Introduction
Atherosclerosis is an inflammatory procedure
characterized by the appearance of fatty deposits known as
atheromatous plaques in the inner layers of arteries. The
synergistic action of increased serum lipid levels, lipid
peroxidation as well as disturbances in nitric oxide (NO)
homeostasis and inflammation facilitate the initiation of
atherosclerosis (1).
CD163 is a 130-kDa member of the scavenger receptor
cysteine rich (SRCR) family expressed on the surface of
monocytes/macrophages. The soluble form of CD163 normally
circulates in plasma, and is produced by the proteolytic cleavage
of CD163 at the cell surface. CD163 is involved in the uptake of
hemoglobin-haptoglobin and mediates the clearance of hemoglobin
(2). Patients with severe coronary
atherosclerosis present increased plasma soluble CD163 (sCD163)
levels (3).
Monocyte chemotactic protein-1 (MCP-1) has been
reported to have a significant role in the pathogenesis of
atherosclerosis (4). MCP-1 is produced
by different cell types within the arterial wall. In addition to
its role in promoting the migration of monocytes into tissues,
MCP-1 is involved in the expression of various proinflammatory
genes. Increased MCP-1 expression has been immunohistochemically
detected in atherosclerotic lesions, implicating this protein in
the recruitment of monocyte-macrophages during the atherosclerosis
process (5).
Apart from their known hypolipidemic properties,
statins also exhibit antiatherosclerotic effects that are
cholesterol-lowering independent. The clinical benefits of these
agents are correlated with the improvement in endothelial
dysfunction, with reduced blood thrombogenicity, as well as with
their anti-inflammatory and immunomodulatory activities (6).
Simvastatin is usually co-administered along with
the atherogenic diets used in experimental protocols of
atherosclerosis in animals or administered following the induction
of hyperlipidemia (7–9). The aim of the present study was to
investigate the potential preventive hypolipidemic activity of
simvastatin in rabbits that initially received simvastatin along
with normal chow, following which they received an atherogenic diet
for the same period.
Materials and methods
Animals and ethical approval
Twenty-two 2-month-old male New Zealand white
rabbits [body weight (mean ± SD) 3743.3±631.6 g; Rabbit Farms,
Farma Trompetas, Megara, Greece] were individually housed in
stainless steel wire-bottom cages. Animals were kept in a
temperature-controlled environment (19±1°C; humidity, 55±5%) under
a 12-h light/dark cycle (5:30 a.m. to 5:30 p.m.) in an
air-conditioned room with free access to standard rabbit chow and
tap water.
The experimental protocol was reviewed and approved
by the Veterinary Directorate of Attica Region and by the Ethics
Committee of the Medicine School of the National and Kapodistrian
University of Athens (Athens, Greece), in accordance with the EU
Legislation for the use of animals for scientific purposes. After 2
weeks of acclimatization, the rabbits were randomly assigned to
three groups.
The control group (group C) consisted of six rabbits
that received standard commercial rabbit chow. The cholesterol
group (group A; n=8) consisted of rabbits fed with a commercial
rabbit chow supplemented with 0.5% w/w cholesterol (a cholesterol
diet) for 8 weeks and then with normal chow for a further 8 weeks.
In group Β (n=8), the rabbits initially received standard
commercial rabbit chow along with 3 mg/kg/day simvastatin (Bennett
Pharmaceuticals SA, Kifisia, Greece) in the drinking water for the
first 8 weeks and subsequently were fed with a cholesterol-enriched
diet for 8 weeks.
Experimental procedure
The rabbit chow [Conigli Svezzamento, S.I.V.A.M.
Società Italiana Veterinaria Agricola Milano S.P.A.,
Casalpusterlengo (LO), Italy] consisted of the following (w/w): 37%
carbohydrates, 16% proteins, 4% fat, 15% fiber, 11% water, 8% Ash
and an appropriate mixture of minerals and vitamins for the healthy
subsistence of the animals in the laboratory (added to the premix
by the manufacturer). The high-cholesterol diet was prepared by
dissolving the appropriate quantity of cholesterol in diethyl ether
(without butylated hydroxytoluene as an inhibitor) and thoroughly
coating the pellets of chow with the mixture. After ether
evaporation, the special diet was stored at −20°C until use. Fresh
food from the freezer was provided to the animals every morning
between 8:30 and 10:30 a.m.
The administration of cholesterol through the
enrichment of a standard diet was performed according to the widely
used method described in rodent models of diet-induced
atherosclerosis (10). The statin
administration through drinking water is a procedure used in a
number of experimental studies with rabbits (11). Furthermore, the quantity of food
provided to the rabbits was not restricted, as dietary stress
severely affects immune and cell functions in rabbits (12).
Simvastatin treatment
In order to ensure that all simvastatin-treated
rabbits received equal doses (3 mg/kg/day), a small volume of water
supplemented with 3 mg/kg simvastatin (based on body weight) was
placed in a separate bottle in each rabbit cage on a daily basis,
in the morning, while the water bottle without simvastatin was
removed during this time. During the following hours, water
consumption was regularly checked and when the bottles were empty,
the initial bottles, filled with water free of simvastatin, were
again placed in the cages.
