Introduction
Ovarian cancer is one of the major causes of
cancer-associated fatalities among gynecological malignancies
(1). In recent years, discovery and
diagnosis of ovarian cancer at an early stage is limited, and there
is no effective therapy at advanced stages. The gold standard for
diagnosis of ovarian cancer is pathological evidence. Due to the
invasive nature of this procedure, it is not suitable for screening
patients of early-stage cancer. Therefore, the identification of
effective biomarkers for ovarian cancer is necessary.
Currently, cancer antigen 125 (CA125) is a
well-accepted serum biomarker for ovarian cancer, but is neither
specific nor sensitive enough for accurate diagnosis (2–4). Bast et
al (5) detected CA125 in 1983. The
serum CA125 level is also affected by numerous primary tumors and
female benign diseases (BD). Therefore, it is necessary to identify
novel biomarkers to improve the diagnostic efficiency for ovarian
cancer and to monitor high-risk patients, such as those with breast
cancer 1 (BRCA1) and BRCA2, infertility or menstrual disorders.
Kallikrein-related peptidase (KLK) (6) is a family of 15 members encoded by a
group of genes tandemly localized on chromosome 19q13.3–4 (7). KLK6 is the protein that is encoded by the
KLK gene and one of the KLKs. The mature KLK6 consists of
223 amino acids with trypsin-like activity (8). It is highly expressed in reproductive
organs such as the breast, ovary, prostate and testis. In 2000,
KLK6 was verified in various biologic fluids, including
cerebrospinal fluid, nipple aspirate fluid, breast cyst fluid, male
and female serum, seminal plasma, amniotic fluid and breast cancer
cytosols (9). A previous study showed
that KLK6 is a new potential serum biomarker for diagnosis and
prognosis of ovarian cancer (10).
Serum KLK6 levels were shown to increase in patients with
late-stage ovarian cancer with and without correlation to serum
CA125 levels, respectively (11).
There are numerous published studies that have evaluated the
diagnostic value of KLK6; however, there are no consistent results.
Therefore, a meta-analysis to ascertain the diagnostic value of
KLK6 systemically is required.
The aim of the present study was to systematically
assess whether the level of serum KLK6 could be used in the
detection of ovarian cancer, with a view to aid in the diagnosis of
ovarian cancer and choose the effective therapy.
Materials and methods
Screening process
Studies that were published before April 29, 2015,
were screened on a number of platforms, such as PubMed/Medline,
Embase, EBSCO, ScienceDirect, Cochrane library and Web of Science.
All the studies were selected using keywords as follows: ‘KLK6’
(kallikrein-related peptidase 6), ‘hk6’ (human kallikrein 6),
‘protease M,’ ‘zyme,’ ‘neurosin,’ ‘ovarian cancer’ (OC), ‘ovarian
carcinoma,’ ‘oophoroma,’ ‘ovarian neoplasm’ and ‘ovarian
neoplasms’.
Study selection
The inclusion criteria were as follows: i) All the
patients with ovarian cancer were diagnosed by pathology clearly;
ii) the predictive value of serum KLK6 was obtained by
enzyme-linked immunosorbent assay or chemiluminescent microparticle
immunoassay; and iii) studies must have the following terms:
False-negative (FN), false-positive (FP), true-negative (TN) and
true-positive (TP).
The exclusion criteria were as follows: i) The
information of the control group was not described clearly; ii)
review, animal experiment and seminars were not included; and iii)
the studies were not published in English or Chinese.
Data extraction and quality
assessment
All five studies were reading intensively to obtain
the number of ovarian cancer patients, non-ovarian cancer patients,
the values of sensitivity (SEN) and specificity (SPE) reported. The
values of FN, FP, TN, TP, positive-likelihood ratio (LR+),
negative-likelihood ratio (LR-) were calculated according to the
following formulas:
TP = Number of ovarian cancer patients × SEN
FN = Number of ovarian cancer patients × (1-SEN)
TN = Number of non-ovarian cancer patients × SPE
FP = Number of non-ovarian cancer patients ×
(1-SPE)
LR+ = SEN/(1-SPE)
LR- = (1-SEN)/SPE (12)
The blinded method was used by two investigators
(ChengJin Hu and Fan Yang) for reviewing and screening the included
studies. The widely used Quality Assessment of Diagnostic Accuracy
Studies (QUADAS2) tool was applied to assess the quality of all
included studies (13).
Statistical analysis
Meta-DiSc statistical software (version 1.4) was
used to obtain all the data (14).
