Introduction
The development of dialysis technology makes the
long-term survival of the end-stage renal disease patient possible.
However, mortality rates for the dialysis patients remain, mainly
due to malnutrition, inflammation and cardiovascular problems.
According to the latest statistics, 35–50% of the dialysis patients
succumbed due to cardiovascular complications, which is 15 times
greater compared to the general population. As a newly discovered
growth hormone-releasing peptide, the association of ghrelin with
cardiovascular disease and malnutrition in dialysis patients is
receiving increased attention.
The molecular weight of ghrelin is 3.3 kD and its
particle diameter ranges 90–150 nm. The human ghrelin gene is
located on chromosome 3 q25–26. Ghrelin mRNA is translated into the
protein precursor containing 117 amino acids and following slicing
becomes ghrelin containing 28 amino acids (1). Ghrelin has two types of molecular forms;
symplectic acylated ghrelin (acyl ghrelin) and des-acyl ghrelin.
Acyl ghrelin has an n-octanoylated serine in position 3, and this
modification is essential for the activity of ghrelin (2). The majority of animal and human tests
have proved that the total ghrelin level can stimulate growth
hormone secretion, enhance appetite, improve feeding, adjust the
positive balance of energy, regulate gastric motility, promote
gastric acid secretion, protect gastric mucosa, provide direct and
indirect protection for the cardiovascular system, and improve the
immune system function (3).
The present study aimed to understand the
association between ghrelin and cardiovascular function by
measuring the plasma ghrelin levels of the hemodialysis (HD)
patients, continuous ambulatory peritoneal dialysis (CAPD) patients
and the control group, by analyzing the correlation between ghrelin
and the associated indexes and by exploring the correlation between
the plasma ghrelin levels and their corresponding echocardiography
indicators in these three groups. A previous study indicates that
ghrelin is closely associated with malnutrition, inflammation and
atherosclerosis (4). The study of the
correlation between ghrelin and malnutrition with dialysis patients
has been reported previously (5), but
the correlation between ghrelin and cardiovascular function in
patients with dialysis remains to be assessed. Therefore, the
association between ghrelin levels, cardiac function and
malnutrition in dialysis patients remains to be further studied.
The present study aimed to further clarify these associations to
provide better protection for the cardiovascular function of
patients by adopting ghrelin as a primary timing indicator of the
nutritional intervention for the therapy of dialysis patients,
which can reduce the mortality rate and improve the patient quality
of life.
Materials and methods
Patients and healthy controls
A total of 30 CAPD patients were selected for the
CAPD group from the Inner Mongolia People's Hospital (Hohhot,
China) between March 2013 and March 2014. The CAPD time of the
patient was ≥3 months (19 males and 11 females, with an average age
of 55.43±13.82 years) using the peritoneal fluid produced by
Guangzhou Baxter Healthcare (Guangzhou, China) and their dialysis
doses were between 6–8 litres every 24 h. For the HD group,
clinical data was selected from the patients who underwent the
dialysis treatment with bicarbonate dialysate at the same time as
the CAPD group in our blood purification center for >3 months.
The control group consisted of 30 healthy physical examinees in our
hospital during the same period who were age- and gender-matched to
the CAPD group. For all three groups, the possibility was excluded
of developing during the observation period severe infection,
malignant tumor, blood disease, active lupus erythematosus,
undergoing hormone and immunosuppressive therapy, rheumatic heart
disease, cor pulmonale, congenital heart disease and
hyperthyroidism heart disease. The protocol was approved by the
Medical Ethics Committee of the Guangdong General Hospital, Inner
Mongolia People's Hospital and the Southern Medical University.
Informed written consent was obtained from all the participants
prior to participation in the study.