The body weight of the animals was measured at
baseline, and at 8 and 16 weeks. Blood samples (1.5–2 ml) were
collected, at baseline, 4, 8 and 16 weeks of the experimental
period, from the central ear artery without anesthesia after a 14-h
fast. At the end of the study, the animals were anesthetized by
intramuscular injection of ketamine and xylazine (35 and 10 mg/kg
of body weight, respectively). Subsequent to anesthesia, the
rabbits were intravenously administered with an overdose of sodium
pentobarbital. The aorta from the arch to the iliac bifurcation and
myocardial tissue samples were rapidly removed.
Serum lipids measurements
The blood serum was separated by centrifugation at
1,000–2,000 × g for 10 min in a refrigerated centrifuge (4°C) and
was stored at −80°C until analysis. Serum concentrations of total
cholesterol and of triglycerides were determined at baseline, at
the 8th and 16th week of the experimental period, using the
enzymatic 4-aminophenazone-phenol commercial kit (Biotechnological
Applications, Athens, Greece). High-density lipoprotein (HDL)
cholesterol was determined using a cholesterol enzymatic
photometric method (HDL cholesterol reagent; Medicon Hellas,
Gerakas, Greece) according to the manufacturer's instructions and
the low-density lipoprotein (LDL) cholesterol was determined
according to the following mathematic model: LDL cholesterol =
total cholesterol - (HDL cholesterol + triglycerides/5). All
samples were analyzed at the Laboratory of Experimental Surgery and
Research of the Medical School of Athens (Athens, Greece).
MCP-1 and CD163 measurements in
serum
MCP-1 and CD163 serum levels were determined in the
serum samples collected during the first 4 and 8 weeks of the
experimental period, using enzyme-linked immunosorbent assay
(ELISA) and commercially available kits, according to the
manufacturer's instructions (MCP-1: USCN Life Science Inc., Wuhan,
China; CD163: BG Bluegene, Shanghai, China). Samples were run in
96-well plates on a plate reader (Elisa reader BIORAD 680; Bio-Rad
Laboratories, Inc., Hercules, CA, USA) to determine absorbance at
wavelengths of 450 and 550 nm.
Histological evaluation of aortic and
myocardial tissue
The isolated aorta was quickly transferred in
ice-cold 0.9% w/v NaCl solution, cleaned to remove surrounding
tissue fragments and sliced open along its length. The vessel was
then fixed in neutral 10% v/v formalin. A similar procedure was
followed for the myocardial tissue. Two days after fixation in
formalin, representative sections of aorta (thoracic and abdominal)
and heart were obtained, and processed for paraffin incubation.
Sections of 5-µm thickness were cut and stained with hematoxylin
and eosin for microscopic examination. Image analysis was performed
in order to evaluate intimal thickening in aorta specimens of
aortic, and myocardial tissues. Slides were digitized using a
microscope eclipse 80i (Nikon Corporation, Tokyo, Japan) attached
to a digital camera (DS-2 MW; Nikon Corporation). The images were
transferred to a computer equipped with Image ProPlus version 5.1
(Media Cybernetics, Inc., Rockville, MD, USA) and the layers of the
vessel wall (intima, media and serosa) were traced. The intimal
thickening was then calculated semi-automatically.
The evaluation of myocardial damage and
atherosclerotic alterations was qualitative and was performed by
two independent pathologists. The atherosclerotic damage in the
aortic tissue was classified into three groups according to the
grade of alteration severity as follows: Grade 1, mild; grade 2,
moderate; and grade 3, severe (13,14). The
classification of the three groups depended on the thickness of the
atherosclerotic plaque/lumen stenosis and the perimetrical area of
the vessel occupied by the plaque. The histopathological results
were consistent with the observations of the two independent
pathologists. The pathologists were absolutely blind to the
experimental treatment.
Statistical analysis
Data are expressed as medians (interquartile range).
Comparisons between more than two groups were performed using
Kruskal-Wallis's test while the Mann-Whitney U test was used for
post hoc/multiple comparisons. Comparisons between more than two
measurements of the same group were performed using Friedman's test
and Wilcoxon's signed rank test as a post hoc test. The Fisher's
exact test was used in order to analyze the data from the
histopathology. All tests were two-sided. P<0.05 was considered
to indicate a statistically significant difference and analyses
were conducted using SPSS statistical software version 19.0 (SPSS,
Inc., Chicago, IL, USA).
Results
Serum lipid levels
At baseline, total serum cholesterol and LDL
cholesterol levels were similar between the three study groups
(P>0.05). Total cholesterol and LDL cholesterol levels were
significantly increased in group A from baseline to 8 weeks
(P=0.043 for total and LDL cholesterol levels), and subsequently a
significant reduction was identified from 8 to 16 weeks (P=0.043
for total and LDL cholesterol levels). No significant changes were
found in total cholesterol and LDL cholesterol levels during the
follow up for group C. In group B, the total cholesterol levels
decreased from baseline to 8 weeks (P=0.012) and subsequently a
significant increase was observed for the total cholesterol
(P=0.012) and LDL cholesterol levels (P=0.017) from 8 to 16 weeks.
