Introduction
Astragaloside IV (Fig.
1), a small molecular saponin, is usually selected as one of
the marker compounds for chemical assessment and standardization of
Astragalus membranaceus (AM) and its products (1). Numerous studies have indicated that
astragaloside IV causes multiple pharmacological effects, including
antioxidant (2), anti-inflammatory
(3), antitumor (4), anti-fibrotic (5), antivirus (6), anti-radiation (7) and anti-scar (8) effects, and promotes angiogenesis
(9). Additionally, astragaloside IV
can also relax the aortic artery, which is possibly the pivotal
mechanism for why AM, at present, has been widely used to treat
numerous disorders, including cardiovascular diseases, in
traditional Chinese medicine (10,11).
The vascular smooth muscle contracts in response to
the activation on voltage-dependent Ca2+ and
receptor-operated Ca2+ channels (12,13), whilst
vascular endothelial cells have an important role in monitoring
tension by synthesizing and secreting various bioactive substances,
particularly dilating factors, such as nitric oxide (NO) (14), prostanoids (PGI2) (15) and endothelium-derived hyperpolarizing
factors (EDHFs) (16,17). A number of studies have suggested that
astragaloside IV relaxed aortic vessels in a
concentration-dependent manner. The comprehensive mechanisms were
associated with endothelium-dependence through the NO and
PGI2 pathways, and inhibiting extracellular
Ca2+ influx and intracellular Ca2+ stores
release (18–20). However, this mechanism has not been
reported on EDHFs mediating astragaloside IV-induced vasodilatation
and influences of astragaloside IV on voltage-dependent
Ca2+ channels and receptor-operated Ca2+
channels. Additionally, studies have reported the relaxation
response to astragaloside IV presenting in the endothelium-denuded
(E-) aortic rings; however, the relative mechanisms remain to be
elucidated. The expression levels of inducible NO synthase (iNOS)
and cyclic guaosine monophosphate (cGMP) were reported to be
markedly increased when AM was acting in cultured vascular smooth
muscle cells (VSMC) (21), suggesting
that the direct action target of AM-induced vasodilatation is
vascular smooth muscle through the NO-soluble guanylate cyclase
(sGC) signaling pathway without endothelium mediating. These
results propose a hypothesis that astragaloside IV, as a major
constituent extracted from AM, may produce relaxant abilities
through the generation of NO from the vascular smooth muscle
system.
Thus, the present study was designed to elucidate
the effects of EDHFs and the NO from VSMC on astragaloside
IV-induced relaxation and further explore the effects of
astragaloside IV on the voltage-dependent Ca2+ and
receptor-operated Ca2+ channels.
Materials and methods
Animals
Healthy male Sprague-Dawley rats weighing 180–220 g,
were purchased from Dashuo Biotechnology Co., Ltd. (Chengdu,
Sichuan, China). All the rats were raised in cages with commercial
solid foods and tap water available under identical conditions. The
temperature was maintained at 25±1°C, the humidity at 50±5% and the
artificial illumination was for 12 h (light period 7:00 a.m.-7:00
p.m.). All the experimental procedures were performed under the
guidelines of the Management Committeef from Chengdu University of
Traditional Chinese Medicine (Chengdu, Sichuan, China).
Chemicals and reagents
Astragaloside IV (chemical structure in Fig. 1, purity 98%;
3-O-β-D-xylopyranosyl-6-O-β-D-glucopyranosyl-cycloastragenol) was
purchased from Sichuan Weikeqi Biotech Co., Ltd. (Chengdu, Sichuan,
China). Nifedipine was obtained from Shanxi Taiyaun Pharmaceutic
Co., Ltd. (Taiyuan, Shanxi, China). Phenylephrine (PHE),
acetylcholine (Ach), Nω-nitro-L-arginine methyl ester
(L-NAME), indomethacin, tetraethtylamine (TEA), carbenoxolone
(CBX), propargylglycine (PPG), catalase (CAT) and proadifen
hydrochloride (SKF525A) were purchased from Sigma-Aldrich (St.
Louis, MO, USA). The other inorganic salts were provided by Chengdu
Kelong Chemical Reagent (Chengdu, Sichuan, China).
The stock solution of astragaloside IV in dimethyl
sulfoxide (DMSO; 10 g/l) was stored at −20°C and used within 1
week. Indomethacin was dissolved in 95% ethanol and protected from
light with aluminum foil. The highest concentrations of DMSO and
ethanol were ≤0.01% in each of the chambers. Other chemicals and
reagents were dissolved in distilled water and diluted with
Krebs-Henseleit (K-H) solution prior to use. In the present study,
all the concentrations noted were the final concentration in the
bath chambers.
Preparation of thoracic aorta
rings
Rats underwent cervical vertebrae dislocation, and
subsequently the thoracic aortic artery was removed rapidly from
the carotid artery and immediately placed into 4°C oxygenated K-H
solution in which the aorta was cleaned of residual fat and
connective tissue and separated 3–5 mm in length. Aortic rings were
mounted with two stainless steel hooks and suspended in the 10-ml
organ chambers containing K-H solution (pH 7.4), which was composed
of 118.4 mM NaCl, 4.7 mM potassium chloride (KCl), 2.5 mM
CaCl2, 1.2 mM KH2PO4, 1.2 mM
MgSO4, 25.0 mM NaHCO3, 11.0 mM glucose and
0.03 mM ethylenediaminetetraacetic acid, aerated with 5%
CO2 and maintained at 37°C. The isometric tension of the
aortic rings was monitored by four-channels of physiological force
transducers. All the rings were stretched to 1 g resting tension in
normal K-H solution for 1 h, and contracted repeatedly with 60 mM
KCl or 1 µM PHE successively. The rings were allowed to rest until
the baseline was restored following each contraction. When the
contraction was stable and the concentration-contraction curve to
KCl or PHE was repeatable, the effects of the drugs to be tested
were observed. The endothelium in each arterial ring was denuded
mechanically by forceps cannulating the lumen of the ring and
gently rolling the vessel on the moist absorbent gauze. The
endothelium was removed judging by the <10% relaxant response to
10 µM Ach subsequent to precontraction of the arterial rings with 1
µM PHE.