Heterogeneity was checked by Spearman correlation analysis and
meta-regression. The index of the P-value and inconsistency index
(I-squared, I2) were used to estimate the heterogeneity.
When there was no significant heterogeneity between studies
(P>0.05, I2≤50%), the analysis was performed using a
fixed-effects model and analyzed bias to obtain SEN, SPE,
positive-predictive and negative-predictive values. When there was
statistical heterogeneity among studies, the analysis was performed
using the random-effects model (P≤0.05, I2>50%). The
pooled SEN, pooled SPE, LR+, LR- and their 95% confidence interval
(CI) were calculated using the DerSimonian-Laird random-effects
method. The summary receiver operating characteristic (sROC), the
area under the sROC curve (AUC) and the Q* value were calculated to
estimate the diagnostic accuracy of index test in the
meta-analysis. An AUC close to 1.0 signifies that the test has
almost perfect discrimination, while an AUC close to 0.5 suggests
poor discrimination. The index Q* corresponds to the upper most
point on the sROC curve in which true positivity (or SEN) equals
SPE. The sROC summarized the joint distribution of SEN and SPE.
This can be shown graphically by drawing a ‘line of identity’ in
which true positivity equals SPE on the sROC graph (13). P<0.05 by χ2 was
considered to indicate a statistically significant difference.
Results
Included studies
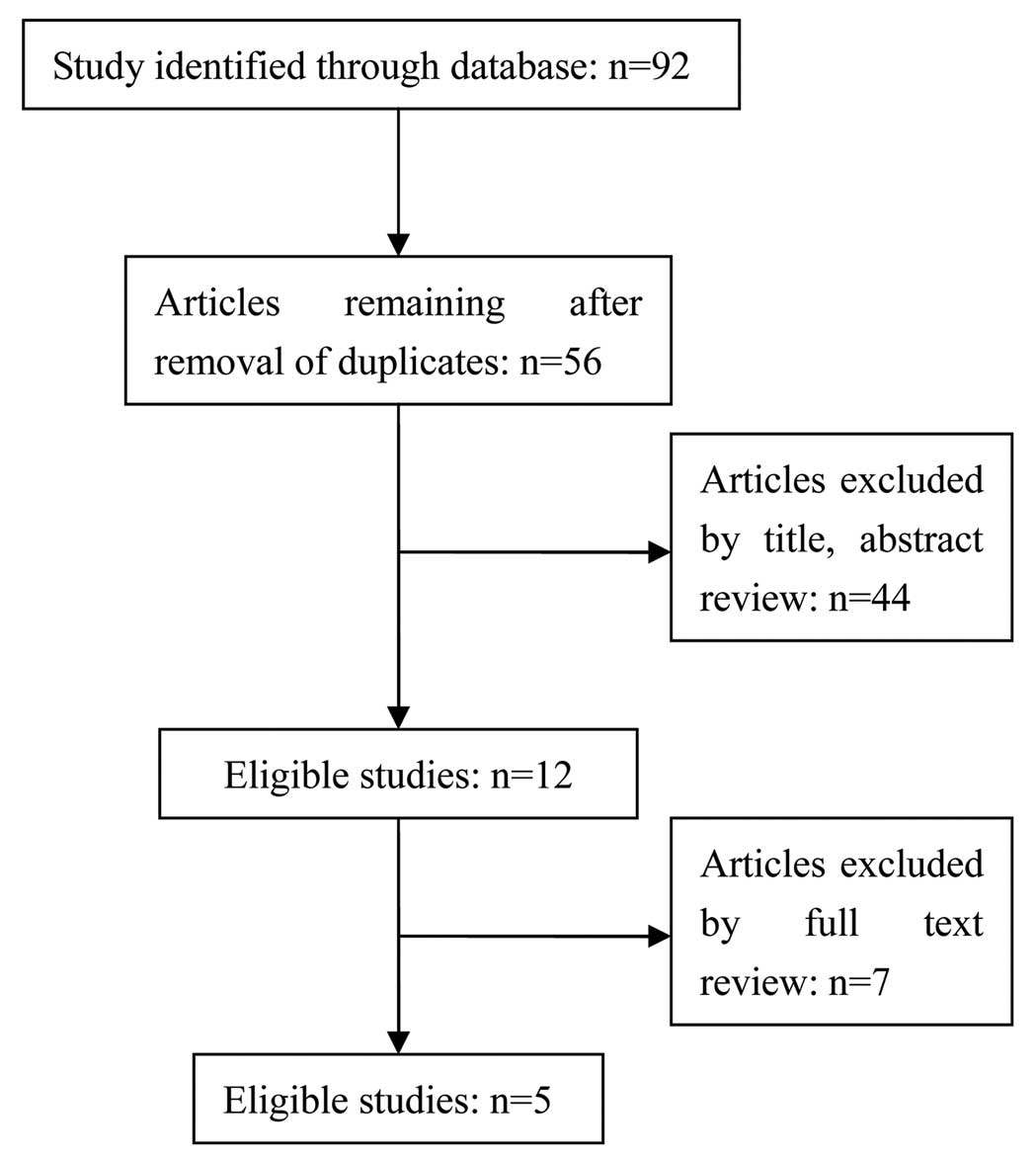
Following intensive assessment of the 92 retrieved
studies and taking the inclusion and exclusion criteria into
account, there were 5 studies selected (8,10,15–17). A
flowchart depicting the study selection is shown in Fig. 1. These 5 studies were published before
2015. The methodology quality of these 5 studies was assessed using
QUADAS2 (the description of QUADAS2 is shown in Table I). In the 5 included studies there were
1,150 samples, and there were 485, 420 and 245 patients with
ovarian cancer, BD and the healthy controls, respectively. All the
patients with ovarian cancer were diagnosed clearly by pathology.
