Introduction
Depression, primarily characterized by a persistent
depressed mood, seriously threatens human health, with a suicide
rate of 15–20% and a cure rate of only ~30%. To improve the cure
rate of depression, current research has focused on investigation
of the brain mechanisms of depression patients and targets for
treatment (1). Many previous studies
using positron emission tomography (PET) and single-photon emission
computed tomography (SPECT) techniques have reported that regional
cerebral blood flow (rCBF) aberrations may be one of the
pathological characteristics of depression. Drevets (2) suggested that the brain rCBF in patients
with depression showed a general tendency to decrease and that the
brain regions with abnormal rCBF in depression patients
predominantly included the frontal lobe, temporal lobe, the
cingulate cortex, amygdala, caudate nucleus, and paralimbic
system.
The application of SPECT and PET to clinical studies
relatively accurately identifies the location of the affected brain
areas (2). However, these techniques
have certain risks, such as allergic response to the injected
radioactive tracer. Such risk factors limit the use of SPECT and
PET imaging in depression patients in the research and clinical
settings. Arterial spin labeling (ASL) involves labeling of the
hydrogen atoms in the blood, which are then used as an endogenous
contrast agent; thus, no tracer injection is required, and the
cerebral perfusion of the subject is monitored in a non-invasive
state with high spatial resolution, good accuracy and
repeatability. Based on these advantages, ASL technology has become
the mainstream method in recent years for investigating the
characteristics of rCBF in depression patients (3,4).
The recently developed pseudo-continuous ASL (pCASL)
technique more effectively balances the signal-to-noise ratio (SNR)
than SPECT or PET techniques and has an improved labeling
efficiency that is characteristic of the developing trend of the
ASL technique (5). Certain studies
have used pCASL to investigate the rCBF of treated patients with
severe depression and found that cerebral blood perfusion
alterations are predominantly located in the anterior cingulate
cortex, right medial prefrontal cortex, temporal cortex,
hippocampus, thalamus and cerebellum (6,7). However,
the results of the two above-mentioned studies may have been
influenced by antidepressants (8).
Selecting untreated patients with first-episode depression and
using pCASL technology to detect the changes in the rCBF in
depression patients would avoid the impact of confounding factors,
such as antidepressants on rCBF. Thus, the specific aberrant rCBF
pattern in patients with depression may be accurately
characterized, which would provide an objective basis for the early
qualitative diagnosis of depression. However, to the best of our
knowledge, no study on the rCBF of drug-naive patients with
first-episode depression using pCASL technology has previously been
reported.
Therefore, the pCASL technique using a 3T MR system
was performed in the current study to investigate blood perfusion
at the whole brain level in drug-naïve patients with first-episode
major depression, with the aim of improving the understanding of
the characteristics of rCBF in patients with depression.
Subjects and methods
Study subjects
The inclusion criteria were patients who met the
DSM-IV diagnostic criteria for major depressive disorder at the
first onset and were diagnosed by two senior psychiatrists in a
structured clinical interview based on the DSM-IV (SCI-D) (9). The Hamilton Rating Scale for Depression
(17-item version) (10) was used to
assess disease severity in patients who were not taking any
antidepressants or mood stabilizers. A total of 10 patients were
enrolled from January to December 2015 in the current study. There
were seven males and three females, with an age range of 18–60
years, a mean age of 38.70±9.41 years, and a disease duration of
2–12 months. A total of 15 healthy volunteers (control group) were
recruited from the hospital staff of Tianjin Anning Hospital
(Tianjin, China), and included six males and nine females with an
age range of 20–59 years and a mean age of 38.42±9.12 years. Two
senior psychiatrists ruled out a diagnosis of a potential mental
disorder, and the volunteers were only enrolled if they did not
have a positive family history of mental disorder. There were no
significant differences between the two groups with regard to age
or the level of education.
The common exclusion criteria were patients with a
history of unconsciousness for ≥5 min, a neurological disease
diagnosis, severe mental illness, drug abuse, serious physical
illness, or endocrine disease. Furthermore, patients who were
pregnant, lactating, had participated in any other research study,
had received treatment within one month, or that had any magnetic
resonance imagine (MRI) contraindications were excluded.
The Ethics Committee of Tianjin 4th Central Hospital
(Tianjin, China) approved the current study. Prior to
experimentation, all subjects were fully informed of the
experimental purposes, methods and risks of possible discomfort,
and all patients provided written informed consent.