Effects of astragaloside IV on basal
tension
When the thoracic aortic rings were only with 1 g
resting tension in the absence of KCl or PHE, cumulative
concentrations of astragaloside IV (0.1, 0.3, 1, 3, 10, 30, 100 and
300 mg/l) and equal doses of DMSO were added to the chamber,
respectively. The vascular response to each concentration of
astragaloside IV or DMSO was allowed to develop until the stable
plateau was reached.
Effects of astragaloside IV on
contraction
Two different experiments was performed. In one
experiment, when the contraction response to 60 mM KCl or 1 µM PHE
was repeated and the last contraction was maintained steadily, the
different concentrations of astragaloside IV were added stepwise in
a cumulative manner. The maximal contraction induced by 60 mM KCl
or 1 µM PHE was taken as 100%. In the second experiment, a
concentration-contraction curve for KCl (10–90 mM) or PHE
(1×10−9-3×10−5 µM) was constructed,
respectively. When successive curves were repeatable, the arterial
rings were preincubated with astragaloside IV prior to a repeat of
the reconstruction of the contractile curves for the two cases.
Effects of inhibitors and denudation
on astragaloside IV-induced relaxation
To study the roles of EDHF and NO, effects of
relative inhibitors on astragaloside IV-induced relaxation in
endothelium-intact (E+) arterial rings were analyzed. When the
contraction induced by 1 µM PHE was repeatable and the last
contraction was sustained, the rings were respectively preincubated
with NO synthase inhibitor L-NAME (100 µM), and L-NAME (100 µM)
together with cyclooxygenase inhibitor indomethacin (10 µM),
Ca2+-dependent K+ channels blocker TEA (1
mM), gap junction blocker CBX (10 µM), cystathionine γ-lyse
inhibitor DL-PPG (100 µM), CAT (500 U/ml), or cytochrome P450
monoamine oxidase inhibitor proadifen hydrachloride (SKF525A, 10
µM) for 20 min before astragaloside IV was cumulatively added to
the chamber. Regarding the investigation of endothelium-denudation,