Regarding serum total and LDL cholesterol levels, the majority of
group comparisons were significant at 8 weeks (total cholesterol
levels: A vs. B, P=0.002; A vs. C, P=0.011; B vs. C, P=0.017; LDL
cholesterol levels: A vs. B, P=0.002; A vs. C, P=0.011), not
including groups C and B that had similar LDL cholesterol levels.
At 16 weeks, groups C and B had similar total cholesterol and LDL
cholesterol levels, while group A had higher total cholesterol and
LDL cholesterol levels when compared with groups C (P=0.014 for
total cholesterol and LDL cholesterol levels) and B (P=0.003 for
total cholesterol levels and LDL cholesterol levels; Table I and Fig.
1).
 | Figure 1.Serum lipid levels, serum MCP-1 and
sCD163 levels. Box plots displaying the second and third quartiles,
as well as the median, of serum triglyceride (mg/dl), HDL and LDL
cholesterol (mg/dl), MCP-1 (ng/ml) and sCD163 (ng/ml) levels among
experimental groups at the different time points. Upon completion
of the study (16 weeks) the box plots indicate that rabbits in
group Β (simvastatin pre-treatment, 3 mg/kg/day) had reduced LDL
cholesterol levels when compared with the atherogenic-fed group Α,
while group B did not differ to the control group. MCP-1, monocyte
chemotactic protein-1; HDL, high-density lipoprotein; LDL,
low-density lipoprotein. *P<0.05, A vs. B; **P<0.05 A vs.
control; ***P<0.05 B vs. control. |
 | Table I.Serum lipid and body weight
levels.a |
Table I.
Serum lipid and body weight
levels.a
| Parameter | Group | Baseline | 8 weeks | 16 weeks |
|---|
| Serum
cholesterol | A | 57.00
(21.00)b,c | 1,865.00
(580.00)c–f | 710.00
(96.50)b,d–f |
| levels (mg/dl) | B | 47.50
(10.75)b,c | 29.00
(5.75)c–e,g | 111.50
(96.75)b,d,e |
|
| C | 54.00 (43.75) | 43.00
(22.25)f,g | 52.00
(22.25)f |
| Serum high-density
lipoprotein | A | 20.00
(10.50)b,c | 23.00
(11.00)d | 24.00
(10.00)d |
| cholesterol levels
(mg/dl) | B | 27.50 (8.00) | 27.50 (8.00) | 26.00 (3.75) |
|
| C | 18.00 (16.00) | 18.50 (14.75) | 20.00 (15.50) |
| Serum low-density
lipoprotein | A | 24.40
(10.80)b,c,e | 1,794.20
(589.70)c–f | 660.80
(110.80)b,d–f |
| cholesterol levels
(mg/dl) | B | 8.90
(10.60)c,e,g | 10.10
(10.30)c,e | 74.60
(93.30)b,d,e |
|
| C | 24.00
(29.30)g | 20.10
(14.35)f | 27.40
(21.65)f |
| Serum
triglyceride | A | 55.00
(47.50)b,c | 152.00
(77.00)d–f | 156.00
(153.50)d–f |
| levels (mg/dl) | B | 49.50 (17.25) | 49.50
(18.50)e | 44.50
(27.25)e |
|
| C | 51.50 (22.75) | 37.00
(21.25)f | 37.50
(19.00)f |
| Body weight
(g) | A | 3,200.00
(495.00)c,e | 3,850.00
(725.00)c,e | 4,180.00
(675.00)b,d,e |
|
| B | 3,800.00
(312.50)b,c,e,g | 4,325.00
(377.50)c,d,e,g | 4,425.00
(410.00)b,d,g |
|
| C | 3,250.00
(232.50)g | 3,555.00
(272.50)g | 4,120.00
(362.50)g |
Triglyceride levels were significantly increased in
group A from baseline to 8 weeks (P=0.043). At 8 and 16 weeks,
group A had higher triglyceride levels when compared with groups C
and B (8 weeks: A vs. B, P=0.039; A vs. C, P=0.019; 16 weeks: A vs.
B, P=0.003; A vs. C, P=0.014; Table I
and Fig. 1).
At 8 weeks, group A rabbits had lower body weights
when compared with rabbits in group B (P=0.002), while group C
rabbits had lower body weights when compared with those in group B
(P=0.006). Body weight was increased from baseline to 8 weeks for
group B (P=0.012) and from baseline to 16 weeks for groups A
(P=0.043) and B (P=0.012). All values and significant differences
between groups and between different time points are presented in
Table I.
Serum MCP-1 and sCD163 levels
The MCP-1 mean values are presented in Table II. MCP-1 values did not differ
significantly among groups regarding the two measurements. Although
no significant changes were detected in rabbit MCP-1 values between
the 4th and 8th week, a trend towards increase was observed over
time, in the mean MCP-1 levels in group A (1.08 vs. 1.56 ng/ml for
4th and 8th week MCP-1 levels, respectively; Table II and Fig.