Image capture
A Philips Achieva 3.0 T TX whole body MRI system
(Philips Medical Systems Nederland B.V., The Netherlands) was used
to perform the scans. An 8-channel parallel coil was used to
collect the signals. Subjects were placed in a supine position and
wore non-magnetic earplugs to reduce the impact of noise during the
experiment, and a supplied foam pad was fixed around the subject's
head to reduce head movement. The scanning scope covered the whole
brain, and a connecting line between the front and back of the
brain served as a baseline during the scanning.
The MRI protocol order for the subjects was as
follows: T1 weighted images (WI), T2WI and pCASL images.
Conventional T1WI and T2WI were captured to exclude organic
diseases, such as craniocerebral disorders and brain malformations.
The pCASL scan included a single-shot echo-planar sequence (EPI),
and the parameters were as follows: Echo time/repetition time =
4,000 msec/14 msec; matrix = 80×80; layer thickness = 7 mm; number
of layers = 17; interlayer spacing = 1 mm; voxel size = 3×3×7 mm;
label spacing = 20 mm; duration of labeling = 1,650 msec; label
delay = 1,525 msec; and field of view = 240×240 mm.
Functional MRI (fMRI) data
preprocessing
For the current study, common data processing
software for brain functions were used, including Statistics
Parameter Mapping (SPM8; Wellcome Department of Imaging
Neuroscience, London, UK) and the Relative Expression Software Tool
to conduct the data preprocessing. The specific process consisted
of the following: i) Data format conversion. The ASL images from
the scans were processed with the built-in software for digital
subtraction to obtain the perfusion map, and the format was then
converted to a dual file format for SPM processing. This step was
performed using MRICroN software 1.40 (http://www.cabiatl.com/mricro/mricro/mricro.html).
ii) Spatial normalization. The individual perfusion image was input
into a standard PET template provided by SPM8. iii) Removal of
non-brain tissues. The normalized image was computed with a
standard mask to remove the non-brain tissue. This process was
achieved using the ImCalc function of SPM8. iv) Gaussian smoothing.
A Gaussian kernel of 4×4×4 mm3 full width at half
maximum (FWHM) was used to perform the spatial smoothing of the
images, reduce the remaining inter-individual differences after the
normalization, and improve the SNR. v) The mean of the whole brain
perfusion was extracted to obtain the normalized image for
individuals. The inter-individual differences in brain perfusion
were removed using the individual whole brain perfusion
information/average individual whole brain perfusion information.
This was achieved using the REST and the ImCalc functions of
SPM8.
Statistical analysis
SPM8 was used to conduct the statistical analysis of
the data of brain perfusion in the two groups and to present the
results. An independent one-sample t-test was conducted for the
healthy control and patient groups, with age and gender as the
statistical covariates to produce the difference map of cerebral
blood perfusion between the two groups. The results were
superimposed onto a single T1 template using the xjView plug-in.
The brain regions demonstrating statistical significance
[P<0.05, with false discovery rate (FDR) correction and cluster
size >30 voxels] were noted. Montreal Neurological Institute
coordinate positioning (x, y, z; http://www.mcgill.ca/neuro/) and Brodmann area
(http://www.talairach.org) identification were
performed, and the intensity of the rCBF was recorded (presented by
the statistical value ‘t’ in the t-test). The rCBF images of the
healthy controls were first displayed layer by layer, followed by
the cerebral perfusion images of the two groups displayed in
layers.
SPSS 19.0 statistical analysis software (SPSS, Inc.,
Chicago, IL, USA) was used for the input, sorting and statistical
analysis of the basic data for the two groups. The statistical
analysis was performed using two independent sample t-tests, and
P<0.05 was considered to indicate a statistically significant
difference. The data are presented as the mean ± standard
deviation.
Results
Social and demographic data of the
participants
Ten patient participants were enrolled in the
current study, including seven males and three females, with an age
range of 18–60 years and a disease duration of 2–12 months.
Simultaneously, 15 healthy volunteers were enrolled in the study,
including six males and nine females, with an age range of 20–59
years. The detailed clinical information is presented in Table I.
 | Table I.Social and demographic data for all
participants. |
Table I.