Research methods
The subjects provided detailed clinical data,
including history and laboratory examination results. The Vivid T
Color Doppler Ultrasonic Diagnostic instrument produced by GE
Healthcare (Pittsburg, PA, USA) was used for analysis of all groups
and 2 ml of fasting venous blood was obtained from all participants
the morning after clinical treatment or hospitalization. Within 30
min of obtaining the sample, the ghrelin blood specimens were
placed in an anticoagulant tube with an appropriate amount of
ethylenediaminetetraacetic acid and were centrifuged for 15 min at
1,509.3 × g. The supernatant fluid was removed and stored in the
refrigerator at −80°C. The ghrelin enzyme-linked immunosorbent
assay kit was produced by R&D Systems, Inc. (New Brunswick, NJ,
USA).
Statistical analysis
Statistical software was used to analyze the data
with all the values expressed as mean ± standard error of the mean.
The independent sample t-test was adopted for the comparison of the
mean of the two samples and the Pearson coefficient was used for
the correlation analysis. SPSS version 13.0 software (SPSS, Inc.,
Chicago, IL, USA) was used for these analyses and P<0.05 was
considered to indicate a statistically significant difference.
Results
Comparison of plasma ghrelin levels
between the three groups
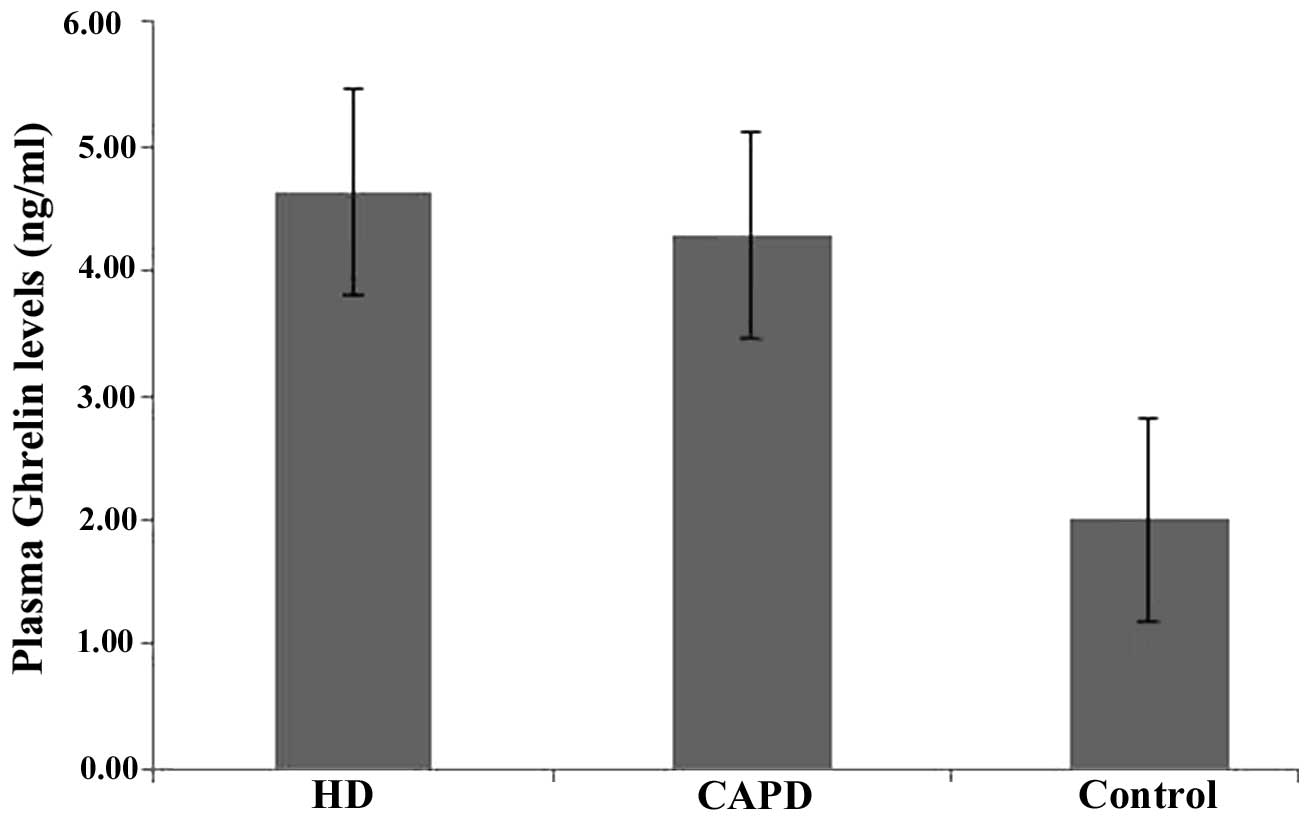
Differences obtained by single-factor analysis of
variance between the three groups were considered to indicate a
statistically significant difference (F-value, 75.106;
P<0.0001), and the comparison between two groups also exhibited
statistical significance (P<0.05). The plasma ghrelin levels of
the HD and CAPD groups were significantly higher compared to that
of the controls (Table I and Fig. 1).