1).
 | Table II.Serum MCP-1 levels [median
(interquartile range)] at the 4th and 8th week of the study. |
Table II.
Serum MCP-1 levels [median
(interquartile range)] at the 4th and 8th week of the study.
|
| Serum MCP-1 levels
(ng/ml) |
|---|
|
|
|
|---|
| Group | 4 weeks | 8 weeks |
|---|
| A | 1.20 (1.12) | 1.66 (1.21) |
| B | 1.30 (0.56) | 1.36 (1.39) |
| C | 1.66 (2.31) | 1.25 (1.05) |
Between 4 and 8 weeks, there were no significant
changes in rabbit sCD163 values at the two time points (Table III). At 4 weeks there were no
significant differences in sCD163 values among all study groups. At
8 weeks, group C had significantly higher sCD163 values than group
B (P=0.011; Table III and Fig. 1).
 | Table III.Serum sCD163 levels [median
(interquartile range)] at the 4th and 8th week of the study. |
Table III.
Serum sCD163 levels [median
(interquartile range)] at the 4th and 8th week of the study.
|
| Serum sCD163 levels
(ng/ml) |
|---|
|
|
|
|---|
| Group | 4 weeks | 8 weeks |
|---|
| A | 2.46
(1.24)a | 3.06 (1.01) |
| Β | 2.40 (0.88) | 2.45
(0.91)b |
| C | 2.50 (1.51) | 4.08
(1.05)c |
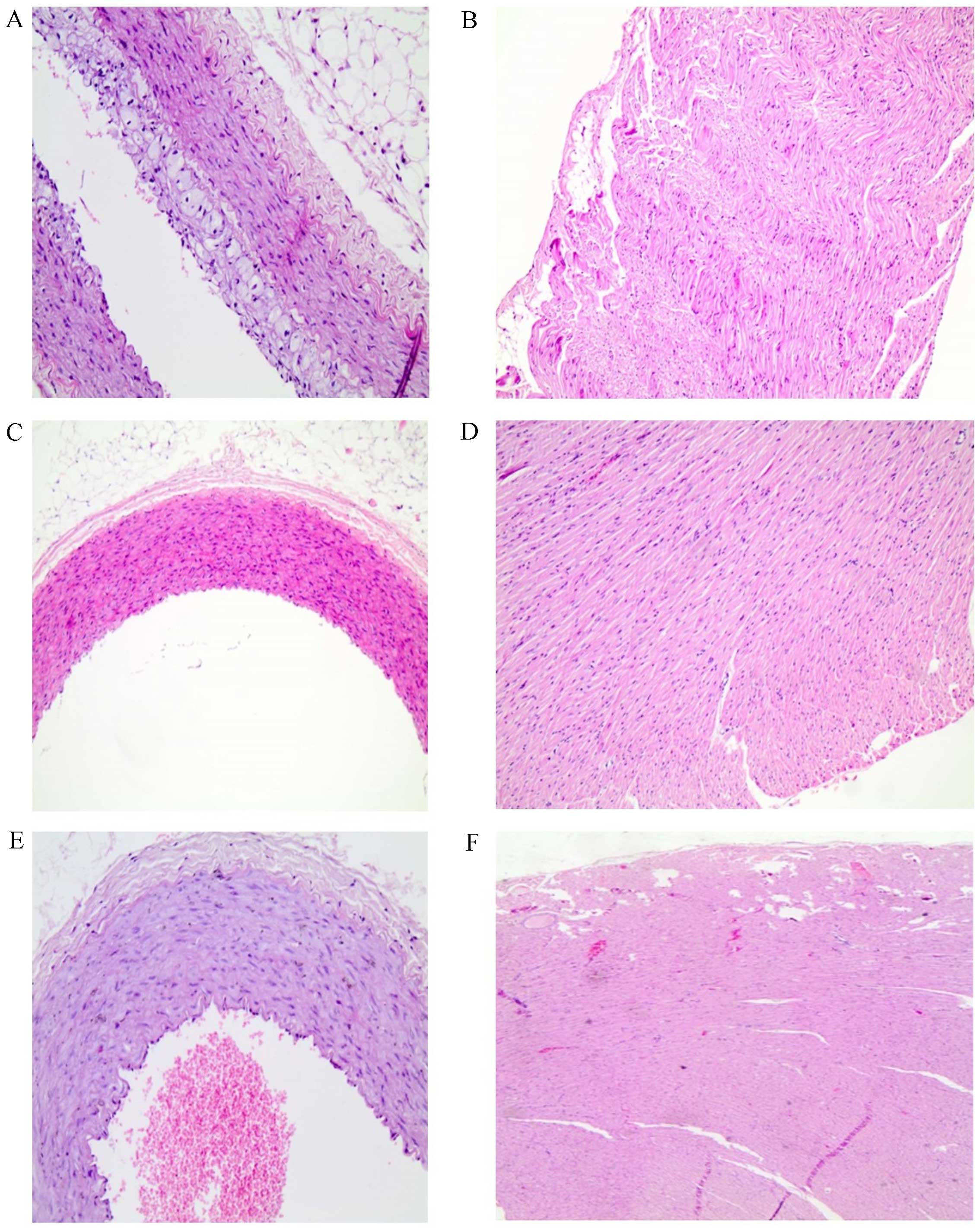
Histopathological analysis
In groups C and B all rabbits exhibited normal
aortic tissue architecture while 40% of the rabbits in group A
presented atherosclerotic alterations in the aorta (grade 1–3).
