Introduction
Ischemia and hypoxia in the inner ear are the main
factors leading to sudden deafness, acute acoustic trauma and
presbycusis. An animal experimental study confirmed that cochlear
tissues were particularly sensitive to hypoxia, and cochlear
compound action potential and endocochlear potential disappeared
after only 8 sec of cochlear hypoxia (1). In addition, in vitro experiments
on organ cultures of the cochlea found that hypoxia causes cochlear
hair cell (HC) loss and neuronal death (2,3). The
mechanisms underlying hypoxia-induced inner ear damage are not
clear. The Qinghai-Tibetan Plateau has high elevation and the
oxygen content of the air in Lhasa is only 70% of that in the
plains. In our previous study, pure tone audiometry tests were
performed on youths (aged 18–25 years) living in Lhasa, and 13% of
youths living at higher elevation areas exhibited hearing loss and
among those individuals, 17% were Tibetan youths, which was a
greater number than Han migrants who had relocated to Tibet
(4). To further study the influence of
high-altitude hypoxic environment on the auditory system, cochlear
samples were collected at different time-points from rats migrating
to high altitude, and the morphological changes of the cochlea were
observed.
Materials and methods
Experimental animals
The current study was performed in accordance with
the recommendations for animal care of Tibet University (Tibet,
China). The care and use of rats in the present study was approved
by the Tibet University Animal Care and Use Committee.
Sixty healthy adult Wistar rats (age, 8 weeks;
clean-grade), half male (weight, 190–200 g) and half female
(weight, 210–220 g) were provide by Charles River Laboratories
[Beijing, China; certificate of conformity, SCXK (Jing) 2012-0001].
All rats were housed (males and females separately) in a room
maintained at a temperature of 20–26°C and a relative humidity of
45–65%, with free access to water and food (complete rat
pellets).
Instruments and reagents
The dissecting microscope and confocal laser
scanning microscope were obtained from Olympus Corp. (Tokyo, Japan)
and the freezing microtome was from Leica Microsystems GmbH
(Wetzlar, Germany). Harris hematoxylin solution, 5% goat serum,
phosphate-buffered saline (PBS) and 4% paraformaldehyde were
purchased from Wuhan Boster Biological Technology, Ltd. (Wuhan,
China). NF-200 antibody (cat. no. N0142; clone N52) and Heochst
33342 were purchased from Sigma-Aldrich (St. Louis, MO, USA) and
Invitrogen Alexa 488-conjugated goat anti-mouse IgG (cat. no.
A10684) was from Thermo Fisher Scientific (Waltham, MA, USA).
Experimental groups
Thirty male and 30 female rats were randomly divided
into seven groups, with a control group (n=6) with samples obtained
from rats one day after moving to the plateau area, and six groups
of samples obtained from rats 30, 60, 90, 120, 150 and 180 days
after moving to the plateau area (nine rats per group). Each
included 4 or 5 male and 4 or 5 female rats. Rats were observed
daily and the following were monitored: Movement, diet, drinking,
defecation and hair quality and density. The rats were weighed once
every three days.
Cochlear tissue collection
Rats were sacrificed by decapitation and the
bilateral otocysts were removed under a dissecting microscope. The
cochleae were placed in culture dishes containing 4%
paraformaldehyde and the bony portion behind the otocysts was
clamped using forceps. The promontorium tympani were fully exposed
via opening of the otocysts via the hypotympanum. A drill hole was
made in the apex of the cochlea using a 1-ml syringe needle, the
bone wall at the lower edge of the round window was poked with a
separating needle and the stapes were pushed through the oval
window. The cochleae were perfused with 4% paraformaldehyde via the
small hoe in the apical portion using the 1-ml syringe. After
passing through the scala tympani and scala vestibuli, the
solutions were flushed through the apex toward the base of the
cochlea, and flushed out through the round and oval windows. The
perfusion was performed three times. Otocysts were fixed in 4%
paraformaldehyde, and placed in the refrigerator at 4°C for 48
h.
Surface preparation of cochlear
basilar membrane and hematoxylin staining
The fixed otocysts were then decalcified in 10% EDTA
for 7 days, fixed in culture dishes containing PBS and placed under
the dissecting microscope. The bony shell of the cochlea were
gently dissected from the apex to the base of the cochlea under the
dissection microscope, then the basilar membranes were separated
from the osseous spiral lamina, which was performed from the apex
toward the base of the cochlea. The basilar membranes were spread
onto glass slides and the slides were immersed in absolute ethanol
twice for 5 min each time, followed by immersion in 80% ethanol and
distilled water for 5 and 2 min, respectively. The slides were
subsequently soaked in distilled water for 5 min and stained with
Harris hematoxylin for 5 min. The slides were washed under running
water for 1–3 sec, differentiated in 1% hydrochloric acid alcohol,
fixed in gradient ethanol and mounted with glycerol.
