Introduction
Asthma is a common chronic respiratory disease,
affecting >300 million individuals worldwide, and its prevalence
is rising, particularly in developing countries (1,2).
Approximately 8.3% of the US population suffers from asthma
(3). Airway hyperresponsiveness (AHR)
leading to excessive narrowing of the airways and, thus, causing
dyspnea is considered to be the primary cause of asthma, though the
mechanisms underlying AHR remain unclear (4). However, it is known that acute narrowing
of the airway lumen is caused by contraction of the airway smooth
muscle cells (ASMCs) (5–7). The counteracting therapy for AHR is,
thus, to relax the ASMCs using bronchodilators. Currently, the most
common bronchodilators are β2-adrenoreceptor agonists;
however, various issues have been raised regarding their use, such
as tachyphylaxis and long-term safety. In addition, these agents
are often ineffective in severe cases of asthma and can cause
serious side effects, including abnormal heart rhythm and increased
blood pressure in the patients (8,9).
A study by Deshpande et al (10) discovered that human ASMCs express
bitter taste receptors (TAS2Rs), and accordingly provide potential
treatment of AHR using bitter substances. It has also been
demonstrated that bitter substances, such as saccharin and
chloroquine, are more effective in comparison with existing
bronchodilators (β agonists) in terms of the extent of drug-induced
relaxation of ASMCs. The relaxation mechanism of ASMCs induced by
bitter substances is still debated; however, it is generally
considered to involve the binding of bitter substance to TAS2Rs on
the membrane of ASMC, causing membrane hyperpolarization and thus
leading to localized intracellular calcium flux, namely
[Ca2+]i, responses (8,10).
An important advantage of using bitter substances as
bronchodilators is that they are vastly available in nature
(8). Among them, the natural product
naringin, a compound extracted from common grapefruit, is of great
interest, as it is an effective bitter substance, and also widely
available and relatively inexpensive. Previous studies have
demonstrated that naringin is a flavanone with various
bioactivities, including anti-inflammatory, expectorant and
antitussive effects on asthma and acute lung injury (11,12).
However, those studies focused on airway inflammation, and the drug
was administered orally. In the present study, we focused on the
relaxation effect of naringin on ASMCs via inhalation, with the aim
to evaluate this natural bitter substance as a potential
bronchodilator for the treatment of AHR in asthma.
In order to study the in vitro and in
vivo effects of naringin on ASMC relaxation, either cultured
ASMCs were directly exposed to naringin solutions, or an ovalbumin
(OVA)-induced asthma model of Balb/c mice was treated with
aerosolized naringin via nasal inhalation. Subsequently, the
traction force and the [Ca2+]i generated by the cultured
ASMCs were measured in the absence or presence of naringin. In
addition, the airway resistance (Rrs) of the mice was evaluated in
response to naringin inhalation.
Materials and methods
Chemicals and reagents
Naringin was purchased from Tokyo Chemical Industry
Co., Ltd. (Tokyo, Japan). Collagen type I, anti-mouse IgG (Fab
specific)-FITC antibody, 4′,6-diamidino-2-phenylindole, Fluo-4, AM,
and Pluronic® F-127 were purchased from Sigma-Aldrich
(St. Louis, MO, USA). Cell culture reagents including Dulbecco's
modified Eagle's medium (DMEM), 10% fetal bovine serum (FBS),
penicillin and streptomycin solution, were obtained from Thermo
Fisher Scientific, Inc. (Waltham, MA, USA), unless stated
otherwise.
Experimental animals
Female Balb/c mice (n=55; weight, 20–22 g; age, 6–8
weeks) were purchased from Cavens Laboratory Animal Co., Ltd.
(Changzhou, China), an authorized supplier of experimental animals
for use in medical research. The animals were maintained in a
specific pathogen-free environment at room temperature 25±3°C,
relative humidity of 40–60%, 12-h light/dark cycle and free access
to food and water. All animal experiments were performed according
to the Institutional Guidelines for Animal Care and Use Committee
of Changzhou University (Changzhou, China). Adequate measures were
taken to minimize the suffering of the experimental animals.