However, the observed differences among groups (groups C and B vs.
group A) did not reach statistical significance (Table IV and Fig.
2). No alterations were observed in the myocardial tissue
samples of all groups.
 | Table IV.Histopathological analysis of aorta
and heart tissue samples.a |
Table IV.
Histopathological analysis of aorta
and heart tissue samples.a
|
|
| Group |
|---|
|
|
|
|
|---|
| Atherosclerotic
damage | Grade | A (%) | B (%) | C (%) |
|---|
| Aorta | 0 |
60.00 | 100.00 | 100.00 |
|
| 1–3 |
40.00 |
0.00 |
0.00 |
| Heart | 0 | 100.00 | 100.00 | 100.00 |
|
| 1–3 |
0.00 |
0.00 |
0.00 |
Discussion
Hypercholesterolemia in combination with increased
LDL cholesterol levels represent dominant risk factors for the
development and progression of atherosclerosis and consequently of
cardiovascular disease (CVD) (15,16).
Dyslipidemia, characterized by raised levels of atherogenic
lipoproteins are recognized as major risk factors for the
development of atherosclerotic CVD (17).
Rabbit is the most common animal model for
evaluating the pathophysiology of atherosclerosis. In addition, it
is extensively used in experimental protocols investigating the
beneficial action of hypolipidemic and anti-inflammatory agents in
atherosclerotic processes. The induction of atherosclerosis in
rabbits is easily achieved after feeding the animals a
high-cholesterol diet, which is not the case in other animal
species, such as the wild type C57bl/6 mice that develop
hypercholesterolemia with no atherosclerotic plaque formation
(18). Various knockout rodent models
have been described in the field of spontaneous atherosclerosis,
without the need for dietary intervention (including apolipoprotein
E−/− and LDL-r−/−) (19). However, rodent models suffer from
disadvantages, which are associated with various discrepancies from
the pathophysiologic processes of the disease in humans. Rabbits
are particularly sensitive to cholesterol-enriched diets
accumulating large quantities of cholesterol in their plasma, thus,
simulating the course of human atherosclerotic disease (20). Furthermore, the extent of the lesions
developed in the aortic wall in rabbits is proportional to the
quantity of cholesterol consumed (21).
Statins have been extensively investigated for their
hypolipidemic and antiatherosclerotic potential (22). Based on the findings of numerous
clinical studies (23,24), statins have been proposed to be
particularly effective, and constitute one of the most critical
treatment options against atherogenic dyslipidemia (25). Simvastatin is a
3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitor, known to
reduce serum lipid levels (26,27). A
number of studies have been conducted to elucidate the underlying
mechanisms involved in the beneficial action of statins against the
induction of hyperlipidemia and the process of atherogenesis
(13,28–30). Statins
improve endothelial function, diminish oxidative stress and
platelet adhesion and enhance atherosclerotic plaque stability
(31). The implicated mechanistic
pathways, depending on the type of statin used, continue to be
investigated.
In a previous clinical study, CVD patients were
treated either with simvastatin or atorvastatin for a 4-week
period. Blood lipid levels were improved in the two groups of
patients, while mean platelet volume levels (an indicator of
platelet activation) significantly decreased in all patients
(32). In another study investigating
the effects of simvastatin or pravastatin administration in
hypercholesterolaemic rabbits, it was observed that only
pravastatin decreased the size of the infarction; an effect that is
possibly associated with reduced nitrotyrosine and the increased
endothelial NO synthase levels measured in the myocardial tissue
(33). The same conclusions regarding
the role of simvastatin on the size of infarcts in hyperlipidemic
rabbits, through the attenuation of oxidative stress, have been
reported in a study by Iliodromitis et al (34).
Animals in group A exhibited increased serum total
cholesterol and LDL cholesterol levels at week 8 and at the end of
the experimental protocol as compared with the baseline
measurements for this group.
Notably, at the end of the experimental period, in
the group B rabbits, which received simvastatin during the first 8
weeks of the study, the serum total and LDL cholesterol levels were
lower than the levels recorded in the rabbits from goup A, although
they did not differ when compared with the respective lipid levels
of rabbits in the control group. This observation supports the
hypothesis that simvastatin exerts a preventive role against
diet-induced hypercholesterolemia.
At the end of the study, the animals that were
treated with simvastatin before administration of a
cholesterol-enriched diet, presented reduced triglyceride levels as
compared with the untreated group. In addition to their total
cholesterol- and LDL cholesterol-lowering effects, statins are
effective in improving hypertriglyceridemia (35). Although fibrates remain the most
important treatment for patients suffering from triglyceride levels
>500 mg/dl, statins are considered as first-line treatment for
those with high triglyceride levels, which are maintained below
this threshold (36).
