Introduction
Galectin-3, a β-galactoside-binding lectin, exhibits
pleiotropic biological functions and has been implicated in cell
growth, differentiation, apoptosis, adhesion, malignant
transformation and RNA processing (1–4). Galectin-3
is expressed intracellularly and extracellularly by numerous cell
types.
Cytoplasmic galectin-3 acts as an apoptosis
inhibitor in the cytoplasm and, in certain conditions, regulates
trafficking between the cytoplasm and the nucleus (3). Nuclear galectin-3 has been reported to
function as an mRNA splicing promoter (5). When expressed on the surface of tumor
cells, galectin-3 has a role as an adhesion molecule in
cell-to-cell or cell-to-matrix interactions (6). Circulating galectin-3 has been reported
to be produced by activated macrophages, mast cells, eosinophils
and tumor cells (7).
Galectin-3 also has a role as an immunological
modulator by regulating cytokine production, phagocytosis,
chemotaxis and apoptosis induction (8–12). As for
cytokine production, galectin-3 has been reported to have
inhibitory effects on interleukin-12 (IL-12) production by
dendritic cells (9). Macrophages from
galectin-3 deficient mice produce higher amounts of IL-10 compared
with those from wild-type mice (11).
The production of IL-6 has been reported to be influenced by
galectin-3 through the tumor-producing galectin-3 binding protein
(13). As for cellular immunity,
galectin-3 has been shown to reduce the affinity of T-cell
receptors (14), influence the
strength of antigen activation in dendritic cells (15,16),
internalize T-cell receptors (17) and
induce apoptosis of T cells (12).
Galectin-3 also inhibits natural killer (NK) cell-mediated tumor
immunity by binding the natural cytotoxicity receptor, NKp30 or
NKG2D (18,19).
Previous studies have revealed an elevated
concentration of serum galectin-3 in various cancers, including
breast (20), colorectal (21,22), stomach
(23), lung (20), bladder (24), head and neck (25), liver (26), thyroid (27), pancreatic (28) and melanoma (29). These studies demonstrated higher
amounts of galectin-3 in the sera of patients and its association
with poorer prognosis. However, the associations between galectin-3
and immunological and nutritional parameters remain to be
elucidated.
The aim of the present study was to clarify the
associations between circulating galectin-3 and host immunity and
nutritional status. IL-10, IL-12 and IL17 production were examined
by peripheral blood mononuclear cells (PBMCs) and lymphocyte
stimulation assay as immunological parameters,
neutrophil/lymphocyte ratio (NLR), white blood cell count (WBC) and
C-reactive protein (CRP) as markers of inflammation, and rapid
turnover proteins (RTP), such as retinol-binding protein (RBP),
prealbumin (PA) and transferrin (TF) as parameters for nutritional
condition.
Materials and methods
Patients
Blood samples were collected from 50 patients with
colorectal cancer before starting treatment between April 2011 and
August 2013. The patients included 8 with stage I disease, 12 with
stage II disease, 18 with stage III disease, and 12 with stage IV
disease. The enrolled patients underwent surgery or chemotherapy
for the treatment of histologically confirmed cancer at the
Department of Organ Regulatory Surgery, Fukushima Medical
University (Fukushima, Japan). In addition, samples from 20 healthy
volunteers of similar age and gender distributions were used as
controls. The study protocol was approved by the ethics committee
of Fukushima Medical University and written informed consent was
obtained from the enrolled patients and healthy volunteers.
Blood samples
PBMCs were separated on Ficoll-Hypaque
(Pharmacia-Biotech, Uppsala, Sweden) columns. The isolated PBMCs
were washed twice with RPMI-1640 (Wako Pure Chemical Industries
Ltd., Osaka, Japan). Sera from patients were stored at −80°C until
use.
Galectin-3 measurement
Serum concentrations of galectin-3 were measured
using an enzyme-linked immunosorbent assay (ELISA; R&D Systems,
Minneapolis, MN, USA) according to the manufacturer's protocol.
