hitzeroth II, Passmore JA, Shephard E,
Stewart D, Müller M, Williamson AL, Rybicki EP and Kast WM:
Immunogenicity of an HPV-16 L2 DNA vaccine. Vaccine. 27:6432–6434.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rubio I, Bolchi A, Moretto N, Canali E,
Gissmann L, Tommasino M, Müller M and Ottonello S: Potent anti-HPV
immune responses induced by tandem repeats of the HPV16 L2 (20-38)
peptide displayed on bacterial thioredoxin. Vaccine. 27:1949–1956.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kondo K, Ishii Y, Ochi H, Matsumoto T,
Yoshikawa H and Kanda T: Neutralization of HPV16, 18, 31, and 58
pseudovirions with antisera induced by immunizing rabbits with
synthetic peptides representing segments of the HPV16 minor capsid
protein L2 surface region. Virology. 358:266–272. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Palmer KE, Benko A, Doucette SA, Cameron
TI, Foster T, Hanley KM, McCormick AA, McCulloch M, Pogue GP, Smith
ML, et al: Protection of rabbits against cutaneous papillomavirus
infection using recombinant tobacco mosaic virus containing L2
capsid epitopes. Vaccine. 24:5516–5525. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huh WK and Roden RB: The future of
vaccines for cervical cancer. Gynecol Oncol. 109(Suppl 2): S48–S56.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gambhira R, Jagu S, Karanam B, Gravitt PE,
Culp TD, Christensen ND and Roden RB: Protection of rabbits against
challenge with rabbit papillomaviruses by immunization with the N
terminus of human papillomavirus type 16 minor capsid antigen L2. J
Virol. 81:11585–11592. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Green M and Loewenstein PM: Autonomous
functional domains of chemically synthesized human immunodeficiency
virus tat trans-activator protein. Cell. 55:1179–1188. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gupta B and Torchilin VP: Transactivating
transcriptional activator-mediated drug delivery. Expert Opin Drug
Deliv. 3:177–190. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dietz GP and Bähr M: Delivery of bioactive
molecules into the cell: The Trojan horse approach. Mol Cell
Neurosci. 27:85–131. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schwarze SR, Ho A, Vocero-Akbani A and
Dowdy SF: In vivo protein transduction: Delivery of a biologically
active protein into the mouse. Science. 285:1569–1572. 1999.
View Article : Google Scholar : PubMed/NCBI
|