Introduction
Acute myocardial infarction (AMI) is associated with
considerable morbidity and mortality worldwide (1,2). The
majority of myocardial infarcts result from coronary
atherosclerosis, complicated by acute rupture of an atherosclerotic
plaque and subsequent formation of coronary thrombus. Coronary
artery occlusion causes ischemia of the anatomic region supplied by
the vessel, ultimately leading to death of a large quantity of
cardiomyocytes. Generally, the heart possesses little regenerative
capacity; therefore, repair of the infarcted myocardium is
dependent on the sequence of cellular events that lead to the
formation of a collagen-based scar (3–5). The
necrotic cardiomyocytes elicit an intense inflammatory cascade that
serves to clear the infarct site from dead cells and matrix debris,
and eventually contributes to replacement of damaged tissue with
collagen-based scar tissue (6).
Cardiac injury generates endogenous signals that
activate the innate and adaptive immune system; these molecules
belong to the α2-macroglobulin family of mediators that warn the
body of injury and are termed complement factors C3, C4, C5b9
(7). The complement system mediates
inflammation by generating anaphylatoxins (to trigger chemotaxis
and cell activation) and promoting phagocytosis, degranulation and
cell lysis (8). Prolonging
inflammatory signaling in the infarcted heart may have numerous
consequences, including loss of cardiomyocytes, suppression of
systolic function, enhanced matrix-degrading processes leading to
chamber dilation, increased tissue breakdown causing loss of
ventricular wall integrity and cardiac rupture, as well as extended
fibrotic changes beyond the initial infarct. Based on this
mechanism, it has been verified that the primitive innate immune
system is activated in coronary heart disease (CHD) by the acute
phase response components, of which high-sensitivity C-reactive
protein (hs-CRP) increases the inflammatory response with high
specificity. Human hs-CRP binds to damaged cells and activates the
complement system by an alternate signaling pathway (9). Experimental models have indicated that
complement component C3 presents in atherosclerotic plaques, and
participates in activation of the inflammatory system and promotes
the process of phagocytosis by macrophages (10). Various complements are directly or
indirectly involved in myocardial cell lysis or damage via
formation of the membrane attack complex in the terminal pathway
(11).
Recent studies revealed that the systemic
inflammatory response, including the cascade of inflammatory and
cytokine complement activation, is key in the development of
myocardial infarction (3). hs-CRP is a
marker of inflammation that predicts incident myocardial
infarction, recurrent events and mortality in patients with AMI.
However, the association between serum complement C3, C4, C5b9 and
hs-CRP changes in patients with AMI and the severity of myocardial
injury remains unclear. The present study aims to investigate the
changes in levels of serum complement C3, C4, C5b9 and hs-CRP, and
probe the potential association between the differentially
expressed complement proteins and the severity of tissue damage
following AMI.
Materials and methods
Study population
The current study was approved by the Hospital
Review Board, Tianjin Union Medical Center of Nankai University
Affiliated Hospital (Tianjin, China). A total of 110 consecutive
patients [73 males and 37 females; age 62 years (range, 50–74
years)] with a diagnosis of AMI from May 2013 to February 2015
whose age and gender matched, and who had no personal or family
history of premature cardiovascular disease, were selected. In
addition, 33 healthy individuals were enrolled to serve as the
control group. AMI was confirmed according to current guidelines
(12). Based on their
electrocardiograms (ECGs), AMI patients were categorized into two
groups: ST segment elevation MI (STEMI; n=51) and non-ST segment
elevation MI (NSTEMI; n=59) (13). The
exclusion criteria included the following: Patients with previous
myocardial infarction or heart failure, cardiomyopathy,
pericarditis, cerebral vascular disease, pulmonary embolism,
hepatic insufficiency, renal insufficiency, hyperthyroidism,
anemia, hematologic disease, autoimmune disease or acute
inflammation. Written informed consent was obtained from all
participants.