 | Table I.Comparison of the plasma ghrelin
levels between the three groups. |
Table I.
Comparison of the plasma ghrelin
levels between the three groups.
| Group (n) | Ghrelin, ng/ml | F | P-value |
|---|
| Hemodialysis
(30) | 4.63±1.08 | 75.106 | <0.0001 |
| Peritoneal dialysis
(30) | 4.28±1.07 |
|
|
| Control (30) | 2.00±0.48 |
|
|
Correlation analysis between the
plasma ghrelin levels and echocardiography in the three groups
The plasma ghrelin levels of CAPD patients were
positively correlated with left ventricular ejection fraction
(LVEF) (r=0.506, P=0.004) (Table
II).
 | Table II.Correlation analysis between plasma
ghrelin levels and the echocardiographic in the three groups. |
Table II.
Correlation analysis between plasma
ghrelin levels and the echocardiographic in the three groups.
|
| Haemodialysis | Peritoneal
dialysis | Control |
|---|
|
|
|
|
|
|---|
|
Echocardiographic | r | P-value | r | P-value | r | P-value |
|---|
| IVST | −0.018 |
0.932 |
0.037 | 0.844 | −0.024 |
0.901 |
| LVPWT | −0.041 |
0.567 |
0.142 | 0.455 |
0.060 |
0.754 |
| LVEDD |
0.023 |
0.806 | −0.068 | 0.720 |
0.001 |
0.996 |
| LAD |
0.185 |
0.356 |
0.080 | 0.675 | −0.018 |
0.924 |
| LVM |
0.206 |
0.184 |
0.077 | 0.684 |
0.023 |
0.903 |
| LVMI |
0.132 |
0.508 | −0.080 | 0.676 |
0.104 |
0.583 |
| LVEF |
0.076 |
0.537 |
0.506 | 0.004a | −0.120 |
0.528 |
Correlation analysis between the
plasma ghrelin levels and laboratory index in the three groups
The present study compared the plasma ghrelin levels
and the association between each laboratory index, which indicates
that plasma ghrelin levels showed a positive correlation with serum
creatinine (Scr) (CAPD group, r=0.506 and P=0.004; HD group,
r=0.478 and P=0.008), blood urea nitrogen (BUN) (CAPD group,
r=0.649 and P=0.002; HD group, r=0.582 and P=0.001). The present
study shows that the plasma ghrelin levels of CAPD patients were
negatively correlated with BMI (r=−0.556, P=0.001) (Table III).
 | Table III.Correlation analysis between plasma
ghrelin levels and the laboratory index in the three groups. |
Table III.
Correlation analysis between plasma
ghrelin levels and the laboratory index in the three groups.