Isolation and in vitro culture of
ASMCs from Balb/c mice
Primary ASMCs were isolated from Balb/c mice and
cultured in vitro, as described previously (13). Briefly, the 6–8-week-old mice were
anesthetized by intraperitoneal injection of pentobarbital sodium
(60 mg/kg). The mice were dissected and the trachea was isolated.
The isolated trachea was cleaned of connective tissues, sliced into
small squares (1×1 mm2), which were allowed to attach to
the bottom of the flask. Subsequently, the tissue samples in the
flask were placed in an incubator at 37°C with humidified air and
5% CO2, until the cells migrated out of the tissue
samples. Subsequently, the ASMCs were isolated according to their
attachment time, and maintained in culture with DMEM containing 10%
FBS for 3–8 passages prior to use. The grown cells were verified as
ASMCs by staining with antibodies against α-smooth muscle actin
[α-SMA (cat. no. BM0002; Wuhan Boster Biological Technology, Ltd.,
Wuhan, China); dilution, 1:1,000], as described in a previous study
(14).
Assessment of traction force generated
by cultured ASMCs
The traction force of cultured ASMCs was measured by
the technique of Fourier transformation traction force microscopy
using polyacrylamide substrate embedded with fluorescent microbead
markers, as described previously (15–18).
Briefly, the cultured cells were transferred to polyacrylamide gel
dishes coated with type I collagen (0.1 mg/ml) at a density of
2,000 cells/dish in DMEM containing 10% FBS and incubated for 12–24
h. After the cells were well adhered to the substrate, they were
washed with phosphate-buffered saline and cultured in serum-free
medium for 12 h prior to further investigation. Subsequently,
control images of a single cell and of the fluorescent microbead
markers were recorded by phase contrast and fluorescence
microscopy, respectively. Increasing concentrations of histamine
[0.01, 0.1 and 1 µM (cat. no. 51-45-6); Aladin Industrial Corp.,
Shanghai, China], which served as a positive control, or naringin
(0.125, 0.25, 0.5 and 1 mM) were then added to cell cultures
sequentially at 3-min intervals, and fluorescent images of the
microbead positions were recorded by fluorescence microscopy every
30 sec. When the treatments were completed, the cells were
trypsinized and the cell-free bead positions were recorded as a
reference point for bead displacement.
Assessment of intracellular
[Ca2+]i in cultured ASMCs
Intracellular calcium signals were visualized as
described previously (19,20), using the membrane permeable
[Ca2+]i-sensitive fluorescent dye Fluo-4 acetoxymethyl
ester (Fluo-4 AM). Briefly, cultured ASMCs were inoculated
(~104 cells per dish) into confocal petri dishes with a
glass bottom. Next, the cells were incubated with 5 µM Fluo-4 AM
for 60 min at 37°C in a 5% CO2 incubator. The cells were
then washed with Tyrode's solution and incubated for 20 min to
allow complete de-esterification of the cytosolic dye. The
fluorescence intensity of the sample (Fluo-4-AM labeled ASMCs) was
measured by laser scanning confocal microscopy (Zeiss LSM710; Carl
Zeiss, Jena, Germany) using an excitation wavelength of 488 nm and
emission wavelength of >505 nm, which represented the influx of
the [Ca2+]i.
OVA-induced asthma model of Balb/c
mice
An asthma model of Balb/c mice was prepared as
described previously (21–25). Briefly, female 6-8-week-old Balb/c mice
were intraperitoneally injected with 100 µg OVA in 0.2 ml aluminium
hydroxide (2%) on days 1 and 8, followed by aerosol challenge with
50 g/l OVA for 20 min every day for 2 weeks. Control animals
received 0.2 ml 0.9% saline solution via intraperitoneal injection
and were challenged with aerosolized 0.9% saline solution on the
same days as the animals in the OVA group. In total of 45 mice were
used in these experiments.