The basic information of the included studies is shown in Table II. Other information regarding KLK6 is
described in Tables III–V. Furthermore, 2 studies evaluated the SEN of
KLK6 with different cut-off values.
 | Table I.Quality assessment of the 5 eligible
studies using QUADAS2. |
Table I.
Quality assessment of the 5 eligible
studies using QUADAS2.
|
| Risk of bias | Applicability
concerns |
|
|---|
|
|
|
|
|
|---|
| Authors, year | Patient
selection | Index test | Reference
standard | Flow and timing | Patient
selection | Index test | Reference
standard | Refs. |
|---|
| Bandiera et
al, 2013 | High | Low | Unknown | High | High | Low | Low | (15) |
| Diamandis et
al, 2003 | High | Unknown | Low | Unknown | High | Unknown | Low | (10) |
| Diamandis et
al, 2000 | High | High | Unknown | Low | High | Unknown | Unknown | (8) |
| Koh et al,
2012 | High | High | Unknown | High | High | Low | Unknown | (16) |
| El Sherbini et
al, 2011 | High | Low | Unknown | Low | Unknown | Unknown | Low | (17) |
 | Table II.Main characteristics of the 5
studies. |
Table II.
Main characteristics of the 5
studies.
|
| Control group, n |
|
|---|
|
|
|
|
|---|
| Authors, year | Country | Test method | Study size, n | OC, n | BD | Healthy | Refs. |
|---|
| Bandiera et
al, 2013 | Italy | ELISA | 180 | 60 | 60 | 60 | (15) |
| Diamandis et
al, 2003 | Canada | ELISA | 384 | 146 | 141 | 97 | (10) |
| Diamandis et
al, 2000 | Canada | ELISA | 161 | 80 | – | 81 | (8) |
| Koh et al,
2012 | Singapore | ELISA | 328 | 172 | 156 | – | (16) |
| El Sherbini et
al, 2011 | Egypt | ELISA | 97 | 27 | 63 | 7 | (17) |
 | Table III.Mean age and disease stage of the
samples. |
Table III.
Mean age and disease stage of the
samples.
|
| Age, years | Disease stage, n |
|---|
|
|
|
|
|---|
| Authors, year | Healthy | BD | OC | Early stages (I and
II) | Late stages (III and
IV) | Refs. |
|---|
| Bandiera et
al, 2013 | 52 | 46 | 56 | 43 | 103 | (15) |
| Diamandis et
al, 2003 | – | – | – | – | – | (10) |
| Diamandis et
al, 2000 | 50 | 58 | 62 | 17 | 43 |
(8) |
| Koh et al,
2012 | – | 38.3 | 45.5 | 72 | 100 | (16) |
| El Sherbini et
al, 2011 | 40 | 36 | 43 | 17 | 10 | (17) |
 | Table V.Cut-off value, TP, FP, FN, TN, SEN
and SPE of KLK6. |
Table V.