Clinical and laboratory data
collection
At the time of enrollment, a detailed clinical
history was recorded, which included age, gender, body mass index
(BMI), blood pressure, heart rate and coronary artery disease
history. Blood samples for hematological parameters were obtained
from all patients. Creatine kinase-MB (CK-MB) (14) and Cardiac troponin T were determined by
an electrochemiluminescence immunoassay on the Elecsys 2010 system
(Roche Diagnostics GmbH, Mannheim, Germany) (15). Levels of cholesterol, high-density
lipoprotein (HDL) cholesterol and low-density lipoprotein (LDL)
cholesterol, and triglycerides were measured with the use of
standard hospital assays through enzymatic methods (Roche
Diagnostics GmbH) with freshly drawn lithium-heparin plasma
samples. Blood samples were obtained from all participants at
admission, and at days 1, 3 and 7. Serum levels of C3, C4 and
hs-CRP were measured quantitatively by velocity scattering
turbidimetry using a Siemens BN II automatic protein analyzer
(Siemens AG, Munich, Germany) as previously described (16). In addition, enzyme linked immunosorbent
assays (Kamiya Biomedial Co., Seattle, WA, USA) were performed to
quantify the serum level of C5b9 (8).
Echocardiography
Echocardiographic examination was performed as
previously described (17). M-mode,
two-dimensional, and Doppler echocardiography analysis (Vevo 770;
Visual Sonics Biotechnology Co., Toronto, ON, Canada) provided the
left ventricular (LV) end-diastolic diameter (LVEDD) and LV
end-systolic diameter (LVESD). The LV systolic function was
measured as ejection fraction (LVEF) and fractional shortening
(LVFS) according to the modified Simpson method (6).
Statistical analysis
Data analyses were performed using Graph Pad Prism
5.0 software (GraphPad Software Inc., La Jolla, CA, USA). For
comparisons between the two groups, analysis of variance and
one-way Student's t-test were used. Continuous variables were
expressed as the mean ± standard deviation and P<0.05 was
considered to indicate a statistically significant difference.
Results
Patient characteristics
Statistical analysis of clinical information from
all participants (Table I) revealed
significant differences in CK-MB, troponin T and LVEF between the
AMI patients and control subjects (P<0.05). No significant