experiments to analyze the effects of astragaloside IV were
conducted in the E- arterial rings in the presence or absence of
100 µM L-NAME following a repeatable and sustained contraction.
Effects of astragaloside IV on
Ca2+ channels
To measure the effects of astragaloside IV on
Ca2+ channels, contraction induced by 60 mM KCl or 1 µM
PHE was compared when the rings were respectively preincubated with
astragaloside IV (100 mg/l), nifedipine (100 mg/l), or
astragaloside IV plus nifedipine for 20 min after a repeatable and
sustained contraction with 60 mM KCl or 1 µM PHE.
Statistical analysis
All the values are expressed as mean ± standard
deviation. Relaxant responses were expressed as the percentage
according to the maximal contractile tension induced by KCl (60 mM)
or PHE (1 µM). To evaluate the potency of testing drugs, values of
Emax and pD2 (negative logarithms of value
EC50 which denotes the concentration of drugs induced
50% of maximal effects) were calculated. The data were analyzed
using the Student's t-test or Mann-Whitney U test when appropriate.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effects of astragaloside IV in
thoracic aortic rings at 1 g resting tension
In thoracic aortic rings with 1 g resting tension,
adding cumulative concentrations of astragaloside IV
(1×10−4-3×10−1 g/l) had no significant
vasomotor actions on the baseline (P>0.05, compared with the
vehicle control).
Effects of astragaloside IV on KCl or
PHE-induced contraction
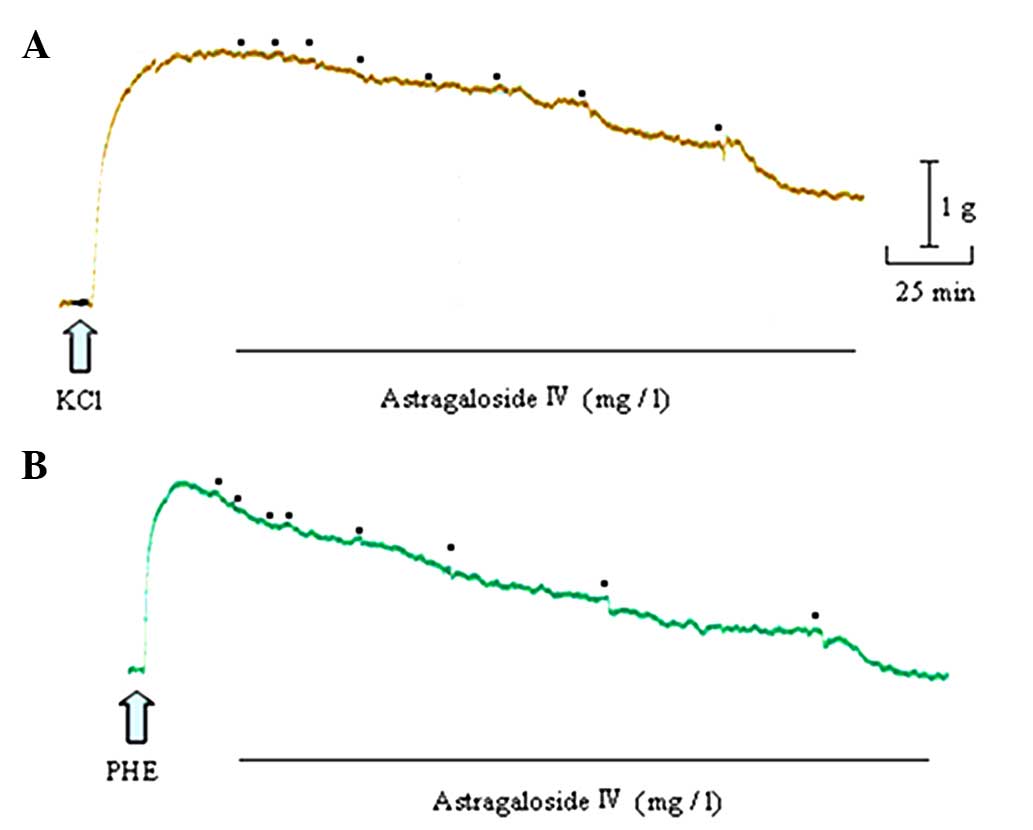
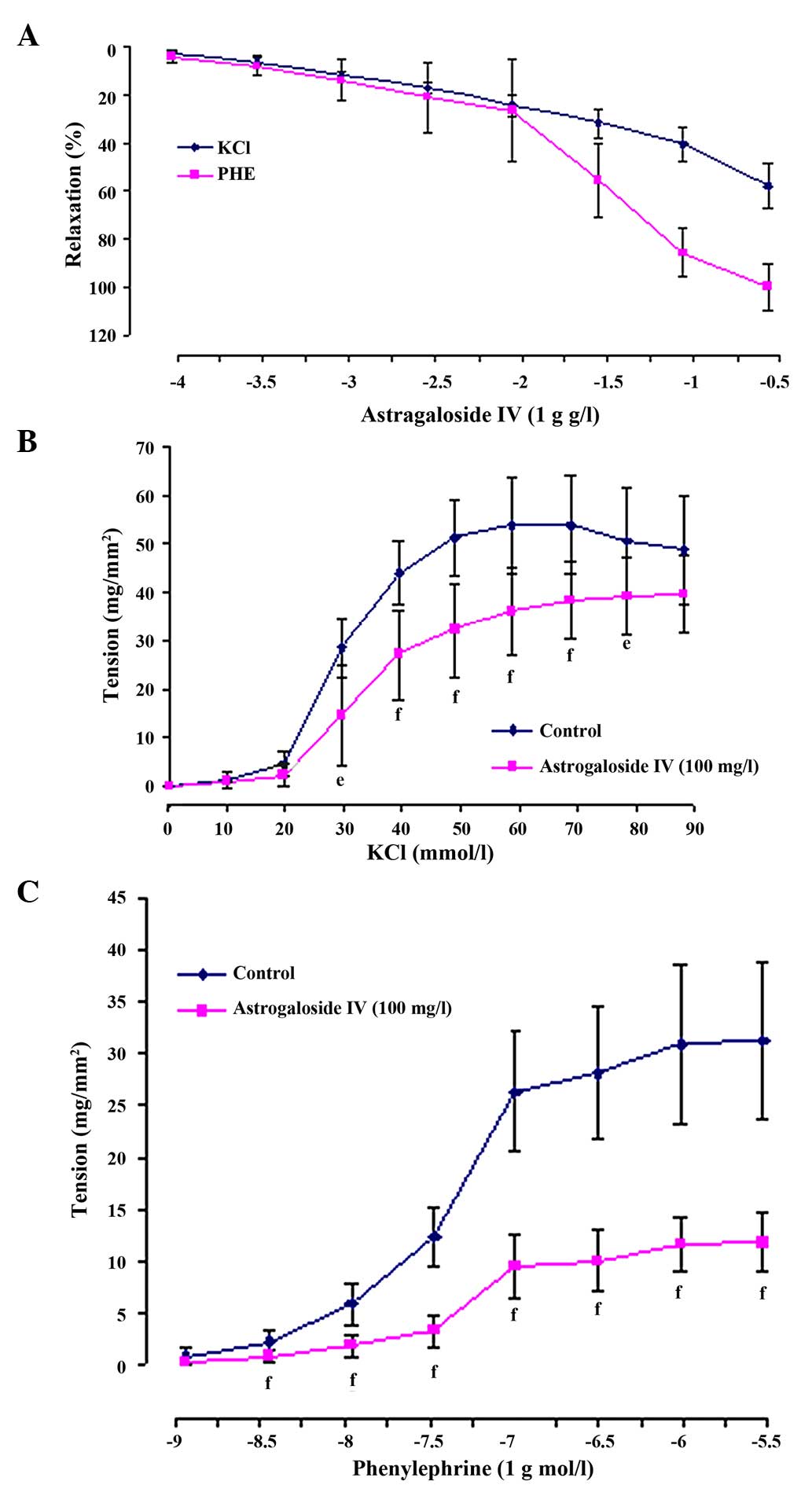
Astragaloside IV produced a concentration-dependent
relaxation in arterial rings precontracted with 60 mM KCl or 1 µM
PHE. When the rings were exposed to KCl, the astragaloside
IV-elicited maximal relaxation magnitude (Emax)
was 57.60±9.55% and the sensitivity (pD2) was 0.79±0.18.
When they were exposed to PHE, the astragaloside IV (300
mg/l)-elicited Emax was 100.01±9.64%, and
pD2 was 1.71±0.27 (Figs. 2 and
3A).