Cut-off value, TP, FP, FN, TN, SEN
and SPE of KLK6.
| Authors, year | Cut off, µg/l | TP, n | FP, n | FN, n | TN, n | SEN, % | SPE, % | Refs. |
|---|
| Bandiera et
al, 2013 | 10.79 | 42 | 7 | 18 | 53 | 70 | 90 | (15) |
|
| 15.31 | 36 | 1 | 24 | 59 | 60 | 96 |
|
|
| 22.94 | 23 | 1 | 37 | 59 | 38 | 100 |
|
| Diamandis et
al, 2003 | 4.2 | 76 | 24 | 70 | 214 | 52 | 90 | (10) |
|
| 4.4 | 69 | 12 | 77 | 226 | 47 | 95 |
|
| Diamandis et
al, 2000 | 15 | 53 | 0 | 27 | 81 | 66 | 100 |
(8) |
| Koh et al,
2012 | 6 | 61 | 12 | 111 | 144 | 35.2 | 92.1 | (16) |
| El Sherbini et
al, 2011 | 3.145 | 17 | 27 | 10 | 36 | 64 |
56.82 | (17) |
SEN, SPE, LR+ and LR- of KLK6
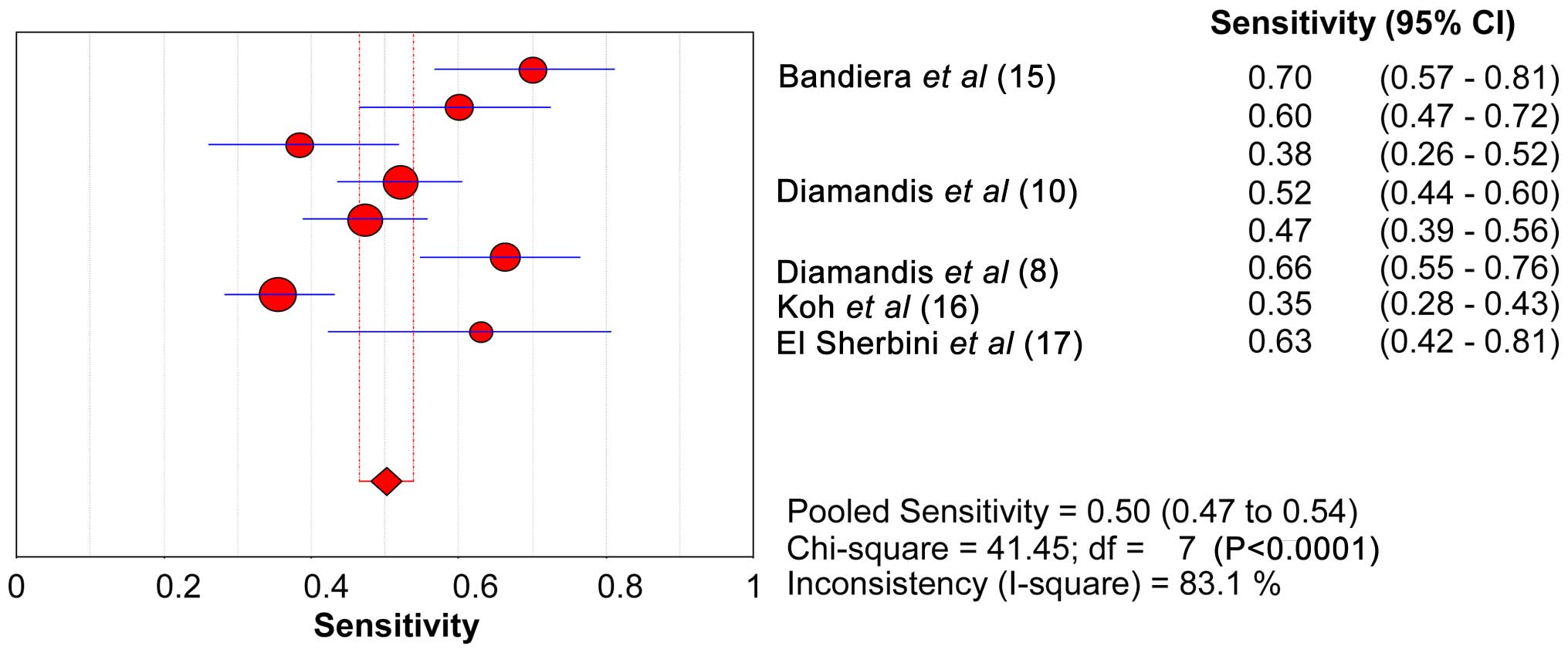
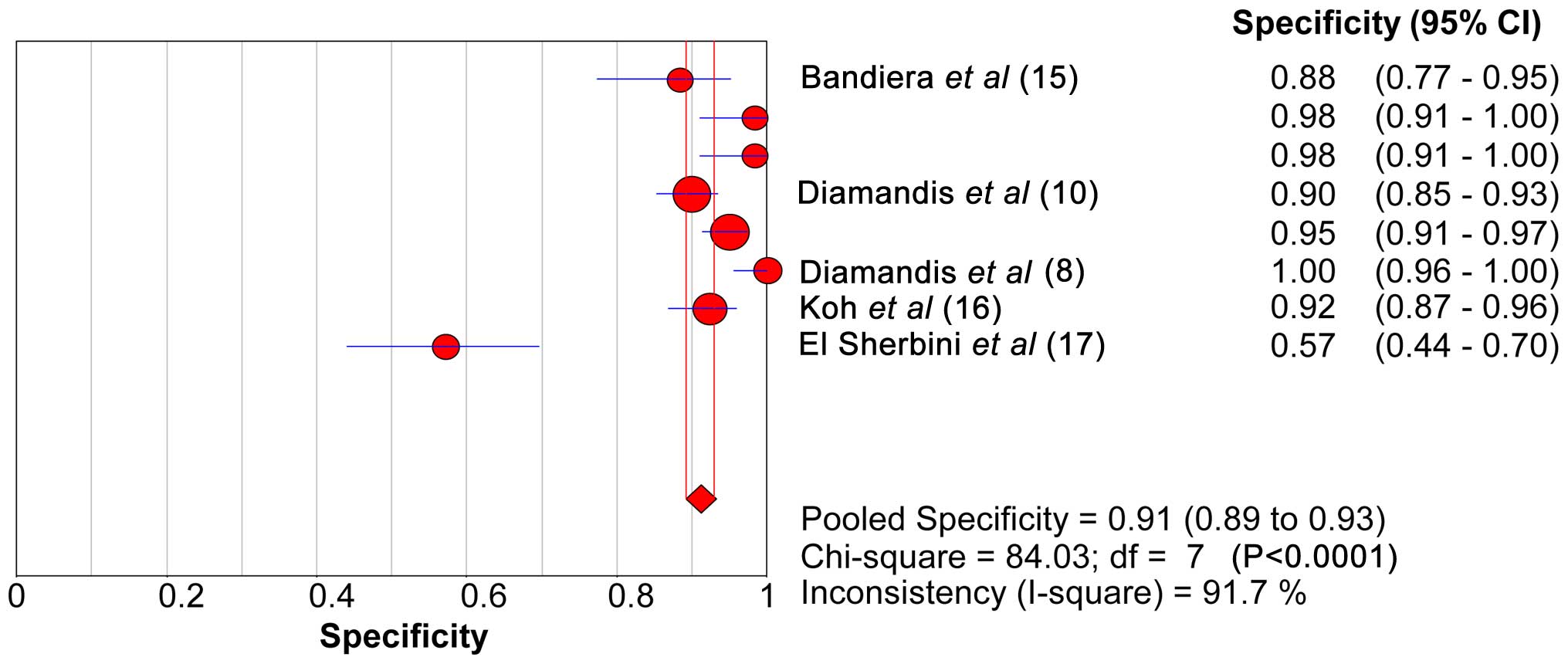
The range of SEN and SPE of KLK6 was 46.6–53.8 and
89.2–92.9%, respectively. The forest plots of KLK6 with SEN, SPE
and the 95% CI are shown in Figs. 2
and 3. The diagnostic SEN range was