Cytokine production by PBMCs
PBMCs, prepared using the aforementioned method,
were incubated in 1 ml of RPMI-1640 at a concentration of
106 cells/ml with 10% heat-inactivated fetal calf serum
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) in 5%
CO2 at 37°C for 24 h with appropriate stimulations: With
20 µg/ml phytohemagglutinin (PHA) for IL-10 and IL-17 production
assays, and with 0.01% of Staphylococcus aureus Cowan-1 for
the IL-12 production assays. Aliquots of these supernatants were
frozen and stored at −80°C until use. Supernatant samples were
subsequently thawed and used for measurements of IL-10, IL-12 and
IL-17 concentrations using ELISA (R&D Systems). Each sample was
used only once subsequent to thawing.
Lymphocyte stimulation assay
A lymphocyte proliferation assay was performed using
prepared PBMCs suspended in RPMI-1640 containing 10% fetal calf
serum. After the addition of 10 µg/ml PHA into PBMC culture wells
stored at 37°C in a 5% CO2 atmosphere, mitogenesis was
observed for 80 h. 3H-thymidine (Japan Radioisotope
Association, Tokyo, Japan) was added to wells for the last 8 h of
incubation. Cells were harvested and 3H-thymidine incorporation was
determined using a liquid scintillation counter (Perkin-Elmer,
Waltham, MA, USA) and expressed as counts per minute (cpm). The
stimulation index (SI) was obtained by calculating total
cpm/control cpm. The controls were PBMCs without PHA addition.
Parameters for nutritional status and
inflammation
The patient nutritional statuses were determined by
measuring serum concentrations of RBP (latex agglutination
immunoassay), PA (turbidimetric immunoassay) (30) and TF (turbidimetric immunoassay)
(31). Neutrophil and lymphocyte
counts, as well as NLR, were used as indicators of
inflammation.
Statistical analysis
Differences between the groups were analyzed using
the Student's t-test. Associations between two variables were
quantified using the Spearman's rank correlation coefficient.
P<0.05 was considered to indicate a statistically significant
difference. All the statistical calculations were performed using
SPSS® version 22 (IBM Corp. Japan, Tokyo, Japan). Not
all blood samples were of sufficient volume for all
measurements.
Results
Serum galectin-3 levels in patients
with untreated colorectal cancer
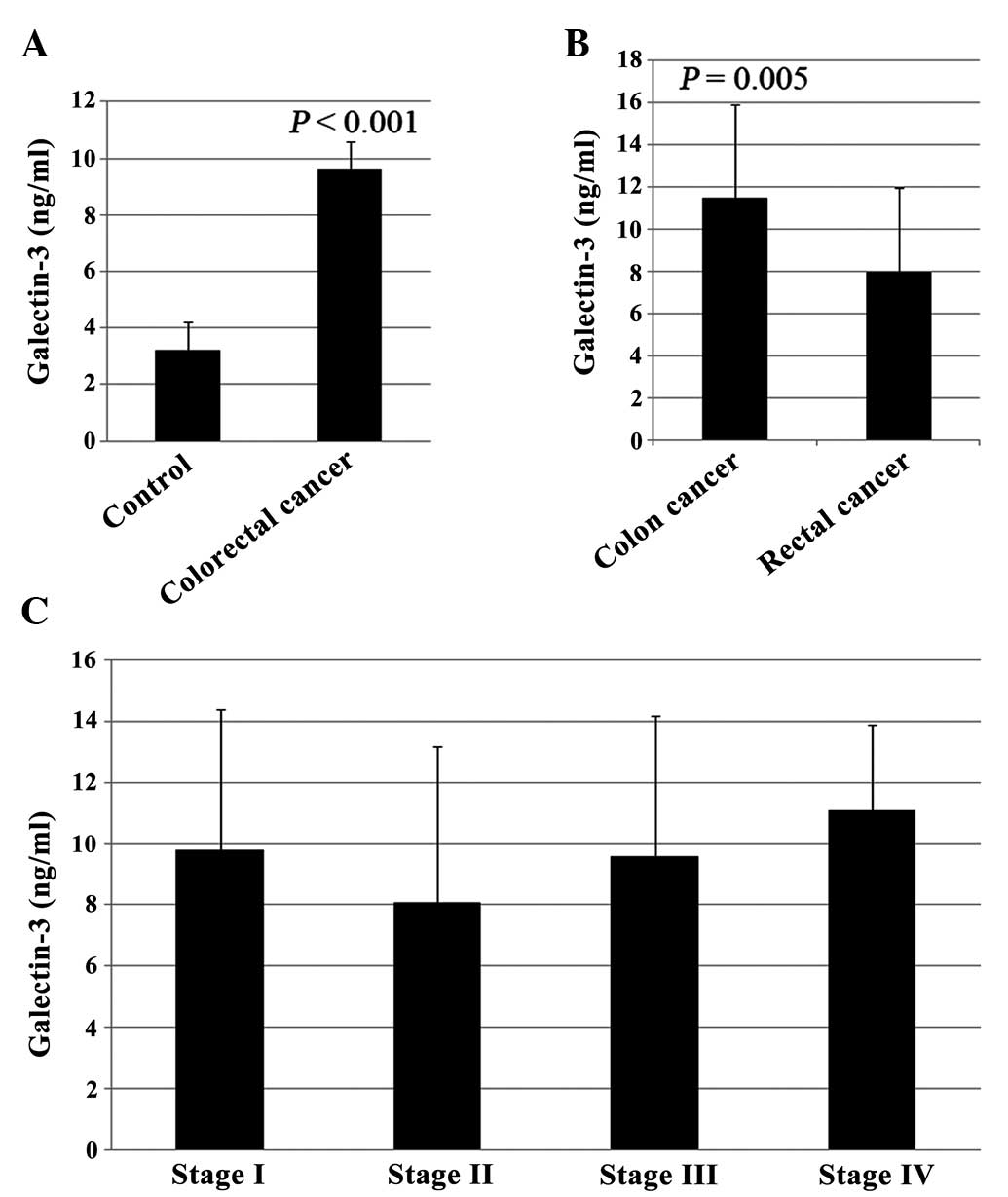
As shown in Fig. 1A,
significant increases (P<0.05) were observed in serum galectin-3
levels in patients with untreated colorectal cancer (9.6±4.5 ng/ml)
compared with normal controls (3.2±1.6 ng/ml). When serum
galectin-3 concentrations were compared between patients with colon