|
| Haemodialysis | Peritoneal
dialysis | Control |
|---|
|
|
|
|
|
|---|
| Laboratory index | r | P-value | r | P-value | r | P-value |
|---|
| Scr, µmol/l |
0.478 |
0.008a |
0.506 |
0.004a |
0.113 | 0.552 |
| BUN, mmol/l |
0.582 |
0.001a |
0.649 |
0.002a |
0.267 | 0.153 |
| Hb, g/l | −0.016 | 0.933 |
0.091 | 0.633 | −0.224 | 0.235 |
| Ca, mmol/l |
0.188 | 0.320 |
0.073 | 0.701 | −0.258 | 0.169 |
| P, mmol/l | −0.150 | 0.429 |
0.177 | 0.351 | −0.339 | 0.067 |
| PTH, pg/ml | −0.045 | 0.814 | −0.223 | 0.235 |
0.092 | 0.630 |
| TP, g/l | −0.139 | 0.464 | −0.042 | 0.824 |
0.001 | 0.997 |
| ALB, g/l | −0.053 | 0.780 |
0.258 | 0.168 | −0.037 | 0.845 |
| TG, mmol/l |
0.173 | 0.362 |
0.035 | 0.854 |
0.062 | 0.747 |
| TC, mmol/l |
0.206 | 0.274 |
0.232 | 0.218 |
0.090 | 0.636 |
| HDL-L, mmol/l |
0.042 | 0.827 | −0.018 | 0.924 | −0.339 | 0.007 |
| LDL-L, mmol/l |
0.209 | 0.269 |
0.063 | 0.740 |
0.288 | 0.123 |
| BMI,
kg/m2 |
|
| −0.556 |
0.001a |
|
|
| Weight, kg |
|
| −0.213 | 0.259 |
|
|
Discussion
Animal experiments and clinical trials have
confirmed that ghrelin is significant in promoting growth
hormone-releasing material, such as the growth hormone secretagogue
receptor (GHSR), and has a dose-effect correlation with growth
hormone secretion. It can also stimulate more growth hormone
secretion compared with dose GHSR and unnatural GHS (6). First ghrelin can enhance appetite,
increase energy intake and maintain a positive balance of energy.
Therefore, ghrelin can improve malnutrition of dialysis patients,
and additionally, ghrelin can enhance myocardial contraction force,
increase LVEF significantly, inhibit vascular calcification,
increase the coronary circulation, improve ischemia reperfusion,
and have a direct or indirect protection on the cardiovascular
system. Therefore, the present study aimed to analyze, using the
plasma ghrelin levels in CAPD, HD and healthy controls, the plasma
ghrelin level and correlation of each index in patients, and the
association between plasma ghrelin levels and each group
echocardiography. The study also aimed to further define the plasma
ghrelin levels, cardiovascular function and malnutrition in
patients with dialysis, assess the conducive use of exogenous
ghrelin to improve malnutrition and protect cardiovascular function
in dialysis patients in the future, and provide novel information
to improve the quality of survival and long-term survival in
dialysis patients.
The majority of the studies have reported that the
plasma ghrelin levels in dialysis patients were significantly
higher compared to the age- and gender-matched control group
(7). The results of the present study
indicate a significantly greater increase in ghrelin levels in the
HD and CAPD groups compared to the control group, which is
consistent with the conclusions of the majority of recent studies.
Pérez-Fontán et al (8),
following the investigation of plasma ghrelin levels in age-matched
control groups, reported that the plasma ghrelin levels of 20 HD
patients and 21 peritoneal dialysis patients were significantly
higher than that of the control group; however, no significant
differences were identified between the former two. Tentolouris
et al (9) studied the ghrelin
levels of 57 healthy controls and 122 chronic renal insufficient
patients (65 HD patients and 57 non-dialysis patients). The results
showed that the fasting total ghrelin plasma levels of the HD group
and non-dialysis patients increased higher in comparison to the
control group, and there was no evident difference between the
dialysis and non-dialysis groups. There are different view points
regarding the reasons for the plasma ghrelin level elevation. For
example, the kidney may be the site where the clearance and
degradation of the hormone occurs. A study conducted by Yoshimoto
et al (10) showed that
compared with the normal controls, ghrelin levels of dialysis
patients increased 2.8-fold. The study also found that ghrelin
levels in rats with partial nephrectomy increased by 1.3-fold, and
3.1-fold in rats with double renal resection. Previous studies have
found that ghrelin can be detected in the urine of healthy
subjects, which suggests that the kidney is an important metabolic
site for ghrelin. The metabolic pathway of ghrelin remains to be
elucidated; however, Mori et al (11) reported that ghrelin and its receptors
exist in the kidney, which means that the kidney is not only the
source of ghrelin, but also its target organ. Another study
suggested that the increase of total plasma ghrelin levels in
patients with chronic kidney disease (CKD) is mainly caused by less
removal and degradation of ghrelin by kidney, which could be one of
the main reasons for the significant increase of the plasma ghrelin
levels in patients with CKD. Certain investigators have suggested
that the adaptive compensatory reaction in the body under the
condition of the inadequate energy intake is another cause of the
rising plasma ghrelin level (8,12). In
addition, others consider that insulin can reduce the plasma
ghrelin levels in the normal population; however, it is common for
non-diabetic dialysis patients to develop insulin resistance.