0.47–0.54 (pooled SEN, 0.50) and the SPE range was 0.89–0.93
(pooled SPE, 0.91). The pooled SEN, pooled SPE, pooled LR+ (7.20)
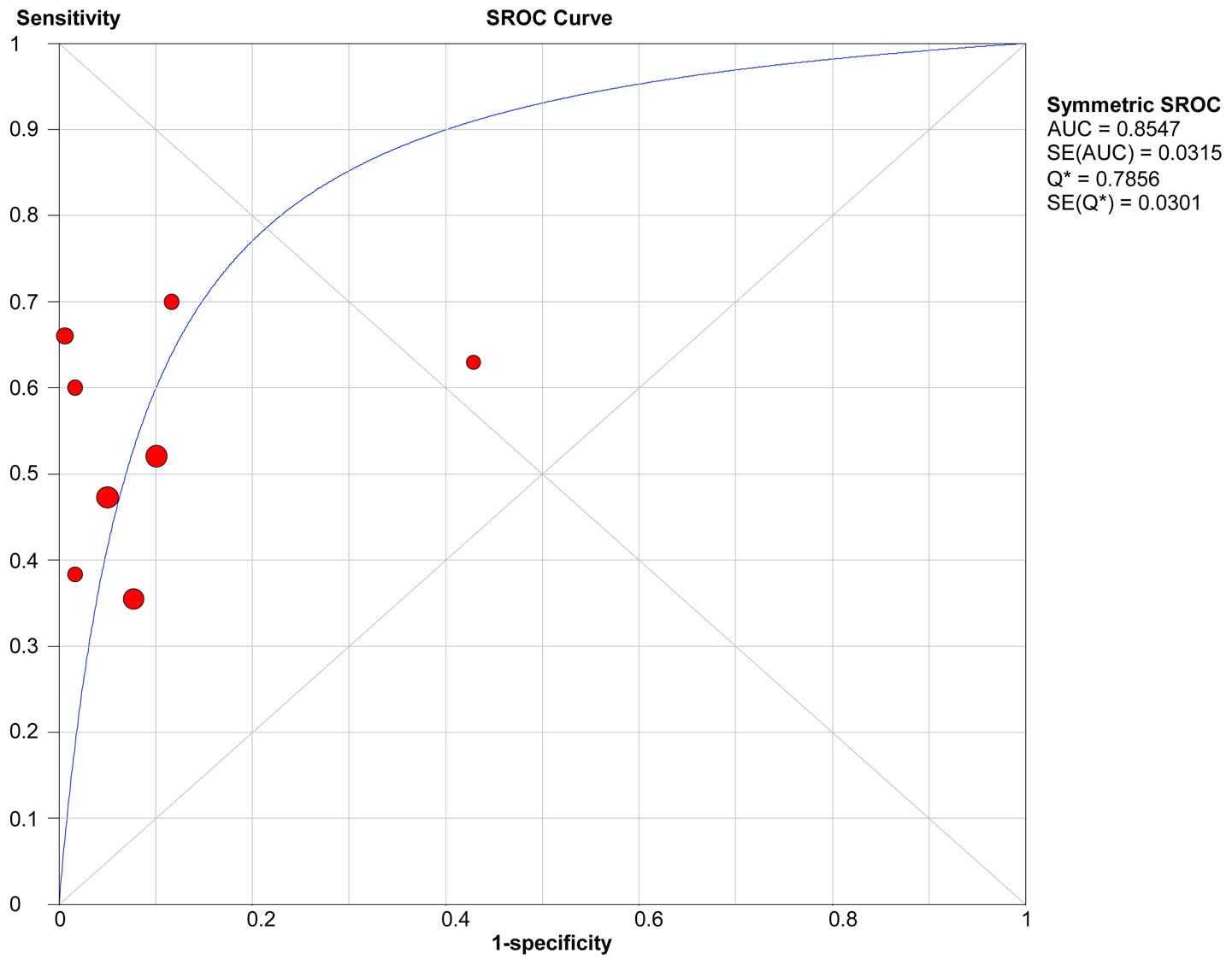
and pooled LR- (0.51) of KLK6 are shown in Table VI. The sROC curve of KLK6 is shown in
Fig. 4. The AUC and Q* index of KLK6
was 0.86 and 0.79, respectively.
 | Table VI.Pooled sensitivity, pooled
specificity, pooled LR+, pooled LR-, AUC and Q* of KLK6. |
Table VI.
Pooled sensitivity, pooled
specificity, pooled LR+, pooled LR-, AUC and Q* of KLK6.
| Gene | Pooled sensitivity,
% (95% CI) | Pooled specificity,
% (95% CI) | Pooled LR+ (95%
CI) | Pooled LR- (95%
CI) | AUC | Q* |
|---|
| KLK6 | 50 (47–54) | 91 (89–93) | 7.20
(3.34–15.51) | 0.51
(0.43–0.62) | 0.855 | 0.786 |
Possible sources of heterogeneity
The KLK6 of the sROC curve shows that the plane
scatter plot does not have a typical ‘shoulder arm-shaped’ style
(Fig. 4), while the Spearman
correlation coefficient was 0.180 and P=0.670, which indicates that
there is no heterogeneity from threshold effects. However, the pool
SEN (I2=83.1%) and SPE (I2=91.7%) indicates
high levels of heterogeneity. The control group, the test method,
the assay kit, a cut-off value, the mean age and the study size
were considered for the source of the heterogeneity (14).
Discussion
Ovarian cancer is a leading cause of
cancer-associated fatality among women in Western Europe and the
USA, and it has the highest mortality rate of all gynecological
malignancies (18).
Diagnosis of ovarian cancer lacks noninvasive tests
or clear biomarkers, and the majority of patients are diagnosed at
advanced stages, which have a poor prognosis. The discovery of new
ovarian cancer biomarkers for early diagnosis, prognosis,
monitoring and prediction of therapeutic response may contribute to
improved clinical outcomes. Therefore, novel biomarkers for
screening ovarian cancer at early stages are required.