In addition, 100 mg/l astragaloside IV shifted the
concentration-contraction curve for KCl (10–90 mM) or PHE
(1×10−9-3×10−5 mM) nonparallel and downwards
to the right. The maximal contraction altitude
(Emax) reduced from 53.88±10.19 to 38.42±8.08
mg/mm2 for KCl (P<0.01) and from 31.26±7.60 to
11.85±2.87 mg/mm2 for PHE (P<0.01). The values of
pD2 also reduced from 1.54±0.05 to 1.47±0.06 for KCl
(P<0.05) and from 7.38±0.07 to 7.26±0.10 for PHE (P<0.01)
(Fig. 3B and C).
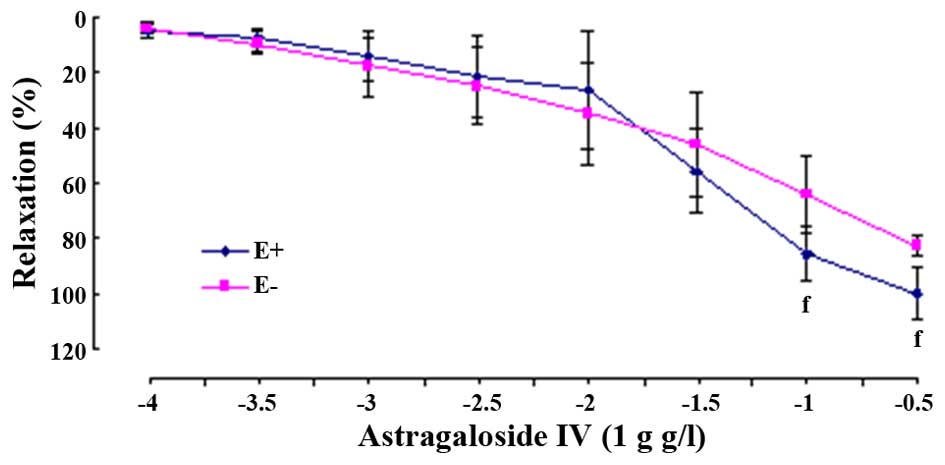
Effects of astragaloside IV in aortic
rings without endothelium
Astragaloside IV also produced
concentration-dependently relaxant effects on PHE-evoked
contraction in E- arterial rings. Although the values of
Emax were significantly decreased (E-,
82.81±3.63%, P<0.01), the values of pD2 (E-, 1.45±0.51,
P>0.05) were not evidently reduced when compared to that in the
E+ rings, respectively (Fig. 4).
Effects of NO on astragaloside
IV-induced relaxation in aortic rings with or without
endothelium
As shown in Table I, in
E+ or E- arterial rings precontracted with PHE, astragaloside
IV-elicited relaxation was evidently depressed by 100 µM L-NAME in
the Emax (P<0.01 for E+ and E-) and pD2
values (P<0.01 for E+ and E-).
 | Table I.Effects of L-NAME on Emax
and pD2 values to astragaloside IV treatment in E+ and E-
aortic rings pretreated with phenylephrine. |
Table I.
Effects of L-NAME on Emax
and pD2 values to astragaloside IV treatment in E+ and E-
aortic rings pretreated with phenylephrine.
|
| E+ | E− |
|---|
|
|
|
|
|---|
| Treatment |
Emax, % | pD2 |
Emax, % | pD2 |
|---|
| Astragaloside
IV |
100.01±9.64 |
1.71±0.27 |
82.81±3.63 |
1.45±0.51 |
| Astragaloside IV +
L-NAME |
66.75±11.05a |
1.17±0.30a |
64.88±13.94a |
0.77±0.16a |
Effects of EDHFs on astragaloside
IV-induced relaxation
Astragaloside IV showed a dilating response to
contraction evoked by PHE, in a concentration-dependent manner,
when the arterial rings were in the presence of preincubation with
L-NAME (100 µM) plus indomethacin (10 µM), and the
Emax values were markedly reduced (41.64±10.52%,
P<0.01) (Fig. 5A). On the basis of
these two inhibitors, arterial rings were preincubated with TEA (1
mM), CBX (10 µM), PPG (100 µM), CAT (500 U/ml) or SKF525A (10 µM),
respectively, showing that astragaloside IV-induced vasodilatation
was not affected by CBX, CAT and SKF525A, but was markedly
inhibited by TEA (P<0.01) and PPG (P<0.05) (Fig. 5B).
 | Figure 5.Relaxant effects of astragaloside IV
(1×10−4-3×10−1 g/l) on preincubation with (A)
L-NAME (100 µM) plus indomethacin (10 µM) and (B) L-NAME plus
indomethacin and TEA (1 mM), CBX (10 µM), PPG (100 µM), CAT (500
U/ml) or SKF525A (10 µM) for 20 min in rat aortic rings after a
contraction induced by PHE (1 µM). The alternation of tension is
expressed as percentage of the active contraction induced by PHE.