differences were identified in age (P=0.054), gender (P=1.000), BMI
(P=0.969), heart rate (P=0.191), systolic blood pressure (P=0.694),
diastolic blood pressure (P=0.663), cholesterol (P=0.326),
triglyceride (P=0.268), or HDL cholesterol (P=0.66) and LDL
cholesterol (P=0.056) between the two groups.
 | Table I.Baseline characteristics of the AMI
and control groups. |
Table I.
Baseline characteristics of the AMI
and control groups.
|
| Group |
|
|---|
|
|
|
|
|---|
| Variable | Control (n=33) | AMI (n=110) | P-value |
|---|
| Age (years) |
57.64±10.48 |
62.06±11.59 | 0.054 |
| Gender
(male/female) | 22/11 | 73/37 | 1.000 |
| BMI
(kg/m2) | 24.42±3.03 | 24.45±1.98 | 0.969 |
| Systolic blood
pressure (mmHg) | 131.30±24.16 | 133.85±25.97 | 0.694 |
| Diastolic blood
pressure (mmHg) | 76.30±8.95 |
75.38±14.26 | 0.663 |
| Heart rate (bpm) |
72.79±12.38 |
75.60±13.93 | 0.191 |
| Cholesterol
(mmol/l) |
4.19±0.45 |
4.79±0.91 | 0.326 |
| Triglyceride
(mmol/l) |
0.97±0.31 |
1.53±0.83 | 0.268 |
| HDL cholesterol
(mmol/l) |
1.31±0.30 |
1.06±0.37 | 0.66 |
| LDL cholesterol
(mmol/l) |
2.65±0.34 |
2.79±0.76 | 0.056 |
| Troponin T
(ng/ml) |
0.012±0.001 |
9.15±1.24 | 0.002 |
| Creatine kinase-MB
(U/l) | 10.67±2.86 | 200.48±64.0 | 0.001 |
| LVEF (%) | 66.87±5.53 | 49.00±6.29 | 0.002 |
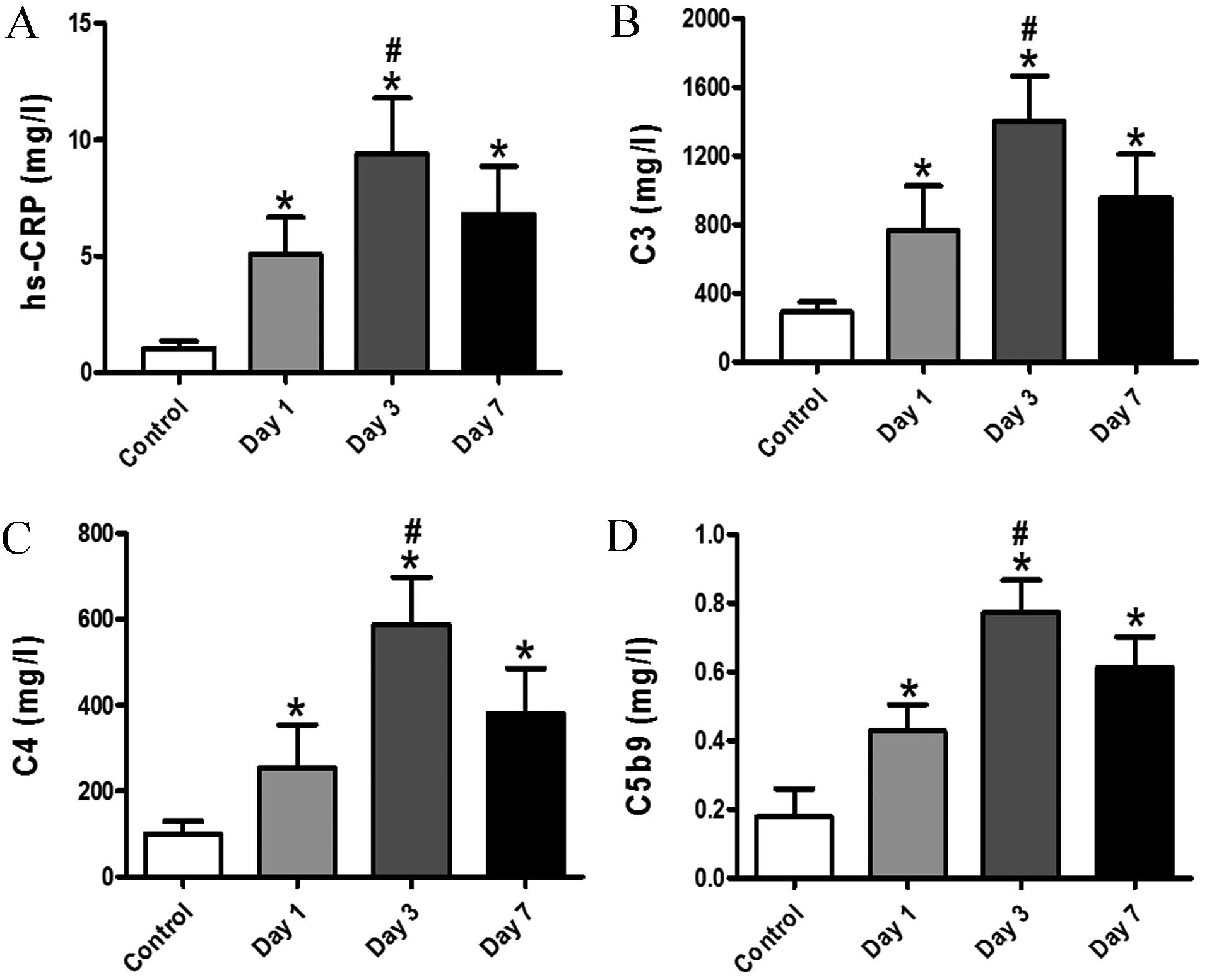
Changes in serum levels of C3, C4,
C5b9 and hs-CRP
It has been proposed that complement proteins are
crucial in the development of AMI. The aim of the current study was
to investigate the association between serum complement and hs-CRP
changes, and the severity of myocardial injury, thus the
differential expression of complement proteins in patients with AMI
was evaluated. This approach may be used to detect changes in
cardiac damage subsequent to interventions in patients with
myocardial injury. In the present study, the results revealed that
the patients with AMI tended to exhibit markedly higher levels of
C3, C4, C5b9 and hs-CRP when compared with the subjects from the
control group at days 1, 3 and 7. On day 3, subsequent to
infarction, a significant enhancement in C3, C4, C5b9 and hs-CRP
serum levels was observed in patients with AMI (Fig. 1; P<0.05). These results indicate
that hs-CRP and activation of complement may be indicators of
myocardiocyte damage.