The results of the present meta-analysis showed that
the serum KLK6 level could aid in the prediction of the presence of
ovarian cancer based on the AUC (AUC=0.85). KLK6 showed SEN 0.50
(0.467 to 0.54) and high SPE 0.91 (0.90 to 0.93). These data
indicate that KLK6 is a useful diagnostic marker for ovarian
cancer. The associated poor SEN of KLK6 clearly limits its value in
its diagnosis of ovarian cancer. However, the high SPE also makes
it a potential biomarker of ovarian cancer.
As a traditional ovarian cancer biomarker, CA125 has
been used for 20 years. In addition to its low SEN for early
disease, CA125 also has low SPE; for example, elevated levels are
observed in numerous benign gynecological diseases (18). However, the diagnostic accuracy of
CA125 compared to KLK6 in the diagnosis of ovarian cancer was not
compared in the present study as CA125 is frequently used
clinically and the test results are not always blinded to
gynecologists. Among these eligible studies, there were 3 original
studies that compared the diagnostic efficiency of KLK6 and CA125.
Koh et al (16) found that the
SEN and the SPE of KLK6 were lower than those of CA125, but
combination of KLK6 and CA125 showed higher diagnostic efficiency.
El Sherbini et al (17) and
Diamandis et al (9) found that
serum KLK6 and may have much lower overall SEN than serum CA125.
However, whereas serum KLK6 may improve the SEN of CA125. Mills
et al (19) suggested that KLK6
is more specific for ovarian cancer than CA125 as elevations were
not observed in BD. Therefore, whether the diagnostic efficiency of
KLK6 is superior to that of CA125 remains to be elucidated.
However, all these studies evidently supported that KLK6 can
improve the diagnostic efficiency of CA125.
The present meta-analysis showed significant
heterogeneity between the selected studies. Therefore, due to the
heterogeneity, the meta-analysis results should be interpreted with
caution. The Spearman correlation coefficient was 0.180 and
P=0.670, which indicates that there is no heterogeneity from
threshold effects. The following reasons may hamper the statistical
analysis of sources of heterogeneity: i) There was no unified
cut-off value. ii) The clinical features of the patients, such as
the mean age and menopausal status, differed among the included
studies. The available data showed that the mean ages ranged from
43 to 62 years. Ages and menopausal status are associated with the
occurrence of ovarian cancer. Therefore, it may be a significant
source of heterogeneity. iii) The nationality of the study
populations differed among the included studies, and the origin of
the study populations has a substantial influence on the diagnostic
SEN (20). iv) Owing to the small
number of patients included in these studies, subgroup analysis was
not possible.
The present systematic review has several strengths.
In recent decades, a series of studies focused on whether KLK6 was
a promising biomarker of ovarian cancer. To the best of our
knowledge, this is the first review of the systematic research and
meta-analysis to evaluate the diagnostic value of KLK6 for ovarian
cancer. All the studies that were published up to April 29, 2015
were searched. There were 485 patients with ovarian cancer, 420
benign cysts and 245 healthy controls involved in the present
study. Study selection, data extraction, evaluation of the risk of
bias and strength of inferences for each outcome were performed by
two investigators independently, to reduce the risk of selection
bias. However, there were several limitations in the meta-analysis.
First, the number of the included studies was too small. Second,
the difference in the mean age and menopausal status may bias the
results. Third, the limitation of the present meta-analysis was
that there was no unified cut-off value.
In conclusion, the high SPE of human KLK6 shows that
it is a potential novel biomarker for ovarian cancer. It can
evidently improve diagnostic efficiency of ovarian cancer and KLK6
can improve the diagnostic accuracy of CA125. However, according to
the present meta-analysis, KLK6 does not meet the standard of an
independent diagnostic biomarker. A combined panel of CA125 and
KLK6 showed a high diagnostic efficiency for advanced ovarian
cancer. Due to the limitations in the present meta-analysis,
additional studies are required to assess the diagnostic accuracy
of KLK6 in the future.
References
|
1
|
Siegel R, Ward E, Brawley O and Jemal A:
Cancer statistics, 2011: The impact of eliminating socioeconomic
and racial disparities on premature cancer deaths. CA Cancer J
Clin. 61:212–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rosenthal AN and Jacobs IJ: The role of CA
125 in screening for ovarian cancer. Int J Biol Markers.