Data are mean ± standard deviation (n=8). fP<0.01
compared with vehicle control; eP<0.05,
iP<0.01 compared with astragaloside IV plus L-NAME
plus indomethacin. PHE, phenylephrine; L-NAME,
Nω-nitro-L-arginine methyl ester; TEA, tetraethtylamine;
CBX, carbenoxolone; PPG, propargylglycine; CAT, catalase; SKF525A,
proadifen hydrochloride. |
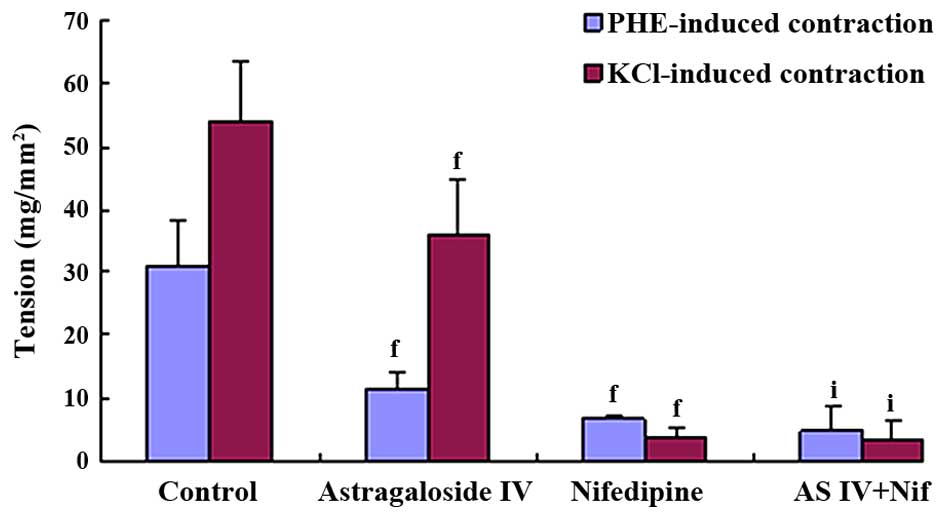
Comparison of astragaloside IV or
nifedipine inhibitory effects on KCl or PHE-induced
contraction
KCl and PHE-induced contraction were significantly
antagonized by 100 mg/l astragaloside IV and 100 mg/l nifedipine.
However, their action potency were different: Astragaloside IV
manifested stronger inhibitory effects on PHE-induced contraction
compared to the KCl-induced contraction (KCl 36.06±9.00
mg/mm2, PHE 11.57±2.65 mg/mm2, P<0.01),
and nifedipine manifested stronger inhibitory effects on
KCl-induced contraction compared to the PHE-induced contraction
(KCl 3.89±1.51 mg/mm2, PHE 6.92±0.38 mg/mm2,
P<0.01). In the combination treatment, synergistically additive
effects were observed and its inhibitory effects on the KCl-induced
contraction were similar to that on the PHE-induced contraction
(Figs. 6 and 7).
Discussion
To the best of our knowledge, the present study
analyzed for the first time the roles of EDHF and NO in denuding
endothelium in vasorelaxation induced by astragaloside IV and
further examined the effects of astragaloside IV on the
voltage-dependent Ca2+ and receptor-operated
Ca2+. The main results were as follows: i) Astragaloside
IV produced a concentration-dependent relaxation response to KCl-
or PHE-induced contraction and inhibited the dose-contraction
curves for KCl or PHE in thoracic aortic rings. ii) Astragaloside
IV-induced relaxation was attenuated by L-NAME in E+ and E-
arterial rings precontracted with PHE. iii) Astragaloside IV, in
the preincubation with L-NAME plus indomethacin, exerted
vasodilatation that was depressed by TEA and PPG, but was not
affected by CBX, CAT or SKF525A. iv) Inhibition of the PHE-induced
contraction by astragaloside IV was more potent in comparison to
inhibition of the KCl-induced contraction, while inhibition of the
KCl-induced contraction by nifedipine was more potent in comparison
to inhibition of the PHE-induced contraction. Additionally,
combined application of astragaloside IV and nifedipine exhibited
synergistic and additive inhibitory effects on the contraction
evoked by KCl similar to PHE. These results added to the
understanding in terms of vasorelaxation caused by astragaloside IV
and simultaneously contribute to improving and enlarging the
clinical application for AM in the field of cardiovascular
diseases.
Contractile activity of vascular smooth muscle
depends on the concentration alternation of
(Ca2+)i, which is recruited from the
extracellular Ca2+ influx through the activation upon
voltage-dependent Ca2+ and receptor-operated
Ca2+ and storage Ca2+ release from
sarcoplasmic reticulum (22–24). In the study, the action of
astragaloside IV in isolated thoracic aortic rings was evidenced to
relax the contraction induced by KCl or PHE in a
concentration-dependent manner, suggesting that astragaloside IV
may act as a vasospasmolytic. The contractile mechanisms on KCl are
different from PHE. KCl contracts vascular smooth muscle in
response to membrane depolarization and openness of
voltage-dependent Ca2+ (25,26), while
PHE induces contraction through the activation upon
receptor-operated Ca2+ without membrane depolarization
(27,28). The results showed that preincubation
with astragaloside IV produced significantly depressant effects on
contraction stimulated by KCl or PHE, suggesting that astragaloside
IV may be a Ca2+ antagonist and reduces the contractile
action via interfering with voltage-dependent Ca2+ and
receptor-operated Ca2+, which is in accordance with the
previous studies on using KCl or PHE (18–20) in rat
thoracic aortic rings preincubated with astragaloside IV. The
inhibitory effects of astragaloside IV on contraction were compared
to the contraction caused by nifedipine, a selective L-type
Ca2+ channels blocker (29). In various types of smooth muscle,
Ca2+ channels blockers strongly decreased high
K+-induced increase in (Ca2+)i
(30). The present study revealed that
astragaloside IV and nifedipine reduced high K+-induced
contraction. Simultaneously, the data also revealed that
astragaloside IV could also reduce the contraction induced by PHE.