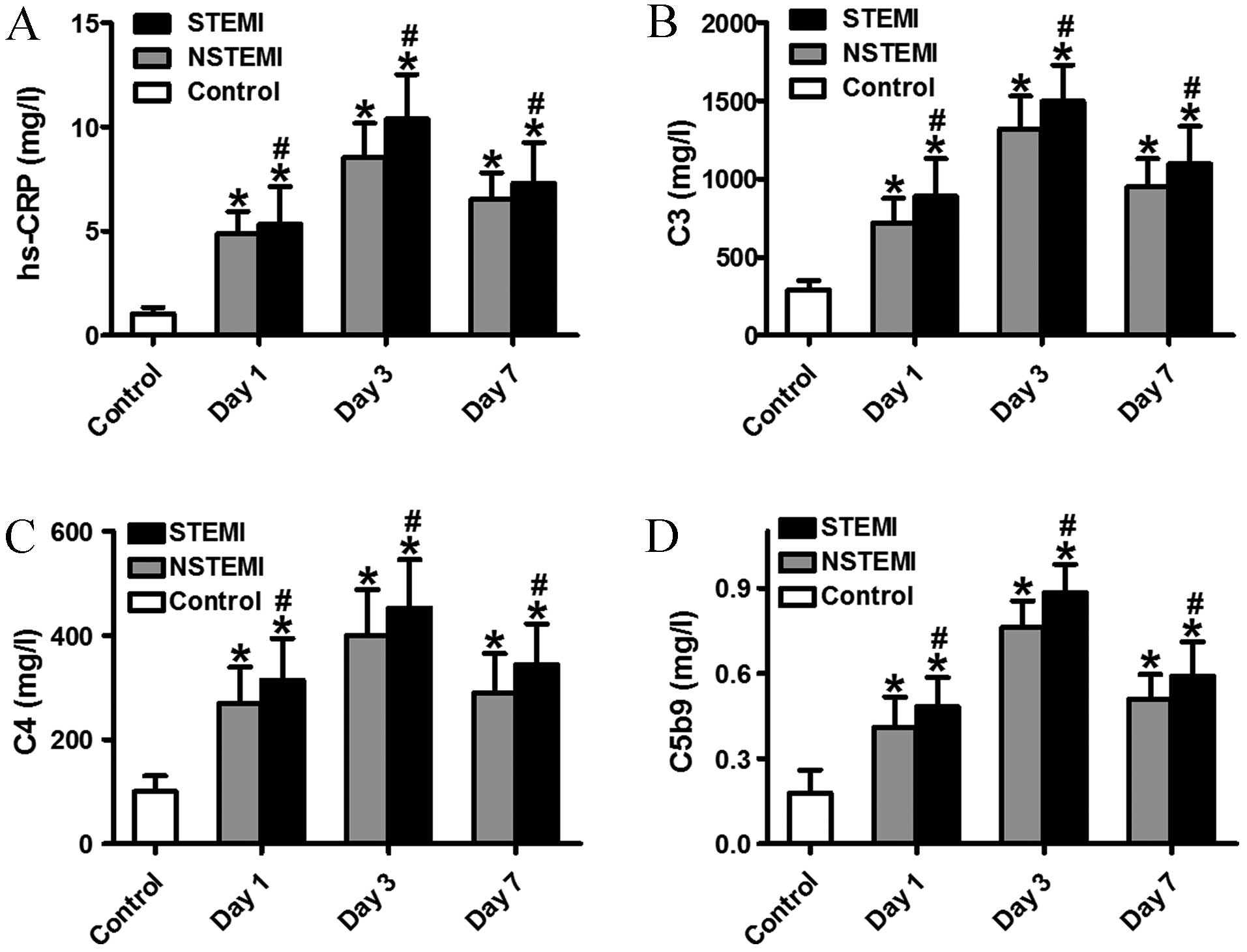
Increased serum levels of C3, C4, C5b9
and hs-CRP in the STEMI subgroup
To date, a small number of studies have demonstrated
the association between serum complement, hs-CRP and heart failure
(8). In the present study, it was
confirmed that there was a more rapid increase in serum levels of
C3, C4, C5b9 and hs-CRP in patients with STEMI when compared with
those with NSTEMI or the control subjects at different time points
(Fig. 2). Activation of the complement
system is an important aspect of the inflammatory process that
regulates injury, repair and remodeling of the infarcted heart.