Cochlear HC count
The surface preparation of cochlear basilar
membranes were placed under the optical microscope (magnification,
×400). The number of cochlear HCs was counted over 0.24-mm
intervals along the basilar membrane with the microscope eyepiece;
three visual fields were selected from each basilar membrane middle
turn and the mean values were taken. The hallmarks of HC death were
considered as indistinct or disappearing cell boundaries and
rupture or loss of cells. Visual fields at the hook and apex of the
cochlea were not counted. All the data were entered into the
computer by the researcher, and the distribution of HC loss was
analyzed.
Cochlear frozen sections and
immunofluorescence staining
The fixed otocysts were decalcified in 10% EDTA
solution, and the solutions were replaced every other day for a
total of 15 days. The cochleae were soaked in 30% sucrose solution
overnight after trimming, embedded in OCT, frozen and then sliced
into sections (thickness, 10 µm) parallel to the modiolus of the
cochlea. Sections were hydrated in 0.1 M PBS for 10 min, followed
by treatment with 0.1% Triton X-100 for 10 min, and blocking with
5% goat serum for 30 min. The sections were incubated with NF-200
antibodies (dilution, 1:400) at 4°C overnight, washed in PBS three
times (10 min each time), then incubated with Alexa 488-conjugated
goat anti-mouse IgG (dilution, 1:400) at room temperature for 60
min, washed in PBS three times for 10 min each and mounted using
glycerol. Sections were observed and photographed using a confocal
microscope. After the modiolus appeared in the sections, the number
of spiral ganglion neurons (SGNs) was counted in every fifth
section, with a total of five sections being continuously counted
under a fluorescent microscope (magnification, ×400).
Statistical analysis
All data are presented as the mean ± standard
deviation and were analyzed using the SPSS 17.0 statistical
software (SPSS, Inc., Chicago, IL, USA). One-way analysis of
variance was used and a P<0.05 was considered to indicate a
statistically significant difference.
Results
General conditions of the rats after
moving to the plateau area
The vital signs and activity of rats were normal one
day after moving to the plateau area, although the activity of rats