These findings indicate that astragaloside IV may block the
voltage-dependent Ca2+ and receptor-operated
Ca2+ channels, which is distinct from the action mode of
nifedipine that mainly blocks voltage-dependent Ca2+
channels. Karaki et al (12)
observed that the contraction triggered by the α1 receptor agonist
was less sensitive to Ca2+ channels blockers compared to
that induced by high K+. However, the present results
demonstrated that the inhibitory effect of astragaloside IV on
PHE-induced contraction was more potent compared to that on
KCl-induced contractile activities, suggesting that astragaloside
IV differs from Ca2+ channels blocker. Therefore,
astragaloside IV is likely to block the superior receptor-operated
Ca2+ channels and inferior voltage-dependent
Ca2+ channels. To confirm the attributes of
astragaloside IV blocking Ca2+ channels, astragaloside
IV and nifedipine were used in combination. The inhibitory effect
of the combined use was enhanced, and its actions on KCl-induced
contraction were similar to that elicited by PHE. Furthermore,
voltage-dependent Ca2+ entry is eliminated in the
presence of nifedipine. Therefore, the reinforced depression
induced by astragaloside IV appears to be the involvement with
receptor-operated Ca2+ channels antagonism. Taken
together, astragaloside IV may act as a Ca2+ antagonist
through blocking the superior receptor-operated Ca2+ and
inferior voltage-dependent Ca2+ channels, but further
and sufficient proof is required to verify its characteristics of
Ca2+ channels antagonism.
There are two vascular relaxant pathways;
endothelium-dependence and -independence. In the present study,
although the Emax produced by astragaloside IV in
the E- arterial rings pre-contracted by PHE was significantly
declined, no notable changes in the sensitivity (pD2) were
observed when compared to those in the E+ arterial rings,
respectively. The results are consistent with those reported by
Wang et al (18) using
astragaloside IV in rat E+ and E- aortic rings precontracted with
PHE or high K+ solution and with those observed by Zhang
et al (20) using astragaloside
IV in normal and stroke-prone spontaneously hypertensive rat
thoracic aortic rings contracted by PHE, high K+
solution or CaCl2, and perivascular fat-intact aortic
rings precontracted with PHE or angiotensin II, which suggests that
astragaloside IV dilates aortic vessels via endothelium-dependence
and -independence.
Vasorelaxation in response to NO converted by its
precursor substance L-arginine under the participation in the NOS
is frequently considered to be mediated by an increased expression
of cGMP in vascular smooth muscle as a result of activation upon
sGC. In the pretreatment PHE-induced contractile E+ arterial rings
with L-NAME, the Emax and pD2 values
produced by astragaloside IV were significantly reduced. The
results indicate that endothelium-derived NO mediates astragaloside
IV-induced vasodilatation, which is in accordance with previous
studies (18–20). However, NO is not only generated from
vascular endothelial cells in the stimulation of bioactive
substance, but is also synthesized from VSMC, particularly in the
condition of endothelial dysfunction in response to the increments
of inflammatory factors (31),
oxidative stress (32), obesity and
insulin resistance (33). In addition,
high content NO (34), secreted by
activating the expression of iNOS, is considered harmful to the
body although it is able to antagonize vasoconstriction in the form
of compensation. In E- arterial rings, endothelium-derived NO is
exhausted. However, preincubation with L-NAME could also
significantly reduce the relaxation of astragaloside IV, suggesting
that astragaloside IV relaxes aortic vessels, which is through the
endothelium-independence and NO signaling pathways. Altogether, NO
generation from the vascular endothelial cells and VSMC mediates
vasorelaxation of astragaloside IV. However, whether astragaloside
IV directly targets at VSMC, which secondly synthesize NO in the
presence of endothelial integrity, remains to be elucidated.
EDHFs, as another vasodilator, are crucial to
regulate vascular tension and maintain homeostasis. Vasodilatation
mediated by EDHFs is considered to be a backup mechanism in case of
endothelial dysfunction or insufficient NO, thus the perspective
that it is more important than NO to a certain extent is accepted
(17). At present, EDHFs were studied
with the common method of adopting preincubation with L-NAME
together with indomethacin in order to exclude the interference of
NO and PGI2. In the present study, astragaloside IV also
showed a concentration-dependent vasodilatation when the rings
exposed to PHE were preincubated with L-NAME and indomethacin,
indicating that vasodilatation response to astragaloside IV is
independent on NO and PGI2. These substances were
considered to possibly be the EDHFs. Therefore, the arterial rings
in the presence of L-NAME plus indomethacin were added to
Ca2+-sensitive K+ channels blocker TEA, which
is a marker used to identify EDHFs. The data revealed that the
vasodilatitation was significantly attenuated by astragaloside IV,
suggesting that astragaloside IV induces endothelium-derived
hyperpolarizing reactions. Although numerous studies on EDHFs have
been published, thus far the detailed substances on EDHFs have not
been identified with potential candidates, including K+,
metabolic products of epoxyeicosatrienoic acid (35), hydrogen peroxide (36), hydrogen sulfide (H2S)
(37) and intracellular gap junction
(38). To determine which of these
substances are possibly responsible for the vasodilatation response
to astragaloside IV, in the present study the rings were pretreated
with L-NAME plus indomethacin and CBX, PPG, CAT or SKF525A,
respectively, following the contraction induced by PHE. The results
indicated that the relaxant actions of astragaloside IV were
affected by PPG and not by CBX, CAT or SKF525A. This appears to
suggest that astragaloside IV-induced vasodilatation is associated
with H2S synthesis. Collectively, astragaloside IV
promotes endothelial cell secretion of K+ and
H2S, which participate in endothelium-derived
hyperpolarizing reactions, and consequently relaxes the
vessels.
In conclusion, astragaloside IV has been shown to
have direct inhibition on aortic contraction induced by KCl or PHE
in vitro. Astragaloside IV acts as a Ca2+ antagonist
inhibiting the contraction by blockade of superior
receptor-operated Ca2+ and inferior voltage-dependent
Ca2+ channels. Astragaloside IV relaxes the vessels
through endothelium-dependent and -independent NO pathways, and
causes vasodilatation in association with K+- and
H2S-mediated endothelium-derived hyperpolarizing
reactions.