cancer (n=23) and patients with rectal cancer (n=27), the levels of
serum galectin-3 in patients with colon cancer (11.5±4.4 ng/ml)
were significantly higher than those in patients with rectal cancer
(8.0±4.0 ng/ml) (Fig. 1B, P=0.005).
However, no statistically significant differences were observed in
the levels of serum galectin-3 between the different stages
(9.8±4.6, 8.1±5.1, 9.6±4.6 and 11.1±2.8 ng/ml for stages 1–4,
respectively; Fig. 1C).
Correlation between the galectin-3
serum concentrations and the immunological parameters
Fig. 2 shows the
results of the correlation analysis between the galectin-3 serum
concentrations and the immunological parameters. The amount of
circulating galectin-3 inversely correlated with the production of
IL-10 (r=−0.59, P<0.001) and IL-12 (r=−0.69, P<0.001). The
serum concentration of galectin-3 also inversely correlated with
the SI (r=−0.42, P=0.021). By contrast, the level of serum
galectin-3 correlated with the production of IL-17 (r=0.67,
P<0.001).
Correlation analysis between the
galectin-3 serum concentrations and inflammation indicators
Fig. 3 shows the
results of the correlation analysis between the galectin-3 serum
concentrations and inflammation indicators. The levels of serum
galectin-3 exhibited significant correlations with NLR (r=0.41,
P=0.009), WBC (r=0.32, P=0.035) and CRP (r=0.63, P<0.001).
Correlation analyses in association
with the nutritional parameters
The results of the correlation analyses in
association with the nutritional parameters are summarized in
Fig. 4. The level of serum galectin-3
exhibited statistically significant inverse correlations with RBP
(r=−0.45, P=0.002), PA (r=−0.46, P=0.001) and TF (r=−0.72,
P<0.001).
Discussion
In accordance with the previous studies, the serum
concentration of galectin-3 in the cancer patients was
significantly higher compared to the healthy volunteers. However,
no statistically significant differences were observed when
comparing disease stages. Immunohistochemicaly, strong expression
of galectin-3 has been reported to be associated with disease
progression and metastasis (32,33). The
sources of circulating galectin-3 are not only tumor cells, but
also macrophages, mast cells and eosinophils (7,20). Thus, the
circulating level of galectin-3 is not always representative of the
expression level of galectin-3 in the tumor microenvironment. The
reason for why the levels of serum galectin-3 in patients with
colon cancer were significantly higher compared to those in
patients with rectal cancer remains to be elucidated.