was reduced, food and water intake increased for prolonged time
periods under hypoxic conditions, and 15 rats succumbed between 10
and 180 days after moving to the plateau area. Monocular or
binocular blindness appeared in six rats after 60 days of moving to
the plateau area. The body weight of the rats continued to increase
throughout the experiment, particularly in the male rats; 27 rats
weighed >500 g after 60 days of entering into the plateau area
and nine rats weighed >600 g after 180 days.
Basilar membrane changes in rat
cochlea at different time-points after moving to the plateau
area
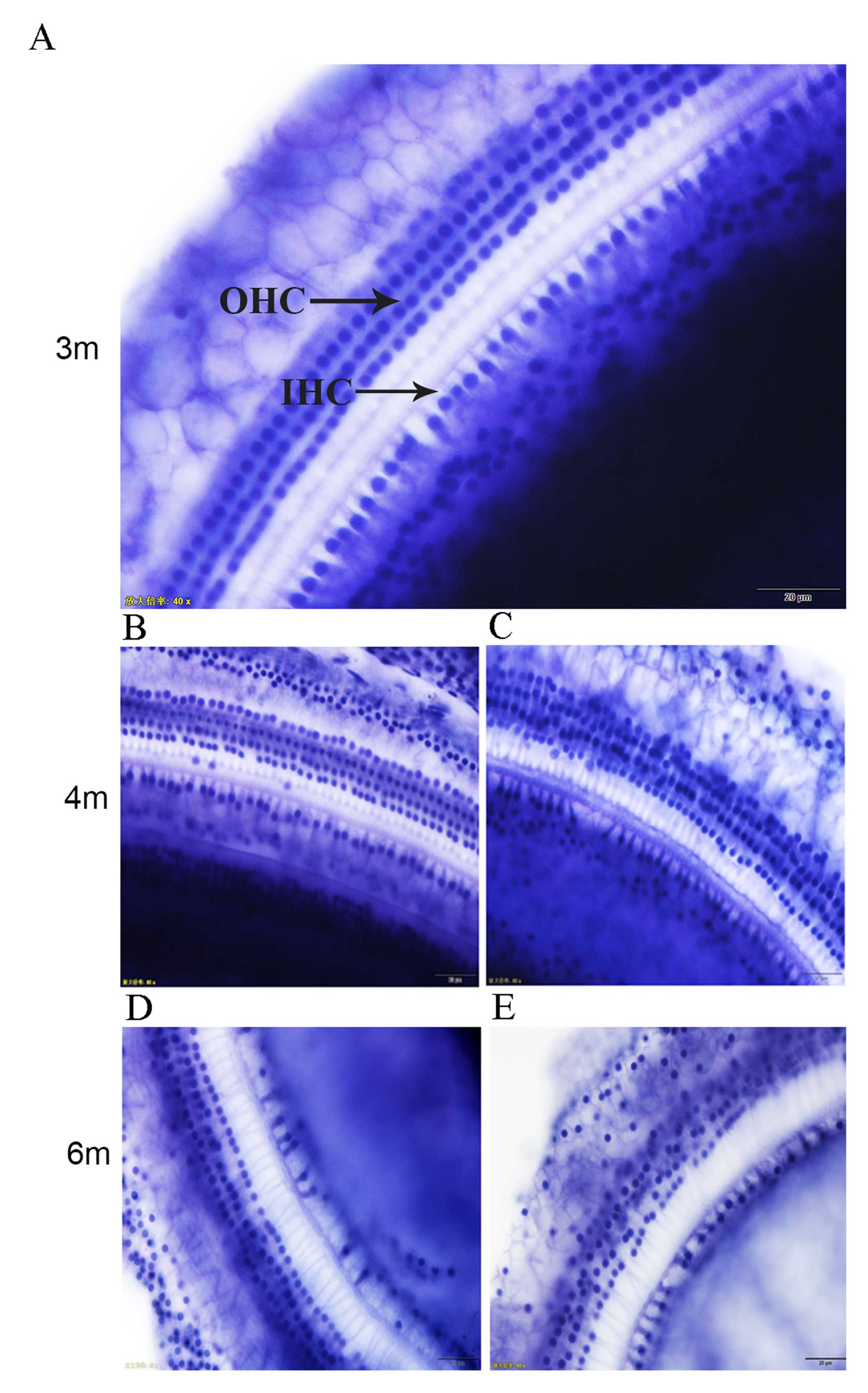
The morphology of cochlear HCs in rats was normal
after one day of moving to the plateau area. The HCs, which
consisted of the three rows of cochlear outer HCs (OHCs) and one
row of cochlear inner HCs (IHCs), were arranged and distributed in
a uniform and regular manner, with integrated and distinct
structures. No cell swelling, deformation or loss was observed.
At 1–3 months after moving to the plateau area, the
arrangement and distribution of IHCs were uniform and regular with
intact and distinct structures, and there were no abnormal cell
changes. OHCs were arranged and distributed regularly, with only a
small number of cells observed to be swollen, distorted and lost
(Fig. 1A). With prolonged periods of
time under hypoxia, cell swelling, dislocation, and deformation
appeared in OHCs after 4–5 months; different degrees of IHC loss
also began to appear (Fig. 1B and C).
Six months after moving to the plateau area, the degree of HC loss
was increased, cell loss was observed in the first row of OHCs,
swelling and deformation of OHCs were observed, and the distance
between the rows of cochlear OHCs increased. In two rats, the
cochlear OHC boundaries became indistinct or disappeared, and the
degree of cell damage was further aggravated (Fig. 1D and E).
The number of cochlear HCs on the cochlear basilar
membrane were counted under a microscope at different time-points
as shown in Table I.
 | Table I.Number of cochlear HCs at different
time-point after moving to the plateau area. |
Table I.
Number of cochlear HCs at different
time-point after moving to the plateau area.