Acknowledgements
The study was financially supported by the Academic
and Technical Leaders Training Funds of Sichuan Province in 2014
(grant no. 003099013003).
Glossary
Abbreviations
Abbreviations:
|
AM
|
Astragalus memeranaceus
|
|
NO
|
nitric oxide
|
|
PGI2
|
prostanoids
|
|
EDHFs
|
endothelium-derived hyperpolarizing
factors
|
|
PHE
|
phenylephrine
|
|
KCl
|
potassium chloride
|
|
iNOS
|
inducible nitric oxide synthase
|
|
sGC
|
soluble guanylate cyclase
|
|
cGMP
|
cyclic guaosine monophosphate
|
|
VSMC
|
vascular smooth muscle cells
|
|
Ach
|
acetylcholine
|
|
L-NAME
|
Nω-nitro-L-arginine methyl
ester
|
|
TEA
|
tetraethtylamine
|
|
CBX
|
carbenoxolone
|
|
PPG
|
propargylglycine
|
|
CAT
|
catalase
|
|
SKF525A
|
proadifen hydrochloride
|
|
NOS
|
nitric oxide synthase
|
|
COX
|
cyclooxygenase
|
|
H2S
|
hydrogen sulfide
|
References
|
1
|
Dong HY, Yang JG, Xiao ZQ, Feng WY, Deng
X, Zhang LT and Wei YX: Pro-angiogenic effects of four Chinese
medicines and three herbal prescription in chicken chorioallantoic
membrane model. Zhong Yao Cai. 36:1297–1300. 2013.(In Chinese).
PubMed/NCBI
|
|
2
|
Ji KT, Tang JF and Chai JD: Effect of
astragaloside against the oxidative damage on endothelial cells.
Zhongguo Zhong Xi Yi Jie He Za Zhi. 31:807–810. 2011.(In Chinese).
PubMed/NCBI
|
|
3
|
Qiu YY, Zhu JX, Bian T, Gao F, Qian XF, Du
Q, Yuan MY, Sun H, Shi LZ and Yu MH: Protective effects of
astragaloside IV against ovalbumin-induced lung inflammation are
regulated/mediated by T-bet/GATA-3. Pharmacology. 94:51–59. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cheng X, Gu J, Zhang M, Yuan J, Zhao B,
Jiang J and Jia X: Astragaloside IV inhibits migration and invasion
in human lung cancer A549 cells via regulating PKC-α-ERK1/2-NF-κB
pathway. Int Immunopharmacol. 23:304–313. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Qi Q, Mao Y, Yi J, Li D, Zhu K and Cha X:
Anti-fibrotic effects of Astragaloside IV in systemic sclerosis.
Cell Physiol Biochem. 34:2105–2116. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shang L, Qu Z, Sun L, Wang Y, Liu F, Wang
S, Gao H and Jiang F: Astragaloside IV inhibits adenovirus
replication and apoptosis in A549 cells in vitro. J Pharm
Pharmacol. 63:688–694. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li YR, Cao W, Guo J, Miao S, Ding GR, Li
KC, Wang J and Guo GZ: Comparative investigations on the protective
effects of rhodioside, ciwujianoside-B and astragaloside IV on
radiation injuries of the hematopoietic system in mice. Phytother
Res. 25:644–653. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shan YH, Peng LH, Liu X, Chen X, Xiong J
and Gao JQ: Silk fibroin/gelatin electrospun nanofibrous dressing
functionalized with astragaloside IV induces healing and anti-scar
effects on burn wound. Int J Pharm. 479:291–301. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang SG, Xu Y, Chen JD, Yang CH and Chen
XH: Astragaloside IV stimulates angiogenesis and increases nitric
oxide accumulation via JAK2/STAT3 and ERK1/2 pathway. Molecules.
18:12809–12819. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang QY, Lu S and Sun HR: Effects of
astragalus on cardiac function and serum tumor necrosis
factor-alpha level in patients with chronic heart failure. Zhongguo
Zhong Xi Yi Jie He Za Zhi. 30:699–701. 2010.(In Chinese).
PubMed/NCBI
|
|
11
|
Liu KZ, Li JB, Lu HL, Wen JK and Han M:
Effects of Astragalus and saponins of Panax notoginseng on
MMP-9 in patients with type 2 diabetic macroangiopathy. Zhongguo
Zhong Yao Za Zhi. 29:264–266. 2004.(In Chinese). PubMed/NCBI
|
|
12
|
Karaki H, Ozaki H, Hori M, Mitsui-Saito M,
Amano K, Harada K, Miyamoto S, Nakazawa H, Won KJ and Sato K:
Calcium movements, distribution, and functions in smooth muscle.
Pharmacol Rev. 49:157–230. 1997.PubMed/NCBI
|
|
13
|
Taggart MJ, Menice CB, Morgan KG and Wray
S: Effect of metabolic inhibition on intracellular Ca2+,
phosphorylation of myosin regulatory light chain and force in rat
smooth muscle. J Physiol. 499:485–496. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bełtowski J and Jamroz-Wiśniewska A:
Hydrogen sulfide and endothelium-dependent vasorelaxation.
Molecules. 19:21183–21199. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jobe SO, Ramadoss J, Wargin AJ and Magness
RR: Estradiol-17β and its cytochrome P450- and
catechol-O-methyltransferase-derived metabolites selectively
stimulate production of prostacyclin in uterine artery endothelial
cells: Role of estrogen receptor-α versus estrogen receptor-β.