Therefore, the weakened inhibition of plasma ghrelin levels caused
by insulin resistance is also a reason for the elevated plasma
ghrelin levels (13).
Cardiovascular disease is one of the major causes of
mortality for dialysis patients. The incidence rate of
cardiovascular disease in patients with CKD is 10–30 times greater
than that of the general population. The early symptom of cardiac
function insufficiency is lower left ventricular diastolic
function. Left ventricular hypertrophy (LVH) is the most common
cardiomyopathy in patients with CKD, which is evident in patients
with mild renal impairment (the incidence of LVH is ~30% in CKD
patients when creatinine clearance is 50–70 ml/min), and the
incidence rate increases (14) with
worsened renal function damage. Renal damage leads to
cardiovascular disease, which can aggravate kidney damage. This
interaction forms a vicious cycle. Cardiovascular disease in
patients with CKD occurs early without clear symptoms, which makes
it easily ignored. Therefore, the damage is difficult to reverse
once the symptom appears. The study results show that left
ventricular myocardial mass index (LVMI) was 151.56±50.56
g/m2 in the HD group, 157.09±46.14 g/m2 in
the CAPD group, and 77.27±18.34 g/m2 in the control
group (data not shown). Compared with the healthy controls, the
LVMI levels in the HD and CAPD groups increased significantly,
which indicate that LVH is widespread among the dialysis patients.
This finding is similar to the result of a Canadian
echocardiographic survey of 432 patients with CKD who just started
dialysis (15). In addition, the study
found that plasma ghrelin levels in CAPD patients were positively
correlated with LVEF (r=0.506, P=0.004), which suggests that the
higher the plasma ghrelin level reaches, the higher the LVEF
scores. This may attribute to the protective effects of plasma
ghrelin on cardiovascular function. No such correlation with LVEF
was observed in the HD group. The increasing plasma ghrelin levels
in CAPD patients also prompted LVEF, increased cardiac output,
increased the end pressure of the left ventricular systolic
function and increased the myocardial contraction ratio. HD
patients have greater hemodynamic fluctuations, which could
undermine the protective effects of ghrelin on the cardiovascular
system. The present study found that LVEF was positively correlated
with plasma ghrelin levels in CAPD patients, which is not
consistent with the result of a study performed in Greece (8) that reported no correlation between the
plasma ghrelin levels and LVEF in patients with end-stage CKD. In
addition, this study showed that there was no significant
correlation between the interventricular septum thickness and LVMI
of CAPD patients with the plasma ghrelin levels.
Protein malnutrition is common among the dialysis
patients, which can be caused by anorexia, nausea and vomiting.
This leads to lack of energy and protein intake. According to
previously reported statistics, every day an abdominal peritoneal
dialysis patient loses, through peritoneal fluid, 8–9 g of protein
and 2.4 g of amino acid. Those who develop peritonitis would lose
15 g of protein (16) and 10–30 g of
amino acids and peptides after 1 HD. When the endocrine disorder
caused by the metabolic acidosis occurs, branched chain amino acids
can inhibit protein synthesis and promote the decomposition of
protein, leading to a negative nitrogen balance. Peritoneal
dialysis patients frequently suffer from micro-inflammatory
conditions due to factors such as oxidative stress, toxin retention
and biological incompatibility. Studies have shown that injections
of growth hormone can evidently improve the nutritional status of
the dialysis patients and reduce their risk of developing
cardiovascular disease (17).