The amount of circulating galectin-3 showed a
significant correlation with IL-17 production, whereas an inverse
correlation was observed with IL-12 production. To the best of our
knowledge, the present study reports for the first time the
association between galectin-3 and IL-17 production, while
galectin-3 has been reported to have inhibitory effects on IL-12
production by dendritic cells (9). Our
previous study reported that increased IL-17 production correlated
with cellular immunosuppression (34).
Thus, cell-mediated immunity may be depressed through Th2-dominant
conditions driven by depressed IL-12 production. By contrast, the
production of IL-10, which is a potent immunosuppressive cytokine
produced primarily by Th2 cells, macrophages and activated B cells,
showed inverse correlation with the amount of circulating
galectin-3. The direct effects of galectin-3 on IL-10, IL-12 and
IL-17 production should be assessed in the future.
As for indicators of inflammation, NLR, WBC and CRP
exhibited significant correlations with the level of serum
galectin-3. The present study supports a previous study that
galectin-3 is associated with systemic inflammation and fibrosis
(7). Systemic chronic inflammation has
been reported to have a role in tumor development and growth, and
importantly in the suppression of tumor immunity (35,36).
The assessment of nutritional status is essential
for a diagnosis of nutritional compromise, and measurements of
serum concentrations of RTPs such as RBP, PA and TF have been
reported to be more accurate for this assessment in comparison to
albumin (37–39). The serum concentration of galectin-3
showed a significant inverse correlation with assessed RTPs. The
key mechanisms leading to cancer cachexia in which nutritional
impairment is a major clinical issue, are mostly immune reactions
caused by chronic inflammation. Galectin-3 may be one of the key
factors in the regulation of immunological, inflammatory and
nutritional conditions.
Glossary
Abbreviations
Abbreviations:
|
IL
|
interleukin
|
|
PBMCs
|
peripheral blood mononuclear cells
|
|
NLR
|
neutrophil/lymphocyte ratio
|
|
WBC
|
white blood cell count
|
|
CRP
|
C-reactive protein
|
|
RBP
|
retinol-binding protein
|
|
PA
|
prealbumin
|
|
TF
|
transferrin
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
References
|
1
|
Akahani S, Nangia-Makker P, Inohara H, Kim
HR and Raz A: Galectin-3: A novel antiapoptotic molecule with a
functional BH1 (NWGR) domain of Bcl-2 family. Cancer Res.
57:5272–5276. 1997.PubMed/NCBI
|
|
2
|
Danguy A, Camby I and Kiss R: Galectins
and cancer. Biochim Biophys Acta. 1572:285–293. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Davidson PJ, Davis MJ, Patterson RJ,
Ripoche MA, Poirier F and Wang JL: Shuttling of galectin-3 between
the nucleus and cytoplasm. Glycobiology. 12:329–337. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lin HM, Pestell RG, Raz A and Kim HR:
Galectin-3 enhances cyclin D(1) promoter activity through SP1 and a
cAMP-responsive element in human breast epithelial cells. Oncogene.