The histological examination of the aortic tissue
samples was consistent with the results obtained from the
evaluation of the serum lipid profile of the rabbits. More
precisely, simvastatin pre-treatment prevented the cholesterol
diet-induced atherosclerotic damage in aortas.
The increased permeability of the endothelium,
developed in the early atherosclerotic process, enhances the
migration of different types of leukocytes to the aortic wall
(37,38). Although various chemotactic substances
induce monocyte migration, MCP-1 constitutes the major regulator
for the recruitment of monocytes into the subendothelial cell layer
(39). The monocytes adhere to the
epithelium, move to the intima, then transform to foam cells and
gradually to fatty streak, which is the first grossly visible
lesion in the development of atherosclerosis (37).
In a study where rabbits were fed with a
cholesterol-enriched diet for a six-week period, the total
cholesterol levels were significantly increased from the third day
of the experimental procedure (40).
However, molecular analysis of the aortic tissue of the animals
revealed that MCP-1 mRNA levels remained unchanged in the early
stages of the atherogenetic process, while raised MCP-1 mRNA
expression levels were detected only after three weeks under high
cholesterol feeding. This implies that the long-term consequences,
due to the increased serum lipid levels, stimulate the alterations
in MCP-1 expression (40).
In the present study, differences in MCP-1 levels
among the experimental groups were not detected at 4 and 8 weeks,
or between the measurements of each animal group at the different
time points, although certain trends were observed. The levels of
MCP-1 in the untreated rabbits (group A) exhibited an increasing
trend at 8 weeks, although this observation was not statistically
significant. Therefore, MCP-1 may be essential in the process of
monocyte migration in the arterial wall during the process of
atherogenesis in these animals.
sCD163 constitutes a marker of monocyte/macrophage
activation. In a study investigating HIV-infected patients, the
sCD163 concentration was associated with certain characteristics of
atherosclerotic plaque, such as the total number of non-calcified
segments and the percentage of non-calcified plaques (41). In another study, increased sCD163
levels were detected in treated HIV-infected men and these were
associated with the extent of atherosclerotic damage (42).
A significant increase in sCD163 levels of rabbits
in group A was recorded at 8 weeks when compared with the
respective levels at 4 weeks. Notably, a decrease in sCD163 serum
levels was not detected in the group B rabbits that were
preventively treated with simvastatin.
The findings of the current study are, however,
partly limited, as the hepatic enzyme activities in the rabbits
were not measured; therefore, potential side effects resulting from
the administration of simvastatin were not observed (43).
The present findings indicate that simvastatin
pre-treatment, before enrichment of the diet with cholesterol in
rabbits, has beneficial hypolipidemic and antiatherosclerotic
effects by inhibiting the increase of total cholesterol, LDL
cholesterol and triglyceride levels in the serum. Therefore
preventing the formation of atherosclerotic lesions in the thoracic
aorta of the rabbits.
Regarding implementations for current clinical
practice and future research, simvastatin pretreatment appears to
exert preventive hypolipidemic activity in rabbits that are due to
receive a high cholesterol atherogenic diet. This observation is
particularly important, as it provides evidence for the beneficial
mode of action of this therapeutic agent. The prevention of
atherosclerosis is considered to be crucial in modern societies
that, generally, are experiencing epidemical increases in obesity
and metabolic syndrome (44,45). Future clinical studies are required to
support the current findings and to provide guidelines for future
clinical practice.
In conclusion, the findings of the present study
indicate for the first time, to the best of our knowledge, that
simvastatin may present as an effective strategy for CVD
prevention. However, the molecular pathways involved in the
prophylactic action of simvastatin against the hypercholesterolemia
and the atherogenesis require further investigation. Furthermore,
improved understanding and increased scientific evidence of the
preventive contribution of simvastatin on the inhibition of the
progression of atherosclerosis are required.
Acknowledgements
The authors would like to thank Mrs. Esmeralda
Ntousi, Mr. Panagiotis Tsakiropoulos and Mr. Nikolaos Tsakiropoulos
for their assistance during the experimental procedures.
References
|
1
|
Rafieian-Kopaei M, Setorki M, Doudi M,
Baradaran A and Nasri H: Atherosclerosis: Process, indicators, risk
factors and new hopes. Int J Prev Med. 5:927–946. 2014.PubMed/NCBI
|
|
2
|
Fabriek BO, Dijkstra CD and van den Berg
TK: The macrophage scavenger receptor CD163. Immunobiology.
210:153–160. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Aristoteli LP, Møller HJ, Bailey B,
Moestrup SK and Kritharides L: The monocytic lineage specific
soluble CD163 is a plasma marker of coronary atherosclerosis.