|
|
| Cell count |
|---|
|
|
|
|
|---|
| Group | Days | Outer HCs | Inner HCs |
|---|
| 1 | 1 | 224.36±5.23 | 67.44±3.43 |
| 2 | 30 | 220.47±6.18 | 66.83±3.15 |
| 3 | 60 | 215.39±4.27 | 66.59±2.97 |
| 4 | 90 | 210.28±4.58 | 65.29±3.47 |
| 5 | 120 |
180.25±5.76a |
53.33±2.53a |
| 6 | 150 |
161.33±4.92a |
41.24±2.86a |
| 7 | 180 |
135.67±5.38a |
29.84±3.52a |
Changes in SGNs of the cochlea in rats
at different time-points after entering the plateau area
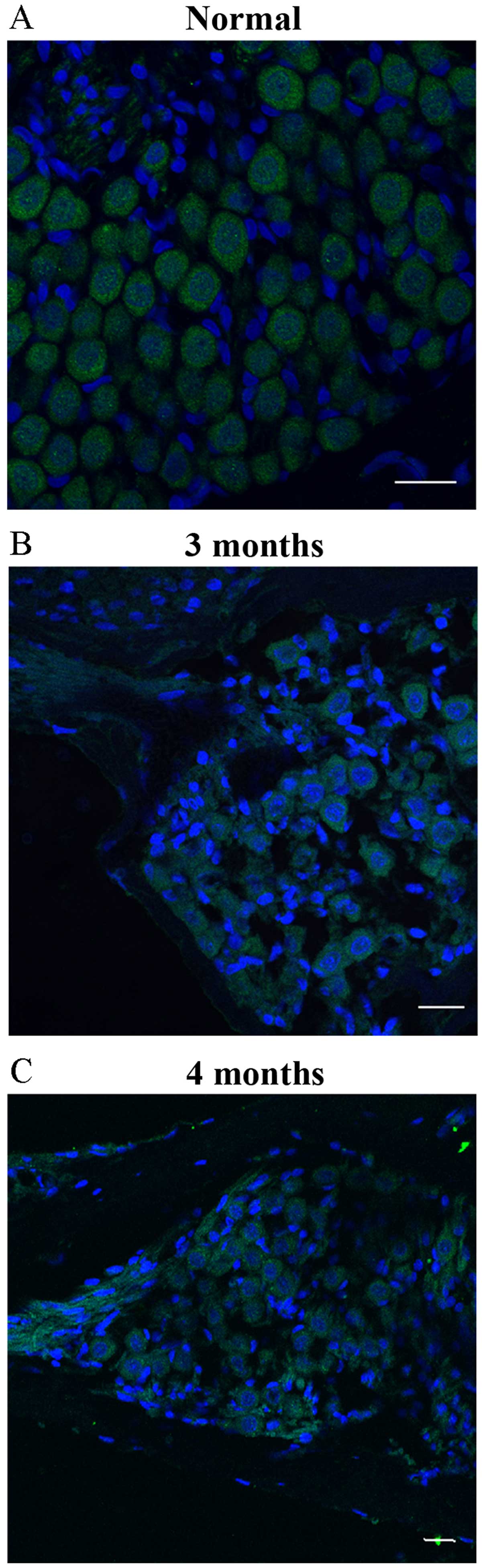
The results, obtained by immunofluorescence staining
of the frozen sections in the 1 day group, showed that the
NF-200-positive cells in the SGNs exhibited green fluorescence in
the cell cytoplasm, which had an intact cell membrane, large, round
nuclei and distinct morphology. In addition, a small quantity of
glial cells and fibroblasts with small, oval or spindle-shaped
nuclei surrounding the neuronal cells were observed (Fig. 2A). No significant histological changes
were observed in SGNs after 1–2 months of moving to the plateau
area. After 3–4 months, the number of SGNs was significantly
decreased, and the number of interstitial cells was increased in
the interstitial space of the ganglion (Fig. 2B and C). The number of SGNs was counted
through serial sections, as shown in Table II. The number of SGNs in the 3–6 month
groups was significantly decreased by 28–35% when compared with the
1 day group (P<0.01), no significant difference in the number of
SGNs was identified between the 3–6 month group.
 | Table II.Number of SGNs in rats at different
time-points after moving to the plateau area. |
Table II.
Number of SGNs in rats at different
time-points after moving to the plateau area.
| Group | Days | SGNs |
|---|
| 1 | 1 | 131.47±15.36 |
| 2 | 30 | 136.94±16.58 |
| 3 | 60 | 129.31±21.28 |
| 4 | 90 |
94.32±14.16a |
| 5 | 120 |
100.29±15.06a |
| 6 | 150 |
90.38±8.94a |
| 7 | 180 |
84.51±11.36a |
Discussion
In the current study cochlea HCs in rats did not
demonstrate obvious changes during the first 3 months of moving to
the plateau area from the low altitude area. However, morphological
changes in HCs were observed with >3 months at high altitude.
Cell swelling, deformation and dislocation were apparent in OHCs,
the space between the rows of OHCs widened and the cell boundaries
became blurred or disappeared; certain morphological changes were
subsequently found in IHCs. OHCs are the first to be damaged in a
hypoxic environment, followed by the IHC as a result of prolonged
hypoxic exposure. Furthermore, it was found that the degree of
damage in the base of the cochlea was greater than that in the apex
and the middle of the cochlea, which indicated that the cochlear
damage caused by hypoxia initiates from the base of the cochlea.