Social and demographic data for all
participants.
| Variable | Patient group
(n=10) | Control group
(n=15) | t-value | P-value |
|---|
| Age (years) | 38.7±9.4 | 38.4±9.1 |
0.350 | 0.557 |
| Education level
(years) | 12.7±2.1 | 12.6±2.2 |
0.041 | 0.840 |
| HAMD-17 score | 35.3±6.9 |
4.6±2.3 | 17.427 | 0.000 |
Comparison of the brain regions with
altered cerebral perfusion between patients with depression and the
healthy control group
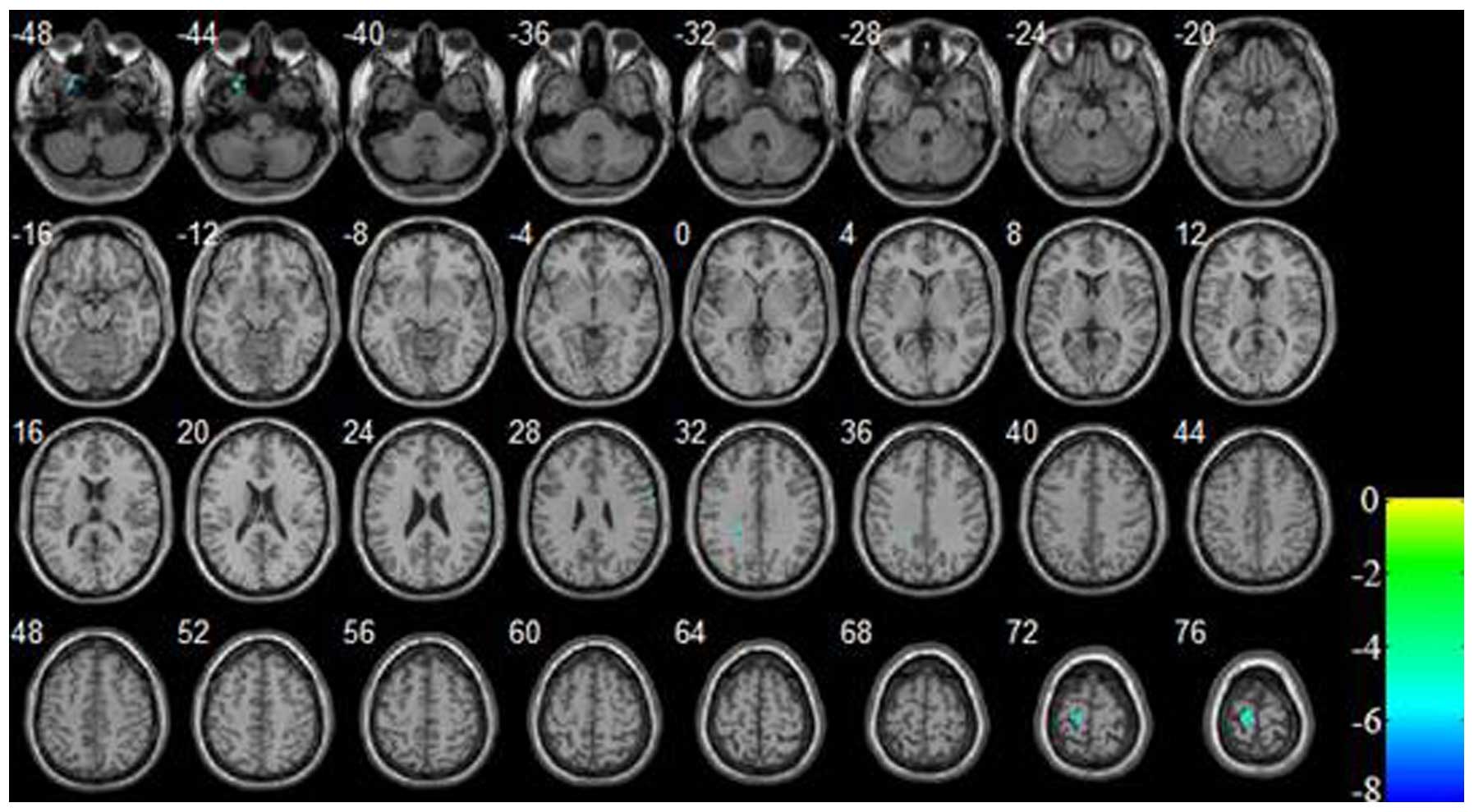
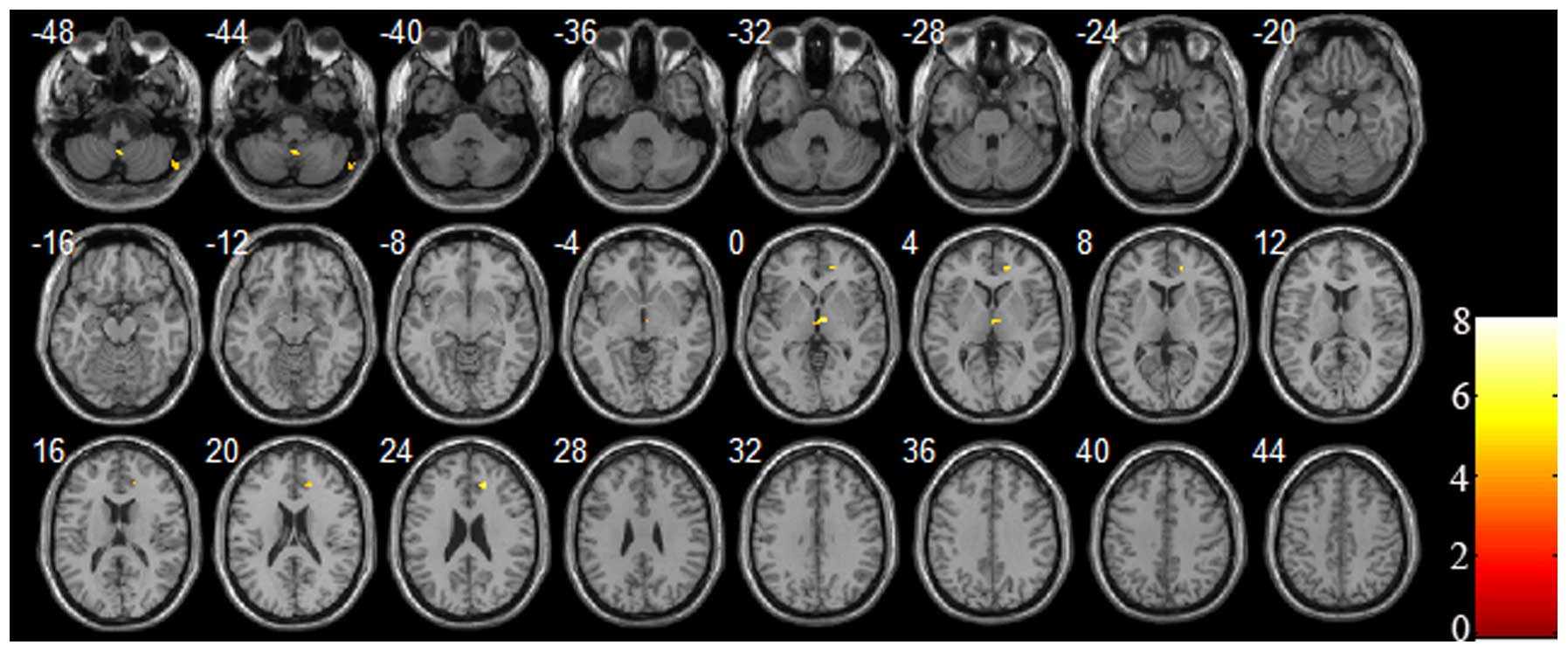
In the current study, increased and decreased CBF
brain regions were observed in the untreated depression patients
when compared with the healthy controls. The significantly
decreased cerebral perfusion brain regions were located in the left
temporal lobe, left frontal lobe, left cingulate cortex and right
parietal lobe (P<0.05 and cluster size ≥30, with FDR correction;
Table II and Fig. 1). Additionally, the significantly
increased cerebral perfusion regions were located in the right
cerebellum, right thalamus, right frontal lobe and right anterior
cingulate cortex (P<0.05 and cluster size ≥30, with FDR
correction; Table III and Fig. 2). Overall, the decreased cerebral
perfusion regions were primarily located in the left hemisphere,
with the exception of the right parietal lobe, and the increased
cerebral perfusion regions were predominantly located in the right
hemisphere.
 | Table II.Comparison of brain regions with
decreased cerebral perfusion between patients with depression and
the healthy control subjects. |
Table II.