Notably, the levels of serum C3, C4, C5b9 and hs-CRP in the
STEMI/NSTEMI groups peaked at day 3. Furthermore, the levels of
complement C3, C4, C5b9 and hs-CRP in the STEMI group were higher
than those in the NSTEMI group. These data indicate that activation
of complement proteins and hs-CRP in patients increases the risk of
complication and severity of AMI.
Heart function analysis
LV function was evaluated using echocardiograms on
day 7 after infarction. Compared with the control groups, the
patients with STEMI displayed more marked LV enlargement and
impaired heart function than those with NSTEMI (Fig. 3A). Normal function in the myocardium is
dependent on optimal preservation of structure, as minimal changes
in cardiac morphology may exacerbate dysfunction. Subsequent to an
infarction, the human heart undergoes a series of structural
changes, which are governed by cytokines, complements and cellular
mechanisms in a pathological metamorphosis. In the present study,
patients with STEMI and NSTEMI demonstrated markedly increased
LVESD and LVEDD of the heart when compared with the control group
(Fig. 3B and C). In addition, patients
with STEMI exhibited significantly exacerbated progression of LV
dysfunction compared with the control group subjects, with
decreased LVFS and LVEF (Fig. 3D and
E). Thus, the results indicate that cardiomyocyte injury was
key in the cardiac remodeling and post infarction heart failure of