Following the measurement of 41 patients with CKD in the study by
Yoshimoto et al (10), the
plasma total ghrelin levels were positively correlated with Scr and
BUN levels, and negatively correlated with the creatinine clearance
rate. However, a study performed in Taiwan suggested that the
plasma ghrelin levels were significantly negatively correlated with
BMI in dialysis patients, and therefore, ghrelin resistance may
exist among dialysis patients, which was closely associated with
the nutritional index (18). The
present study suggests that plasma ghrelin levels were
significantly positively correlated with Scr and BUN, indicating
that the kidney was the important site of metabolism of ghrelin.
The plasma ghrelin levels of CAPD patients were significantly
negatively correlated with BMI, but no correlation was observed for
BMI in HD patients, indicating that the plasma levels of ghrelin
were closely associated with malnutrition in CAPD patients. This
may be associated with the two types of dialysis in a different
way. HD on toxin removal is intermittent and HD for the removal of
the ghrelin is intermittent as ghrelin has a weak fluctuation in
the body. However, toxin removal by CAPD is continuous and smooth,
sustaining high levels of ghrelin, which will have a lasting impact
on the nutritional status of CAPD patients. The plasma ghrelin
levels from different dialysis methods indicate that the
nutritional status of patients has different effects. In addition,
malnutrition, inflammation and atherosclerosis are inseparable
interactions in dialysis patients, and therefore, the effect of
ghrelin should be noted with regards to malnutrition, inflammation
and atherosclerosis, and also the occurrence and development of
cardiovascular disease in dialysis patients. The aforementioned
correlation analysis found that plasma ghrelin levels are
associated with malnutrition in CAPD patients; however, the
specific mechanism remains to be elucidated, and further
investigations are required regarding the association between the
plasma ghrelin levels in dialysis patients and inflammation and
atherosclerosis.
In conclusion, the plasma ghrelin levels may be
associated with protein malnutrition, oxidative stress and the
occurrence and development of cardiovascular disease in end-stage
renal disease and dialysis patients. The significant increase of
ghrelin levels in HD and CAPD patients may be attributed to the
declining function of the kidney to remove and metabolically
degrade the hormone. However, the malnutrition and endocrine
disorders of patients should be considered as another, and possibly
more important, cause for this symptom. The positive correlation
between the plasma ghrelin level and LVEF in CAPD patients
demonstrates a significant correlation. Plasma ghrelin is a
recently identified brain-gut peptide, which can increase appetite,
improve energy intake, regulate weight, protect against
malnutrition, protect the cardiovascular function and adjust immune
system function. Therefore, further study of the cause behind the
increase in plasma ghrelin levels in patients with CKD will be
beneficial for the adoption of exogenous ghrelin for improving
malnutrition, microinflammation and atherosclerosis in end-stage
renal disease and dialysis patients, which will protect their
cardiovascular function. The present study provides a novel
perspective for improving the quality of life in patients and
reducing complications.
Acknowledgements
The authors would like to thank the patients and
healthy controls who participated in the study. The present study
was funded by the Talents Fund of Inner Mongolia Autonomous Region
(no. 2008).
References
|
1.
|
Seim I, Collet C, Herington AC and Chopin
LK: Revised genomic structure of the human ghrelin gene and
identification of novel exons, alternative splice variants and
natural antisense transcripts. BMC Genomics. 8:2982007. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Kojima M and Kangawa K: Structure and
function of ghrelin. Results Probl Cell Differ. 46:89–115. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Kojima M and Kangawa K: Ghrelin: Structure
and function. Physiol Rev. 85:495–522. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Hubacek JA, Bloudícková S, Bohuslavová R,
Táborský P, Polakovic V, Sazamová M, Svítilová E, Vlasák J, Sojková
I, Ryba M, et al: Ghrelin variants influence development of body
mass index and plasma levels of total cholesterol in dialyzed
patients. Clin Chem Lab Med. 45:1121–1123. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Ruperto M, Sánchez-Muniz FJ and Barril G:
A clinical approach to the nutritional care process in
protein-energy wasting hemodialysis patients. Nutr Hosp.