Comparison of brain regions with
decreased cerebral perfusion between patients with depression and
the healthy control subjects.
| Brain region | Cluster size | MNI coordinates (x,
y, z) | t-value | Brodmann area
location |
|---|
| Left temporal
lobe | 50 | −24, 16, −46 | −6.7440 | 38 |
| Right parietal
lobe | 31 | 66, −4, 26 | −6.8639 | 6 |
| Left frontal lobe and
cingulate cortex | 32 | −20, −40, 32 | −7.6494 | – |
| Left paracentral
lobule and precentral gyrus | 119 | −14, −24, 74 | −8.2019 | 6, 4 |
 | Table III.Comparison of the brain regions with
increased cerebral perfusion between patients with depression and
the healthy control subjects. |
Table III.
Comparison of the brain regions with
increased cerebral perfusion between patients with depression and
the healthy control subjects.
| Brain region | Cluster size | MNI coordinates (x,
y, z) | t-value | Brodmann area
location |
|---|
| Right cerebellum
(posterior lobe) | 50 | 62, −68, −48 | 4.6746 | – |
| Right cerebellum
(tonsil) | 36 | 2, −54, −46 | 5.8710 | – |
| Right thalamus | 57 | 4, −10, 2 | 7.9571 | – |
| Right frontal lobe,
anterior cingulate cortex | 38 | 20, 46, 6 | 6.8155 | 32 |
| Right anterior
cingulate cortex | 30 | 18, 36, 24 | 7.0385 | 32 |
Comparison of the brain regions with
altered gray matter volume between patients with depression and the
healthy control group
In the current study, the gray matter volume
alterations in the patients with first-episode untreated depression
were investigated. Although the gray matter volume was found to be
decreased in the bilateral frontal lobe, bilateral occipital lobe,
left thalamus and left cerebellum, the significance of these
findings disappeared following FDR correction.
Discussion
The best of our knowledge, the current investigation
is the first to use advanced pCASL technology to characterize
specific alterations in the rCBF in first-episode drug-naïve
depression patients. Compared with previous studies, the findings
strictly controlled the influence of therapeutic agents and more
objectively characterized the specific alterations of rCBF in the
patients with depression. The most notable finding of our current
study is that the alterations of rCBF in drug-naïve patients with
first-episode depression tended to display an asymmetric pattern.
Almost all of the decreased cerebral blood perfusion brain regions
were located in the left hemisphere, specifically the left
prefrontal cortex, left temporal lobe, and left cingulate cortex,
with the exception of the right parietal lobe. By contrast, all the
increased cerebral perfusion brain regions were located in the
right hemisphere, specifically the right cerebellum, right
thalamus, right frontal lobe and right anterior cingulate cortex.
According to previous studies, the prefrontal cortex, temporal
lobe, cingulate cortex, parietal lobe, thalamus and cerebellum had
been confirmed to be key in emotional perception, memory,
integration, regulation and expression processing (6,7,11–13). The
rCBF alterations in these brain regions cause emotional
disturbances and are proposed to be the neural substrate of
depression.
In the current study, the asymmetric pattern of the
rCBF alterations in drug-naïve first-episode depression patients
were supported by other studies using different techniques. For
example, a study using 320-Slice Computed Tomography found that the
rCBF of the left hemisphere is lower than that in the right
hemisphere in depression patients (14), although the present study did not find
that rCBF increased in the right hemisphere in depression patients
compared with the healthy control subjects. Additionally, numerous
electrophysiology studies confirmed that brain electrical activity
also exhibits an asymmetric pattern. For example, a decreased
pattern was observed in left cerebral electrical activity, and
decreased left hemisphere activity may influence the ability of the
subject to modulate emotion, which is associated with depressive
episodes (15–18). Furthermore, certain studies found that
hypo-neural activity is predominantly exhibited in the left brain
hemisphere and that hyper-neural activity is predominantly
exhibited in the right brain hemisphere in depression patients. For
example, a study using an event related potential technique found
that depression patients exhibited lower activation in the central
and left brain regions (19). Another
study found that electrophysiologic power asymmetry was present in
the depression patients, and that the right brain hemisphere of
depression patients showed higher θ power when compared with the
healthy control subjects (20).