Atherosclerosis. 184:342–347. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lin J, Kakkar V and Lu X: Impact of MCP-1
in atherosclerosis. Curr Pharm Des. 20:4580–4588. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ylä-Herttuala S, Lipton BA, Rosenfeld ME,
Särkioja T, Yoshimura T, Leonard EJ, Witztum JL and Steinberg D:
Expression of monocyte chemoattractant protein 1 in macrophage-rich
areas of human and rabbit atherosclerotic lesions. Proc Natl Acad
Sci USA. 88:5252–5256. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Blanco-Colio LM, Tuñón J, Martín-Ventura
JL and Egido J: Anti-inflammatory and immunomodulatory effects of
statins. Kidney Int. 63:12–23. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Risovic V, Man D, Sivak O, Lee SD and
Wasan KM: Assessing lipid lowering and plasma cholesteryl ester
transfer protein activity of simvastatin following administration
to rabbits fed a high fat/cholesterol diet. Drug Dev Ind Pharm.
32:609–615. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang YH, Zhang Y, Li J, Tong WX and Xu
FQ: Protective effects of Xiongshao Capsule () on anti-inflammatory
function of high-density lipoprotein in atherosclerosis rabbit
model. Chin J Integr Med. 10:102015.
|
|
9
|
Djerrou Z: Anti-hypercholesterolemic
effect of Pistacia lentiscus fatty oil in egg yolk-fed rabbits: A
comparative study with simvastatin. Chin J Nat Med. 12:561–566.
2014.PubMed/NCBI
|
|
10
|
Rasmusen C, Moinard C, Martin C, Tricottet
V, Cynober L and Couderc R: L-arginine plus atorvastatin for
prevention of atheroma formation in genetically
hypercholesterolaemic rabbits. Br J Nutr. 97:1083–1089. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yanni AE, Yatzidis HA, Kavantzas NG,
Agapitos EV, Perrea DN and Karayannacos PE: Dietary L-aspartate and
L-glutamate inhibit fatty streak initiation in cholesterol-fed
rabbit. Nutr Metab Cardiovasc Dis. 13:80–86. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Franci O, Amici A, Margarit R, Merendino N
and Piccolella E: Influence of thermal and dietary stress on immune
response of rabbits. J Anim Sci. 74:1523–1529. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cook DL, Mills LM and Green DM: The
mechanism of alloxan protection in experimental atherosclerosis. J
Exp Med. 99:119–124. 1954. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kamimura R, Suzuki S, Sakamoto H, Miura N,
Misumi K and Miyahara K: Development of atherosclerotic lesions in
cholesterol-loaded rabbits. Exp Anim. 48:1–7. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Verma DR and Brinton EA: Management of
hypercholesterolemia for prevention of atherosclerotic
cardiovascular disease: Focus on the potential role of recombinant
anti-PCSK9 monoclonal antibodies. Rev Cardiovasc Med. 15:86–101;
quiz 101. 2014.PubMed/NCBI
|
|
16
|
Roberts WC: Twenty questions on
atherosclerosis. Proc (Bayl Univ Med Cent). 13:139–143.
2000.PubMed/NCBI
|
|
17
|
Roh E, Ko SH, Kwon HS, Kim NH, Kim JH, Kim
CS, Song KH, Won JC, Kim DJ, Choi SH, et al: Taskforce Team of
Diabetes Fact Sheet of the Korean Diabetes Association: Prevalence
and Management of Dyslipidemia in Korea: Korea National Health and
Nutrition Examination Survey during 1998 to 2010. Diabetes Metab J.
37:433–449. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Korou LM, Agrogiannis G, Koros C, Kitraki
E, Vlachos IS, Tzanetakou I, Karatzas T, Pergialiotis V,
Dimitroulis D and Perrea DN: Impact of N-acetylcysteine and sesame
oil on lipid metabolism and hypothalamic-pituitary-adrenal axis
homeostasis in middle-aged hypercholesterolemic mice. Sci Rep.
4:68062014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kapourchali FR, Surendiran G, Chen L, Uitz
E, Bahadori B and Moghadasian MH: Animal models of atherosclerosis.
World J Clin Cases. 2:126–132. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dornas WC, Oliveira TT, Augusto LE and
Nagem TJ: Experimental atherosclerosis in rabbits. Arq Bras
Cardiol. 95:272–278. 2010.(In Portuguese). View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yanni AE: The laboratory rabbit: An animal
model of atherosclerosis research. Lab Anim. 38:246–256. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Profumo E, Buttari B, Saso L and Rigano R:
Pleiotropic effects of statins in atherosclerotic disease: Focus on
the antioxidant activity of atorvastatin. Curr Top Med Chem.
14:2542–2551. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tousoulis D, Oikonomou E, Economou EK,
Crea F and Kaski JC: Inflammatory cytokines in atherosclerosis:
Current therapeutic approaches. Eur Heart J. 37:1723–1732. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
März W, Scharnagl H, Gouni-Berthold I,
Silbernagel G, Dressel A, Grammer TB, Landmesser U, Dieplinger H,
Windler E and Laufs U: LDL-Cholesterol: Standards of Treatment
2016: A German Perspective. Am J Cardiovasc Drugs. 16:323–336.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ahn CH and Choi SH: New drugs for treating
dyslipidemia: Beyond statins. Diabetes Metab J. 39:87–94. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Toth PP: Drug treatment of
hyperlipidaemia: A guide to the rational use of lipid-lowering
drugs. Drugs. 70:1363–1379. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Toth PP and Davidson MH: Simvastatin plus
ezetimibe: Combination therapy for the management of dyslipidaemia.