21:8001–8010. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dagher SF, Wang JL and Patterson RJ:
Identification of galectin-3 as a factor in pre-mRNA splicing. Proc
Natl Acad Sci USA. 92:1213–1217. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Newlaczyl AU and Yu LG: Galectin-3 - a
jack-of-all-trades in cancer. Cancer Lett. 313:123–128. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
de Boer RA, van Veldhuisen DJ, Gansevoort
RT, Muller Kobold AC, van Gilst WH, Hillege HL, Bakker SJL and van
der Harst P: The fibrosis marker galectin-3 and outcome in the
general population. J Intern Med. 272:55–64. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Acosta-Rodríguez EV, Montes CL, Motrán CC,
Zuniga EI, Liu FT, Rabinovich GA and Gruppi A: Galectin-3 mediates
IL-4-induced survival and differentiation of B cells: Functional
cross-talk and implications during Trypanosoma cruzi infection. J
Immunol. 172:493–502. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bernardes ES, Silva NM, Ruas LP, Mineo JR,
Loyola AM, Hsu DK, Liu FT, Chammas R and Roque-Barreira MC:
Toxoplasma gondii infection reveals a novel regulatory role for
galectin-3 in the interface of innate and adaptive immunity. Am J
Pathol. 168:1910–1920. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ferraz LC, Bernardes ES, Oliveira AF, Ruas
LP, Fermino ML, Soares SG, Loyola AM, Oliver C, Jamur MC, Hsu DK,
et al: Lack of galectin-3 alters the balance of innate immune
cytokines and confers resistance to Rhodococcus equi infection. Eur
J Immunol. 38:2762–2775. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ruas LP, Bernardes ES, Fermino ML, de
Oliveira LL, Hsu DK, Liu FT, Chammas R and Roque-Barreira MC: Lack
of galectin-3 drives response to Paracoccidioides brasiliensis
toward a Th2-biased immunity. PLoS One. 4:e45192009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fukumori T, Takenaka Y, Yoshii T, Kim HR,
Hogan V, Inohara H, Kagawa S and Raz A: CD29 and CD7 mediate
galectin-3-induced type II T-cell apoptosis. Cancer Res.
63:8302–8311. 2003.PubMed/NCBI
|
|
13
|
Silverman AM, Nakata R, Shimada H, Sposto
R and DeClerck YA: A galectin-3-dependent pathway upregulates
interleukin-6 in the microenvironment of human neuroblastoma.
Cancer Res. 72:2228–2238. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Demotte N, Wieërs G, Van Der Smissen P,
Moser M, Schmidt C, Thielemans K, Squifflet JL, Weynand B, Carrasco
J, Lurquin C, et al: A galectin-3 ligand corrects the impaired
function of human CD4 and CD8 tumor-infiltrating lymphocytes and
favors tumor rejection in mice. Cancer Res. 70:7476–7488. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Breuilh L, Vanhoutte F, Fontaine J, van
Stijn CM, Tillie-Leblond I, Capron M, Faveeuw C, Jouault T, van Die
I, Gosset P, et al: Galectin-3 modulates immune and inflammatory
responses during helminthic infection: Impact of galectin-3
deficiency on the functions of dendritic cells. Infect Immun.
75:5148–5157. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu SY, Yu JS, Liu FT, Miaw SC and Wu-Hsieh
BA: Galectin-3 negatively regulates dendritic cell production of
IL-23/IL-17-axis cytokines in infection by Histoplasma capsulatum.
J Immunol. 190:3427–3437. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen HY, Fermin A, Vardhana S, Weng IC, Lo
KF, Chang EY, Maverakis E, Yang RY, Hsu DK, Dustin ML and Liu FT:
Galectin-3 negatively regulates TCR-mediated CD4+ T-cell activation
at the immunological synapse. Proc Natl Acad Sci USA.
106:14496–14501. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang W, Guo H, Geng J, Zheng X, Wei H, Sun
R and Tian Z: Tumor-released Galectin-3, a soluble inhibitory
ligand of human NKp30, plays an important role in tumor escape from
NK cell attack. J Biol Chem. 289:33311–33319. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tsuboi S, Sutoh M, Hatakeyama S, Hiraoka
N, Habuchi T, Horikawa Y, Hashimoto Y, Yoneyama T, Mori K, Koie T,
et al: A novel strategy for evasion of NK cell immunity by tumours
expressing core2 O-glycans. EMBO J. 30:3173–3185. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Iurisci I, Tinari N, Natoli C, Angelucci
D, Cianchetti E and Iacobelli S: Concentrations of galectin-3 in
the sera of normal controls and cancer patients. Clin Cancer Res.
6:1389–1393. 2000.PubMed/NCBI
|
|
21
|
Iacovazzi PA, Notarnicola M, Caruso MG,
Guerra V, Frisullo S, Altomare DF and Correale M: Serum levels of
galectin-3 and its ligand 90k/mac-2bp in colorectal cancer
patients. Immunopharmacol Immunotoxicol. 32:160–164. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen C, Duckworth CA, Zhao Q, Pritchard
DM, Rhodes JM and Yu LG: Increased circulation of galectin-3 in
cancer induces secretion of metastasis-promoting cytokines from
blood vascular endothelium. Clin Cancer Res. 19:1693–1704. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cheng D, Liang B and Li Y: Serum
galectin-3 as a potential marker for gastric cancer. Med Sci Monit.