Furthermore, a meta-analysis of depression fMRI studies indicated
that hypo-activity or hypo-connectivity of brain regions was
primarily localized to the left hemisphere (21). Notably, a previous study found that
neural electrical activity alterations were associated with rCBF
alterations (22). Together, these
previous studies and the current findings suggest that a functional
asymmetric alteration pattern is present in depression patients.
Although this hypothesis has no substantial basis and may seem
unlikely, it provides a point of reference for additional studies
of brain function in depression patients.
Cerebral blood perfusion was found to be
significantly decreased in the left brain hemisphere, which is
consistent with the findings of previous studies (23,24).
However, in the present study, cerebral blood hyper-perfusion
tended to be located in the right brain hemisphere, which is
inconsistent with certain previous studies (7,25). Our
current findings and the findings of previous studies indicate a
sophisticated phenomenon; however, this is not surprising, as the
results of the different studies may have been influenced by
various confounding factors, such as differences in clinical
characteristics of the participants, effects of antidepressants on
the participants, and the technical characteristics of image
acquisition and analysis. Given all of these potential confounding
factors, to the best of our knowledge, there are currently no
consistent findings. Accurately addressing the question of
functional brain asymmetry changes in patients with depression
requires a large sample and long-term follow up studies of
drug-naïve first-episode depression patients. These patients must
be enrolled using uniform diagnosis criteria for uniform
neuroimaging acquisition and analysis to compare rCBF measures
between patients and healthy controls. In addition to large-sample
long-term follow up studies, rCBF measures should be compared
before and after treatment to investigate the association between
the alterations of rCBF and the effects of antidepressants. Thus,
improving our understanding of the mechanisms of depression and
establishing an objective predictor of antidepressant effects may
facilitate the development of tailored treatments and personalized
medicine (26).
In conclusion, the rCBF perfusion alterations in
drug-naïve first-episode depression patients displayed an
asymmetric pattern, where decreased cerebral blood perfusion was
predominantly localized to the left hemisphere and increased rCBF
was predominantly localized to the right hemisphere. The altered
rCBF brain regions overlapped with brain centers associated with
emotional processing. Thus, the pCASL technique revealed specific
alteration patterns that are characteristic of rCBF perfusion in
drug-naïve patients with first-episode depression and may be used
as an auxiliary objective indicator for clinical diagnosis.
Acknowledgements
The present study was supported by grants from the
Science and technology fund of Tianjin Health Bureau (grant no.
2013KR03 to Haiman Bian).
References
|
1
|
Saltiel PF and Silvershein DI: Major
depressive disorder: Mechanism-based prescribing for personalized
medicine. Neuropsychiatr Dis Treat. 11:875–888. 2015.PubMed/NCBI
|
|
2
|
Drevets WC: Neuroimaging studies of mood
disorders. Biol Psychiatry. 48:813–829. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ho TC, Wu J, Shin DD, Liu TT, Tapert SF,
Yang G, Connolly CG, Frank GK, Max JE, Wolkowitz O, et al: Altered
cerebral perfusion in executive, affective, and motor networks
during adolescent depression. J Am Acad Child Adolesc Psychiatry.
52:1076–1091.e2. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang DJ, Chen Y, Fernández-Seara MA and
Detre JA: Potentials and challenges for arterial spin labeling in
pharmacological magnetic resonance imaging. J Pharmacol Exp Ther.
337:359–366. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hartkamp NS, Petersen ET, De Vis JB,
Bokkers RP and Hendrikse J: Mapping of cerebral perfusion
territories using territorial arterial spin labeling: Techniques
and clinical application. NMR Biomed. 26:901–912. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Järnum H, Eskildsen SF, Steffensen EG,
Lundbye-Christensen S, Simonsen CW, Thomsen IS, Fründ ET, Théberge
J and Larsson EM: Longitudinal MRI study of cortical thickness,
perfusion, and metabolite levels in major depressive disorder. Acta
Psychiatr Scand. 124:435–446. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ota M, Noda T, Sato N, Hattori K, Teraishi
T, Hori H, Nagashima A, Shimoji K, Higuchi T and Kunugi H:
Characteristic distributions of regional cerebral blood flow
changes in major depressive disorder patients: A pseudo-continuous
arterial spin labeling (pCASL) study. J Affect Disord. 165:59–63.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nobler MS, Olvet KR and Sackeim HA:
Effects of medications on cerebral blood flow in late-life
depression. Curr Psychiatry Rep. 4:51–58. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
First MB, Spitzer RL, Gibbon M and
Williams JB: Structured Clinical Interview for DSM-IV Axis I
Disorders (SCID)Biometrics Research. New York State Psychiatric
Institute; New York, NY: 1996
|
|
10
|
Hamilton M: A rating scale for depression.