patients in the STEMI group. Furthermore, these data reveal that
activation of complement protein and hs-CRP are indicators of heart
failure.
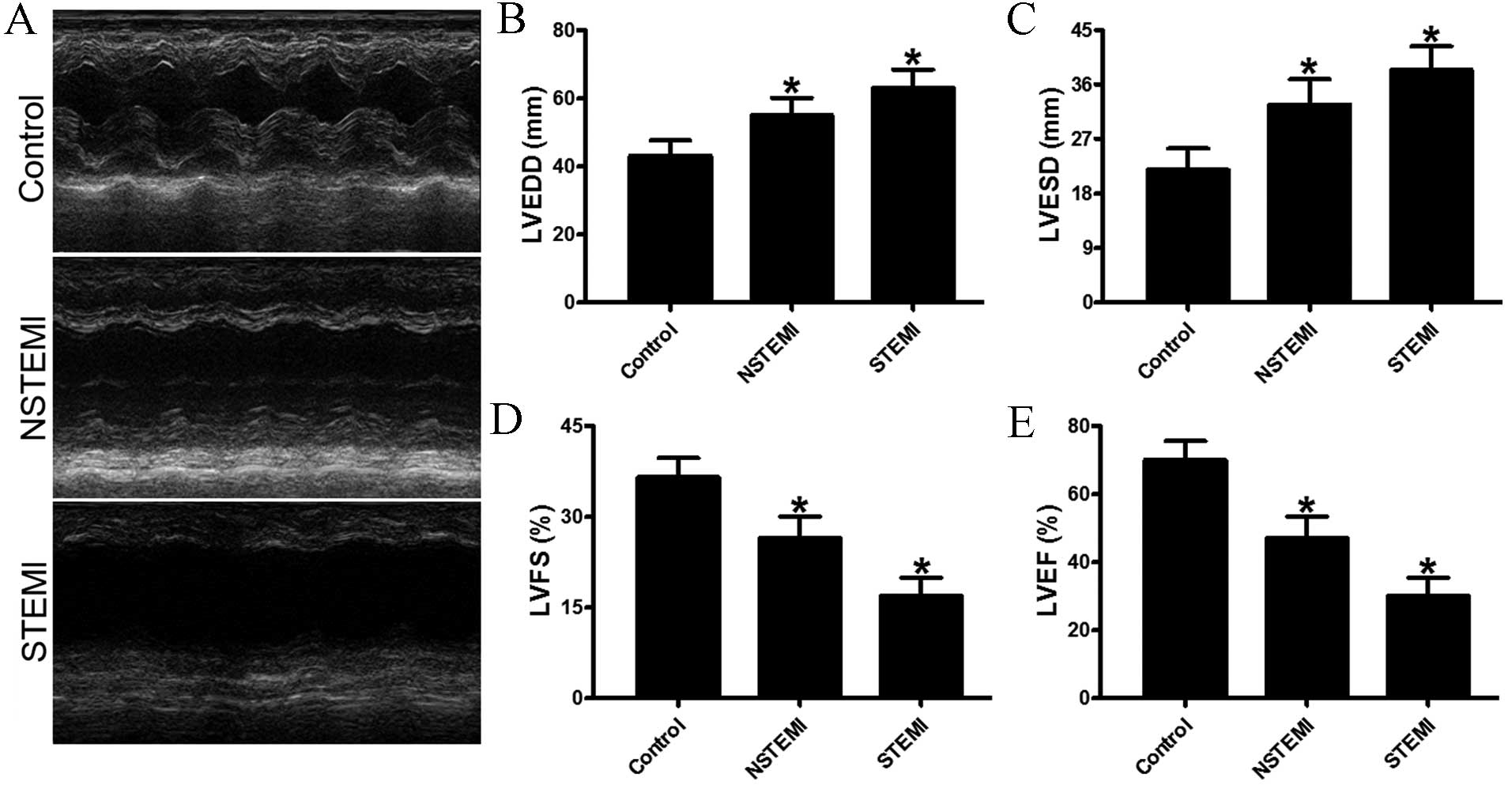 | Figure 3.Echocardiographic evaluation of
cardiac function. (A) Representative M-mode echocardiogram data of
infarcted hearts of the control group, and NSTEMI and STEMI
subgroups at day 7. (B and C) Comparison of LVEDD and LVESD between
control group, NSTEMI subgroup and STEMI subgroup. (D and E)
Quantitative analysis of LVFS and LVEF among the three groups.
Compared with the control, the patients in the STEMI subgroup
exhibited significantly accentuated heart function according to the
LVEF values. *P<0.05 vs. control. AMI, acute myocardial
infarction; STEMI, ST segment elevation MI; NSTEMI, non-ST segment
elevation MI; hs-CRP, high-sensitivity C-reactive protein; LVEDD,
left ventricular end-diastolic diameter; LVESD, left ventricular
end-systolic diameter; LVFS, left ventricular fractional
shortening; LVEF, left ventricular ejection fraction. |
Discussion
In the current study, complement proteins and hs-CRP
levels were demonstrated to be associated with the severity of
myocardial injury in AMI patients. In addition, the high level of
complement proteins and hs-CRP were demonstrated as a strong
predictor of heart failure after AMI. The patients with STEMI
exhibited significantly exacerbated ventricular dilation
accompanied by progression to systolic dysfunction and heart
failure (18).