21:755–760. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sakaki M, Oka N, Nakanishi R, Yamaguchi K,
Fukumori T and Kanayama HO: Serum level of galectin-3 in human
bladder cancer. J Med Invest. 55:127–132. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Saussez S, Lorfevre F, Lequeux T, Laurent
G, Chantrain G, Vertongen F, Toubeau G, Decaestecker C and Kiss R:
The determination of the levels of circulating galectin-1 and −3 in
HNSCC patients could be used to monitor tumor progression and/or
responses to therapy. Oral Oncol. 44:86–93. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ulu M, Alacacioglu A, Yuksel E, Pamukk BO,
Bozkaya G, Ari A, Yuksel A, Sop G and Alacacioglu I: Prognostic
significance of serum galectin-3 levels in patients with
hepatocellular cancer and chronic viral hepatitis. Saudi J
Gastroenterol. 21:47–50. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Išić T, Savin S, Cvejić D, Marečko I,
Tatić S, Havelka M and Paunović I: Serum Cyfra 21.1 and galectin-3
protein levels in relation to immunohistochemical cytokeratin 19
and galectin-3 expression in patients with thyroid tumors. J Cancer
Res Clin Oncol. 136:1805–1812. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xie L, Ni WK, Chen XD, Xiao MB, Chen BY,
He S, Lu CH, Li XY, Jiang F and Ni RZ: The expressions and clinical
significances of tissue and serum galectin-3 in pancreatic
carcinoma. J Cancer Res Clin Oncol. 138:1035–1043. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Vereecken P, Zouaoui Boudjeltia K, Debray
C, Awada A, Legssyer I, Sales F, Petein M, Vanhaeverbeek M, Ghanem
G and Heenen M: High serum galectin-3 in advanced melanoma:
Preliminary results. Clin Exp Dermatol. 31:105–109. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bergström K and Lefvert AK: An automated
turbidimetric immunoassay for plasma proteins. Scand J Clin Lab
Invest. 40:637–640. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kleine TO and Merten B: Rapid manual
immunoturbidimetric and immunonephelometric assays of prealbumin,
albumin, IgG, IgA and IgM in cerebrospinal fluid. J Clin Chem Clin
Biochem. 18:245–254. 1980.PubMed/NCBI
|
|
32
|
Hittelet A, Legendre H, Nagy N, Bronckart
Y, Pector JC, Salmon I, Yeaton P, Gabius HJ, Kiss R and Camby I:
Upregulation of galectins-1 and −3 in human colon cancer and their
role in regulating cell migration. Int J Cancer. 103:370–379. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nakamura M, Inufusa H, Adachi T, Aga M,
Kurimoto M, Nakatani Y, Wakano T, Nakajima A, Hida JI, Miyake M, et
al: Involvement of galectin-3 expression in colorectal cancer
progression and metastasis. Int J Oncol. 15:143–148.
1999.PubMed/NCBI
|
|
34
|
Yazawa T, Shibata M, Gonda K, Machida T,
Suzuki S, Kenjo A, Nakamura I, Tsuchiya T, Koyama Y, Sakurai K, et
al: Increased IL-17 production correlates with immunosuppression
involving myeloid-derived suppressor cells and nutritional
impairment in patients with various gastrointestinal cancers. Mol
Clin Oncol. 1:675–679. 2013.PubMed/NCBI
|
|
35
|
Balkwill F and Mantovani A: Cancer and
inflammation: Implications for pharmacology and therapeutics. Clin
Pharmacol Ther. 87:401–406. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Inoue Y, Nezu R, Matsuda H, Takagi Y and
Okada A: Rapid turnover proteins as a prognostic indicator in
cancer patients. Surg Today. 25:498–506. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Vanlandingham S, Spiekerman AM and Newmark
SR: Prealbumin: A parameter of visceral protein levels during
albumin infusion. JPEN J Parenter Enteral Nutr. 6:230–231. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Delpeuch F, Cornu A and Chevalier P: The
effect of iron-deficiency anaemia on two indices of nutritional
status, prealbumin and transferrin. Br J Nutr. 43:375–379. 1980.
View Article : Google Scholar : PubMed/NCBI
|