J Neurol Neurosurg Psychiatry. 23:56–62. 1960. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Alexopoulos GS, Kiosses DN, Heo M, Murphy
CF, Shanmugham B and Gunning-Dixon F: Executive dysfunction and the
course of geriatric depression. Biol Psychiatry. 58:204–210. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Davies J, Lloyd KR, Jones IK, Barnes A and
Pilowsky LS: Changes in regional cerebral blood flow with
venlafaxine in the treatment of major depression. Am J Psychiatry.
160:374–376. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Galynker II, Cai J, Ongseng F, Finestone
H, Dutta E and Serseni D: Hypofrontality and negative symptoms in
major depressive disorder. J Nucl Med. 39:608–612. 1998.PubMed/NCBI
|
|
14
|
Wang Y, Zhang H, Tang S, Liu X, O'Neil A,
Turner A, Chai F, Chen F and Berk M: Assessing regional cerebral
blood flow in depression using 320-slice computed tomography. PLoS
One. 9:e1077352014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Blackhart GC, Minnix JA and Kline JP: Can
EEG asymmetry patterns predict future development of anxiety and
depression? A preliminary study. Biol Psychol. 72:46–50. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bruder GE, Quitkin FM, Stewart JW, Martin
C, Voglmaier MM and Harrison WM: Cerebral laterality and
depression: Differences in perceptual asymmetry among diagnostic
subtypes. J Abnorm Psychol. 98:177–186. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hannesdóttir DK, Doxie J, Bell MA,
Ollendick TH and Wolfe CD: A longitudinal study of emotion
regulation and anxiety in middle childhood: Associations with
frontal EEG asymmetry in early childhood. Dev Psychobiol.
52:197–204. 2010.PubMed/NCBI
|
|
18
|
Kemp AH, Cooper NJ, Hermens G, Gordon E,
Bryant R and Williams LM: Toward an integrated profile of emotional
intelligence: Introducing a brief measure. J Integr Neurosci.
4:41–61. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu H, Yin HF, Wu DX and Xu SJ:
Event-related potentials in response to emotional words in patients
with major depressive disorder and healthy controls.
Neuropsychobiology. 70:36–43. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Quraan MA, Protzner AB, Daskalakis ZJ,
Giacobbe P, Tang CW, Kennedy SH, Lozano AM and McAndrews MP: EEG
power asymmetry and functional connectivity as a marker of
treatment effectiveness in DBS surgery for depression.
Neuropsychopharmacology. 39:1270–1281. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sundermann B, Olde Lütke Beverborg M and
Pfleiderer B: Toward literature-based feature selection for
diagnostic classification: A meta-analysis of resting-state fMRI in
depression. Front Hum Neurosci. 8:6922014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Moretti DV, Prestia A, Binetti G, Zanetti
O and Frisoni GB: Increase of theta frequency is associated with
reduction in regional cerebral blood flow only in subjects with
mild cognitive impairment with higher upper alpha/low alpha EEG
frequency power ratio. Front Behav Neurosci. 7:1882013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lui S, Parkes LM, Huang X, Zou K, Chan RC,
Yang H, Zou L, Li D, Tang H, Zhang T, et al: Depressive disorders:
Focally altered cerebral perfusion measured with arterial
spin-labeling MR imaging. Radiology. 251:476–484. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Smith DJ and Cavanagh JT: The use of
single photon emission computed tomography in depressive disorders.
Nucl Med Commun. 26:197–203. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vasic N, Wolf ND, Grön G, Sosic-Vasic Z,
Connemann BJ, Sambataro F, von Strombeck A, Lang D, Otte S, Dudek
M, et al: Baseline brain perfusion and brain structure in patients
with major depression: A multimodal magnetic resonance imaging
study. J Psychiatry Neurosci. 40:412–421. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Patrick KS, Corbin TR and Murphy CE:
Ethylphenidate as a selective dopaminergic agonist and
methylphenidate-ethanol transesterification biomarker. J Pharm Sci.
103:3834–3842. 2014. View Article : Google Scholar : PubMed/NCBI
|