Heart failure after AMI may be accompanied by a
variety of effects, including increased apoptosis, decreased
capillary density and increased hypertrophy. Notably, these effects
occurred despite the complete activation of C5b9, which has a
specific and important role in the response to AMI (19). A previous study revealed that injured
or necrotic myocardial tissue releases subcellular constituents,
such as mitochondria, which may be a trigger for complement
activation pathways (20). In
addition, increased complement proteins may impair regenerative
processes to further aggravate myocardium injury (21). In uncomplicated myocardial infarction,
the acute inflammatory reaction begins 4–8 h after onset, with the
invasion of neutrophils from the perfused surrounding tissue, peaks
within 3–4 days and returns to normal within 7 days (22). Therefore, peak complement and hs-CRP
levels in patients with heart failure may be induced by ischemic
necrosis and reperfusion injury may initiate this potent
inflammatory stimulus (23). In
addition, animal studies revealed that hs-CRP enhances ischemic
tissue damage by a complement-dependent mechanism in the heart
(24). Complement proteins have also
been found to be deposited in atherosclerosis plaque (25).
In conclusion, the present study demonstrated that
the serum levels of complement and hs-CRP increased rapidly in
patients with AMI, particularly in early stage STEMI patients, and
hs-CRP and activation of complement responses are immune
inflammatory pathways that may lead to catastrophic consequences.
However, there were certain limitations of the present study; it
reflects a small sample of AMI patients, who were analyzed
retrospectively and the long-term outcomes were not evaluated.
Therefore, the present study may contribute to establishing
biomarker-based approaches to rationally implement clinical
therapeutic strategies for AMI.
Acknowledgements
The present study was supported by the Tianjin
Municipal Bureau of Health for Science and Technology (grant no.
2014KZ052).
References
|
1
|
Du W, Tao H, Zhao S, He ZX and Li Z:
Translational applications of molecular imaging in cardiovascular
disease and stem cell therapy. Biochimie. 116:43–51. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu Y, Ye X, Mao L, Cheng Z, Yao X, Jia X,
Mao D, Ou L, Li Z, Che Y, et al: Transplantation of parthenogenetic
embryonic stem cells ameliorates cardiac dysfunction and
remodelling after myocardial infarction. Cardiovasc Res.
97:208–218. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Frangogiannis NG: The inflammatory
response in myocardial injury, repair, and remodelling. Nat Rev
Cardiol. 11:255–265. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu N, Qi X, Han Z, Liang L, Kong D, Han
Z, Zhao S, He ZX and Li Z: Bone marrow is a reservoir for cardiac
resident stem cells. Sci Rep. 6:287392016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cai M, Shen R, Song L, Lu M, Wang J, Zhao
S, Tang Y, Meng X, Li Z and He ZX: Bone marrow mesenchymal stem
Cells (BM-MSCs) improve heart function in swine myocardial
infarction model through paracrine effects. Sci Rep. 6:282502016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li Z, Lee A, Huang M, Chun H, Chung J, Chu
P, Hoyt G, Yang P, Rosenberg J, Robbins RC, et al: Imaging survival
and function of transplanted cardiac resident stem cells. J Am Coll
Cardiol. 53:1229–1240. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Janssen BJ, Huizinga EG, Raaijmakers HC,
Roos A, Daha MR, Nilsson-Ekdahl K, Nilsson B and Gros P: Structures
of complement component C3 provide insights into the function and
evolution of immunity. Nature. 437:505–511. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wysoczynski M, Solanki M, Borkowska S, van
Hoose P, Brittian KR, Prabhu SD, Ratajczak MZ and Rokosh G:
Complement component 3 is necessary to preserve myocardium and
myocardial function in chronic myocardial infarction. Stem Cells.
32:2502–2515. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zacho J, Tybjaerg-Hansen A, Jensen JS,
Grande P, Sillesen H and Nordestgaard BG: Genetically elevated
C-reactive protein and ischemic vascular disease. N Engl J Med.
359:1897–1908. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Melis JP, Strumane K, Ruuls SR, Beurskens
FJ, Schuurman J and Parren PW: Complement in therapy and disease:
Regulating the complement system with antibody-based therapeutics.
Mol Immunol 67 (2 Pt A). 117–130. 2015. View Article : Google Scholar
|
|
11
|
Epelman S, Liu PP and Mann DL: Role of
innate and adaptive immune mechanisms in cardiac injury and repair.