29:735–750. 2014.(In Spanish). PubMed/NCBI
|
|
6.
|
Nagaya N, Kojima M, Uematsu M, Yamagishi
M, Hosoda H, Oya H, Hayashi Y and Kangawa K: Hemodynamic and
hormonal effects of human ghrelin in healthy volunteers. Am J
Physiol Regul Integr Comp Physiol. 280:R1483–R1487. 2001.PubMed/NCBI
|
|
7.
|
Caliskan Y, Gorgulu N, Yelken B, Yazici H,
Oflaz H, Elitok A, Turkmen A, Bozfakioglu S and Sever MS: Plasma
ghrelin levels are associated with coronary microvascular and
endothelial dysfunction in peritoneal dialysis patients. Ren Fail.
31:807–813. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Pérez-Fontán M, Cordido F,
Rodríguez-Carmona A, Peteiro J, García-Naveiro R and García-Buela
J: Plasma ghrelin levels in patients undergoing haemodialysis and
peritoneal dialysis. Nephrol Dial Transplant. 19:2095–2100. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Tentolouris N, Makrilakis K, Doulgerakis
D, Moyssakis I, Kokkinos A, Kyriaki D, Georgoulias C, Stathakis C
and Katsilambros N: Increased plasma ghrelin levels in chronic
renal failure are not associated with hemodynamic parameters. Horm
Metab Res. 37:646–652. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Yoshimoto A, Mori K, Sugawara A, Mukoyama
M, Yahata K, Suganami T, Takaya K, Hosoda H, Kojima M, Kangawa K,
et al: Plasma ghrelin and desacyl ghrelin concentrations in renal
failure. J Am Soc Nephrol. 13:2748–2752. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Mori K, Yoshimoto A, Takaya K, Hosoda K,
Ariyasu H, Yahata K, Mukoyama M, Sugawara A, Hosoda H, Kojima M, et
al: Kidney produces a novel acylated peptide, ghrelin. FEBS Lett.
486:213–216. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Barazzoni R, Zanetti M, Biolo G and
Guarnieri G: Metabolic effects of ghrelin and its potential
implications in uremia. J Ren Nutr. 15:111–115. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Barazzoni R, Zanetti M, Stulle M, Mucci
MP, Pirulli A, Dore F, Panzetta G, Vasile A, Biolo G and Guarnieri
G: Higher total ghrelin levels are associated with higher
insulin-mediated glucose disposal in non-diabetic maintenance
hemodialysis patients. Clin Nutr. 27:142–149. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Kobayashi S, Oka M, Maesato K, Ikee R,
Mano T, Hidekazu M and Ohtake T: Coronary artery calcification,
ADMA, and insulin resistance in CKD patients. Clin J Am Soc
Nephrol. 3:1289–1295. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Parfrey PS, Foley RN, Harnett JD, Kent GM,
Murray D and Barre PE: Outcome and risk factors of ischemic heart
disease in chronic uremia. Kidney Int. 49:1428–1434. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Mitsnefes MM: Cardiovascular complications
of pediatric chronic kidney disease. Pediatr Nephrol. 23:27–39.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Feldt-Rasmussen B, Lange M, Sulowicz W,
Gafter U, Lai KN, Wiedemann J, Christiansen JS and El Nahas M: APCD
Study Group: Growth hormone treatment during hemodialysis in a
randomized trial improves nutrition, quality of life and
cardiovascular risk. J Am Soc Nephrol. 18:2161–2171. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Chang CC, Hung CH, Yen CS, Hwang KL and
Lin CY: The relationship of plasma ghrelin level to energy
regulation, feeding and left ventricular function in non-diabetic
haemodialysis patients. Nephrol Dial Transplant. 20:2172–2177.
2005. View Article : Google Scholar : PubMed/NCBI
|