Hypertension. 61:509–518. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mathewson AM and Dunn WR: A comparison of
responses to raised extracellular potassium and endothelium-derived
hyperpolarizing factor (EDHF) in rat pressurised mesenteric
arteries. PLoS One. 9:e1119772014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xavier FE, Blanco-Rivero J, Sastre E,
Caracuel L, Callejo M and Balfagón G: Tranilast increases
vasodilator response to acetylcholine in rat mesenteric resistance
arteries through increased EDHF participation. PLoS One.
9:e1003562014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang QH, Zhu L and Chen H: Effect of
astragaloside IV on thoracic aortic rings isolated from fat. Chin
Pharmacol Bull. 22:1319–1323. 2006.
|
|
19
|
Zhang C, Wang XH, Zhong MF, Liu RH, Li HL,
Zhang WD and Chen H: Mechanisms underlying vasorelaxant action of
astragaloside IV in isolated rat aortic rings. Clin Exp Pharmacol
Physiol. 34:387–392. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang WD, Zhang C, Wang XH, Gao PJ, Zhu
DL, Chen H, Liu RH and Li HL: Astragaloside IV dilates aortic
vessels from normal and spontaneously hypertensive rats through
endothelium-dependent and endothelium-independent ways. Planta Med.
72:621–626. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang KH: Aqueous extracts from Chinese
herb Ginseng, Astragalus membranaceus and
Scutellaria baicalensis increase the expression both NO and
cGMP. Foreign Med Sci. 18:38–39. 1996.
|
|
22
|
Hamada H, Damron DS, Hong SJ, Van Wagoner
DR and Murray PA: Phenylephrine-induced Ca2+
oscillations in canine pulmonary artery smooth muscle cells. Circ
Res. 81:812–823. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Su X, Smolock EM, Marcel KN and Moreland
RS: Phosphatidylinositol 3-kinase modulates vascular smooth muscle
contraction by calcium and myosin light chain
phosphorylation-independent and -dependent pathways. Am J Physiol
Heart Circ Physiol. 286:H657–H666. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Carmignoto G, Pasti L and Pozzan T: On the
role of voltage-dependent calcium channels in calcium signaling of
astrocytes in situ. J Neurosci. 18:4637–4645. 1998.PubMed/NCBI
|
|
25
|
Ratz PH and Berg KM: 2-Aminoethoxydiphenyl
borate inhibits KCl-induced vascular smooth muscle contraction. Eur
J Pharmacol. 541:177–183. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kravtsov GM and Kwan CY: A revisitation on
the mechanism of action of KCl-induced vascular smooth muscle
contraction: A key role of cation binding to the plasma membrane.
Biol Signals. 4:160–167. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee CH, Poburko D, Sahota P, Sandhu J,
Ruehlmann DO and van Breemen C: The mechanism of
phenylephrine-mediated [Ca(2+)](i) oscillations underlying tonic
contraction in the rabbit inferior vena cava. J Physiol.
534:641–650. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hirata S, Enoki T, Kitamura R, Vinh VH,
Nakamura K and Mori K: Effects of isoflurane on receptor-operated
Ca2+ channels in rat aortic smooth muscle. Br J Anaesth.
81:578–583. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sensch O, Vierling W, Brandt W and Reiter
M: Effects of inhibition of calcium and potassium currents in
guinea-pig cardiac contraction: Comparison of beta-caryophyllene
oxide, eugenol, and nifedipine. Br J Pharmacol. 131:1089–1096.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Muraki K, Bolton TB, Imaizumi Y and
Watanabe M: Effect of isoprenaline on Ca2+ channel
current in single smooth muscle cells isolated from taenia of the
guinea-pig caecum. J Physiol. 471:563–582. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Denis MC, Furtos A, Dudonné S, Montoudis
A, Garofalo C, Desjardins Y, Delvin E and Levy E: Apple peel
polyphenols and their beneficial actions on oxidative stress and
inflammation. PLoS One. 8:e537252013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang M, Sun S, Tang N, Cai W and Qian L:
Oral administration of alkylglycerols differentially modulates
high-fat diet-induced obesity and insulin resistance in mice. Evid
Based Complement Alternat Med. 2013:8340272013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bak MJ, Hong SG, Lee JW and Jeong WS: Red
ginseng marc oil inhibits iNOS and COX-2 via NFκB and p38 pathways
in LPS-stimulated RAW 264.7 macrophages. Molecules. 17:13769–13786.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ayele Y, Kim JA, Park E, Kim YJ, Retta N,
Dessie G, Rhee SK, Koh K, Nam KW and Kim HS: A methanol extract of
Adansonia digitata L. leaves inhibits pro-inflammatory iNOS
possibly via the inhibition of NF-κB activation. Biomol Ther
(Seoul). 21:146–152. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Campbell WB and Gauthier KM: Inducible
endothelium-derived hyperpolarizing factor: Role of the
15-lipoxygenase-EDHF pathway. J Cardiovasc Pharmacol. 61:176–187.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Garry A, Edwards DH, Fallis IF, Jenkins RL
and Griffith TM: Ascorbic acid and tetrahydrobiopterin potentiate
the EDHF phenomenon by generating hydrogen peroxide. Cardiovasc
Res. 84:218–226. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jamroz-Wiśniewska A, Gertler A, Solomon G,
Wood ME, Whiteman M and Bełtowski J: Leptin-induced
endothelium-dependent vasorelaxation of peripheral arteries in lean
and obese rats: Role of nitric oxide and hydrogen sulfide. PLoS
One. 9:e867442014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fujiwara H, Wake Y, Hashikawa-Hobara N,
Makino K, Takatori S, Zamami Y, Kitamura Y and Kawasaki H:
Endothelium-derived relaxing factor-mediated vasodilation in mouse
mesenteric vascular beds. J Pharmacol Sci. 118:373–381. 2012.
View Article : Google Scholar : PubMed/NCBI
|