Cochlear blood flow is from the apex to the base of the cochlea,
however, the blood flows very slowly due to the spiral structure of
the cochlea; thus, in hypoxic conditions, cochlear HCs are easily
damaged by insufficient blood supply to the cochlea. In addition,
metabolism in the apex of the cochlea is predominantly anaerobic
and the main metabolic pathway at the base of the cochlea is
aerobic. Under hypoxic environments, the base of the cochlea cannot
perform aerobic metabolism due to the lack of blood flow and oxygen
to the brain, which causes damage to cells at the base of the
cochlea (5,6).
A significant loss of SGNs was observed within 3
months of moving to the plateau area, which was accompanied by the
proliferation of glial cells and fibroblasts surrounding the
neurons. Daniel et al (7)
determined the effects of hypoxia on hearing in 10-day-old neonatal
rats, and found that in adulthood, permanent hearing loss occurred
with prolonged wave I–V inter-peak latencies, hypoxia-induced
hearing loss may damage the central auditory pathways (8). In the current study, damage of SGNs
appeared earlier than HC damage, and SGN damage was not aggravated
over time; during 3–6 months, no significant difference was found
in the number of SGNs. It has been speculated that cochlear neurons
are more tolerant to hypooxia, which shows enhanced ability of
survival neuron cells to adapt to the hypoxic microenvironment.
In conclusion, long term, continuous hypoxia was
found to affect the survival of cochlear HCs and SGNs. Cochlear
damage caused by hypoxia initiates at the base of the cochlea and
spreads to the apex, with the OHCs becoming damaged first, followed
by damage to the IHCs. Cochlear SGNs are markedly more sensitive to
hypoxia, but SGNs are able to elicit adaptive mechanisms, which
protect neurons from hypoxia. The current study presents
preliminary results regarding histological changes in the
peripheral auditory system of rats that migrated to a plateau area.
A limitation of the present study was the lack of rat auditory
function test data, due to the temporary conditions of the
experiment. Further investigations are in progress that will
facilitate with investigating the change of the auditory system and
hypoxia adaptation process in humans that have relocated to a
plateau area from a low altitude region.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (grant no.
81260422).
References
|
1
|
Hess A, Bloch W, Huverstuhl J, Su J,
Stennert E, Addicks K and Michel O: Expression of inducible nitric
oxide synthase (iNOS/NOS II) in the cochlea of guinea pigs after
intratympanical endotoxin-treatment. Brain Res. 830:113–122. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Olgun Y, Kırkım G, Kolatan E, Kıray M,
Bağrıyanık A, Şerbetçioğlu B, Yılmaz O, Gökmen N, Ellidokuz H,
Kumral A, et al: Otoprotective effect of recombinant erythropoietin
in a model of newborn hypoxic-ischemic encephalopathy. Int J
Pediatr Otorhinolaryngol. 77:739–746. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Amarjargal N, Andreeva N, Gross J, Haupt
H, Fuchs J, Szczepek AJ and Mazurek B: Differential vulnerability
of outer and inner hair cells during and after oxygen-glucose
deprivation in organotypic cultures of newborn rats. Physiol Res.
58:895–902. 2009.PubMed/NCBI
|
|
4
|
Ren HL, Pan YY, Gao DG, Wang G and Fan DY:
Investigation on high altitude environmental effects on hearing
health. Tibetan Journal of Medicine. 36:4–6. 2015.
|
|
5
|
Dziennis S, Reif R, Zhi Z, Nuttall AL and
Wang RK: Effects of hypoxia on cochlear blood flow in mice
evaluated using Doppler optical microangiography. J Biomed Opt.
17:1060032012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen W, Wang J, Chen J, Chen J and Chen Z:
Relationship between changes in the cochlear blood flow and
disorder of hearing function induced by blast injury in guinea
pigs. Int J Clin Exp Pathol. 6:375–384. 2013.PubMed/NCBI
|
|
7
|
Daniel SJ, McIntosh M, Akinpelu OV and
Rohlicek CV: Hearing outcome of early postnatal exposure to hypoxia
in Sprague-Dawley rats. J Laryngol Otol. 128:331–335. 2014.
View Article : Google Scholar
|
|
8
|
Kim YJ, Kang HH, Ahn JH and Chung JW:
Hypoxic changes in the central nervous system of noise-exposed
mice. Acta Otolaryngol Suppl. 127:73–77. 2007. View Article : Google Scholar
|