Expert Opin Pharmacother. 6:131–139. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Satoh M, Takahashi Y, Tabuchi T, Minami Y,
Tamada M, Takahashi K, Itoh T, Morino Y and Nakamura M: Cellular
and molecular mechanisms of statins: An update on pleiotropic
effects. Clin Sci (Lond). 129:93–105. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tissier F, Mallem Y, Goanvec C, Didier R,
Aubry T, Bourgeois N, Desfontis JC, Dubreuil M, Le Grand Y,
Mansourati J, et al: A non-hypocholesterolemic atorvastatin
treatment improves vessel elasticity by acting on elastin
composition in WHHL rabbits. Atherosclerosis. 251:70–77. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lin CP, Huang PH, Lai CF, Chen JW, Lin SJ
and Chen JS: Simvastatin Attenuates Oxidative Stress, NF-κB
Activation, and Artery Calcification in LDLR-/- Mice Fed with High
Fat Diet via Down-regulation of Tumor Necrosis Factor-α and TNF
Receptor 1. PLoS One. 10:e01436862015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ludman A, Venugopal V, Yellon DM and
Hausenloy DJ: Statins and cardioprotection-more than just lipid
lowering? Pharmacol Ther. 122:30–43. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xian-Yu JB, Feng JF, Chen YC and Yang YW:
Effects of simvastatin and atorvastatin on biochemical and
hematological markers in patients with risk of cardiovascular
diseases. Int J Clin Exp Med. 8:13983–13989. 2015.PubMed/NCBI
|
|
33
|
Andreadou I, Farmakis D, Prokovas E,
Sigala F, Zoga A, Spyridaki K, Papalois A, Papapetropoulos A,
Anastasiou-Nana M, Kremastinos DT, et al: Short-term statin
administration in hypercholesterolaemic rabbits resistant to
postconditioning: Effects on infarct size, endothelial nitric oxide
synthase, and nitro-oxidative stress. Cardiovasc Res. 94:501–509.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Iliodromitis EK, Andreadou I, Prokovas E,
Zoga A, Farmakis D, Fotopoulou T, Ioannidis K, Paraskevaidis IA and
Kremastinos DT: Simvastatin in contrast to postconditioning reduces
infarct size in hyperlipidemic rabbits: Possible role of
oxidative/nitrosative stress attenuation. Basic Res Cardiol.
105:193–203. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Stein EA, Lane M and Laskarzewski P:
Comparison of statins in hypertriglyceridemia. Am J Cardiol.
81(4A): 66B–69B. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Brinton EA: Management of
hypertriglyceridemia for prevention of atherosclerotic
cardiovascular disease. Cardiol Clin. 33:309–323. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ross R: Atherosclerosis - an inflammatory
disease. N Engl J Med. 340:115–126. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Deshmane SL, Kremlev S, Amini S and Sawaya
BE: Monocyte chemoattractant protein-1 (MCP-1): An overview. J
Interferon Cytokine Res. 29:313–326. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Takahashi K, Takeya M and Sakashita N:
Multifunctional roles of macrophages in the development and
progression of atherosclerosis in humans and experimental animals.
Med Electron Microsc. 35:179–203. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen YL, Chang YJ and Jiang MJ: Monocyte
chemotactic protein-1 gene and protein expression in atherogenesis
of hypercholesterolemic rabbits. Atherosclerosis. 143:115–123.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Burdo TH, Lo J, Abbara S, Wei J, DeLelys
ME, Preffer F, Rosenberg ES, Williams KC and Grinspoon S: Soluble
CD163, a novel marker of activated macrophages, is elevated and
associated with noncalcified coronary plaque in HIV-infected
patients. J Infect Dis. 204:1227–1236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
McKibben RA, Margolick JB, Grinspoon S, Li
X, Palella FJ Jr, Kingsley LA, Witt MD, George RT, Jacobson LP,
Budoff M, et al: Elevated levels of monocyte activation markers are
associated with subclinical atherosclerosis in men with and those
without HIV infection. J Infect Dis. 211:1219–1228. 2015.PubMed/NCBI
|
|
43
|
Calderon RM, Cubeddu LX, Goldberg RB and
Schiff ER: Statins in the treatment of dyslipidemia in the presence
of elevated liver aminotransferase levels: A therapeutic dilemma.
Mayo Clin Proc. 85:349–356. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Landecho MF, Moncada R, Valentí V and
Frühbeck G: Cardiovascular Prevention in Obese Patients. Curr Pharm
Des. 22:222016. View Article : Google Scholar
|
|
45
|
Lim S and Eckel RH: Pharmacological
treatment and therapeutic perspectives of metabolic syndrome. Rev
Endocr Metab Disord. 15:329–341. 2014. View Article : Google Scholar : PubMed/NCBI
|