13:216–220. 1998.PubMed/NCBI
|
|
3
|
Maggino T and Gadducci A: Serum markers as
prognostic factors in epithelial ovarian cancer: An overview. Eur J
Gynaecol Oncol. 21:64–69. 2000.PubMed/NCBI
|
|
4
|
Bast RC Jr, Xu FJ, Yu YH, Barnhill S,
Zhang Z and Mills GB: CA 125: The past and the future. Int J Biol
Markers. 13:179–187. 1998.PubMed/NCBI
|
|
5
|
Bast RC Jr, Klug TL, St John E, Jenison E,
Niloff JM, Lazarus H, Berkowitz RS, Leavitt T, Griffiths CT, Parker
L, et al: A radioimmunoassay using a monoclonal antibody to monitor
the course of epithelial ovarian cancer. N Engl J Med. 309:883–887.
1983. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lundwall A, Band V, Blaber M, Clements JA,
Courty Y, Diamandis EP, Fritz H, Lilja H, Malm J, Maltais LJ, et
al: A comprehensive nomenclature for serine proteases with homology
to tissue kallikreins. Biol Chem. 387:637–641. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yousef GM and Diamandis EP: The new human
tissue kallikrein gene family: Structure, function, and association
to disease. Endocr Rev. 22:184–204. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Diamandis EP, Yousef GM, Soosaipillai AR
and Bunting P: Human kallikrein 6 (zyme/protease M/neurosin): A new
serum biomarker of ovarian carcinoma. Clin Biochem. 33:579–583.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Diamandis EP, Yousef GM, Soosaipillai AR,
Grass L, Porter A, Little S and Sotiropoulou G: Immunofluorometric
assay of human kallikrein 6 (zyme/protease M/neurosin) and
preliminary clinical applications. Clin Biochem. 33:369–375. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Diamandis EP, Scorilas A, Fracchioli S,
Van Gramberen M, De Bruijn H, Henrik A, Soosaipillai A, Grass L,
Yousef GM, Stenman UH, et al: Human kallikrein 6 (hK6): A new
potential serum biomarker for diagnosis and prognosis of ovarian
carcinoma. J Clin Oncol. 21:1035–1043. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Luo LY, Bunting P, Scorilas A and
Diamandis EP: Human kallikrein 10: a novel tumor marker for ovarian
carcinoma? Clinica Chim Acta. 306:11–118. 2001. View Article : Google Scholar
|
|
12
|
Chen X, Li WL, Zhang YL, Wu Q, Guo YM and
Bai ZL: Meta-analysis of quantitative diffusion-weighted MR imaging
in the differential diagnosis of breast lesions. BMC Cancer.
10:6932010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Whiting PF, Rutjes AW, Westwood ME,
Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA and Bossuyt
PM: QUADAS-2 Group: QUADAS-2: A revised tool for the quality
assessment of diagnostic accuracy studies. Ann Intern Med.
155:529–536. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zamora J, Abraira V, Muriel A, Khan K and
Coomarasamy A: Meta-DiSc: A software for meta-analysis of test
accuracy data. BMC Med Res Methodol. 6:312006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bandiera E, Zanotti L, Fabricio AS, Bucca
E, Squarcina E, Romani C, Tassi R, Bignotti E, Todeschini P, Tognon
G, et al: Cancer antigen 125, human epididymis 4, kallikrein 6,
osteopontin and soluble mesothelin-related peptide immunocomplexed
with immunoglobulin M in epithelial ovarian cancer diagnosis. Clin
Chem Lab Med. 51:1815–1824. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Koh SC, Huak CY, Lutan D, Marpuang J,
Ketut S, Budiana NG, Saleh AZ, Aziz MF, Winarto H, Pradjatmo H, et
al: Combined panel of serum human tissue kallikreins and CA-125 for
the detection of epithelial ovarian cancer. J Gynecol Oncol.
23:175–181. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
El Sherbini MA, Sallam MM, Shaban EA and
El-Shalakany AH: Diagnostic value of serum kallikrein-related
peptidases 6 and 10 versus CA125 in ovarian cancer. IInt J Gynecol
Cancer. 21:625–632. 2011. View Article : Google Scholar
|
|
18
|
Campos SM and Ghosh S: A current review of
targeted therapeutics for ovarian cancer. J Oncol. 2010:1493622010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mills GB, Bast RC Jr and Srivastava S:
Future for ovarian cancer screening: Novel markers from emerging
technologies of transcriptional profiling and proteomics. J Natl
Cancer Inst. 93:1437–1439. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Whiting P, Rutjes AW, Reitsma JB, Glas AS,
Bossuyt PM and Kleijnen J: Sources of variation and bias in studies
of diagnostic accuracy: A systematic review. Ann Intern Med.
140:189–202. 2004. View Article : Google Scholar : PubMed/NCBI
|