Nat Rev Immunol. 15:117–129. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Thygesen K, Alpert JS, Jaffe AS, Simoons
ML, Chaitman BR, White HD, Thygesen K, Alpert JS, White HD, Jaffe
AS, et al: Joint ESC/ACCF/AHA/WHF Task Force for Universal
Definition of Myocardial Infarction; Authors/Task Force Members
Chairpersons; Biomarker Subcommittee; ECG Subcommittee; Imaging
Subcommittee; Classification Subcommittee; Intervention
Subcommittee; Trials & Registries Subcommittee; Trials &
Registries Subcommittee; Trials & Registries Subcommittee;
Trials & Registries Subcommittee; ESC Committee for Practice
Guidelines (CPG); Document Reviewers: Third universal definition of
myocardial infarction. J Am Coll Cardiol. 60:1581–1598. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
White H, Thygesen K, Alpert JS and Jaffe
A: Universal MI definition update for cardiovascular disease. Curr
Cardiol Rep. 16:4922014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Karahan Z, Uçaman B, Uluğ AV, Aydınalp Ö,
Uğurlu M, Çevik K, Kaya İ and Öztürk Ö: Effect of hematologic
parameters on microvascular reperfusion in patients with ST-segment
elevation myocardial infarction treated with primary percutaneous
coronary intervention. Angiology. 67:151–156. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu Z, Tang Q, Wen J, Tang Y, Huang D,
Huang Y, Xie J, Luo Y, Liang M, Wu C, et al: Elevated serum
complement factors 3 and 4 are strong inflammatory markers of the
metabolic syndrome development: A longitudinal cohort study. Sci
Rep. 6:187132016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zethelius B, Berglund L, Sundström J,
Ingelsson E, Basu S, Larsson A, Venge P and Arnlöv J: Use of
multiple biomarkers to improve the prediction of death from
cardiovascular causes. N Engl J Med. 358:2107–2116. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dai S, Yuan F, Mu J, Li C, Chen N, Guo S,
Kingery J, Prabhu SD, Bolli R and Rokosh G: Chronic AMD3100
antagonism of SDF-1alpha-CXCR4 exacerbates cardiac dysfunction and
remodeling after myocardial infarction. J Mol Cell Cardiol.
49:587–597. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Frangogiannis NG: The immune system and
the remodeling infarcted heart: Cell biological insights and
therapeutic opportunities. J Cardiovasc Pharmacol. 63:185–195.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Thorp EB: Mechanisms of failed apoptotic
cell clearance by phagocyte subsets in cardiovascular disease.
Apoptosis. 15:1124–1136. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Giasuddin AS, ElMahdawi JM and ElHassadi
FM: Serum complement (C3, C4) levels in patients with acute
myocardial infarction and angina pectoris. Bangladesh Med Res Counc
Bull. 33:98–102. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mellbin LG, Bjerre M, Thiel S and Hansen
TK: Complement activation and prognosis in patients with type 2
diabetes and myocardial infarction: A report from the DIGAMI 2
trial. Diabetes Care. 35:911–917. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ribeiro DR, Ramos AM, Vieira PL, Menti E,
Bordin OL Jr, Souza PA, Quadros AS and Portal VL: High-sensitivity
C-reactive protein as a predictor of cardiovascular events after
ST-elevation myocardial infarction. Arq Bras Cardiol. 103:69–75.
2014.PubMed/NCBI
|
|
23
|
Timmers L, Pasterkamp G, de Hoog VC,
Arslan F, Appelman Y and de Kleijn DP: The innate immune response
in reperfused myocardium. Cardiovasc Res. 94:276–283. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Christenson E and Christenson RH: The role
of cardiac biomarkers in the diagnosis and management of patients
presenting with suspected acute coronary syndrome. Ann Lab Med.
33:309–318. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wildgruber M, Swirski FK and Zernecke A:
Molecular imaging of inflammation in atherosclerosis. Theranostics.
3:865–884. 2013. View Article : Google Scholar : PubMed/NCBI
|