Assessment of bronchial Rrs by force
oscillatory technique
Bronchial Rrs of the mice was measured by a force
oscillatory technique using a computer-controlled FlexiVent system
(SciReq, Montreal, QC, Canada) as described elsewhere (26). Briefly, the mice were anesthetized by
intraperitoneal injection of pentobarbital sodium (100 mg/kg),
intubated and connected to the adapter of the instrument. Next, the
mice were first challenged with the bronchoconstrictor agent
methacholine (Mch) at concentrations of 0, 2, 8, 32 or 64 mg/ml via
inhalation, until the sustained Rrs was approximately 4–5-fold
greater than the baseline value. The mice then inhaled naringin
(15, 30 or 60 µg) or albuterol (3 µg), which was used as a
conventional short acting β2 agonist for comparison.
Subsequently, Rrs was measured using the FlexiVent system every 30
sec after atomization, for a duration of 5 min. The results are
expressed as the percentage of Rrs following treatment with the
respective drug (Mch, Mch + naringin or Mch + albuterol) at each
dose relative to the baseline value.
Statistical analysis
Dose response curves for [Ca2+]i were
analyzed by iterative non-linear least squares fitting. Results
from all studies were compared using one-way analysis of variance
followed by post ad-hoc Student's t-tests for multiple comparisons
using OriginPro 8.5.1 SR2 (OriginLab Corporation, Northampton, MA,
USA), with P<0.05 considered to indicate a statistically
significant difference. Data are presented as the mean ± standard
error.
Results
Immunohistochemical characterization
of the ASMCs cultured in vitro
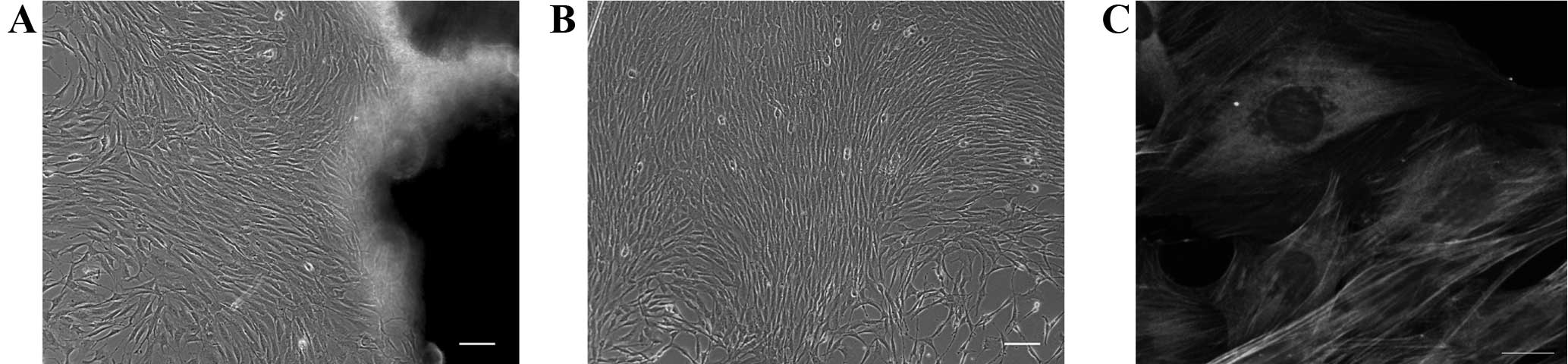
Phase contrast microscopic images showed that the
isolated cells had a long spindle shape and grew collectively into
confluence with a ‘peak-valley’ structure, which are typical
features of ASMCs in culture (Fig. 1A and
B). Subsequent to a few cycles of purification, the cells were
further authenticated by immunofluorescence appraisal method, which
uses the fluorescently labeled α-SMA antibodies to distinguish
ASMCs. The results indicated that the purified cells exhibited
uniform staining of α-SMA with stress fiber structures (Fig. 1C). The purity of ASMCs was confirmed to
be ~99% based on the α-SMA staining.
Effect of naringin on the traction
force generated by the cultured ASMCs
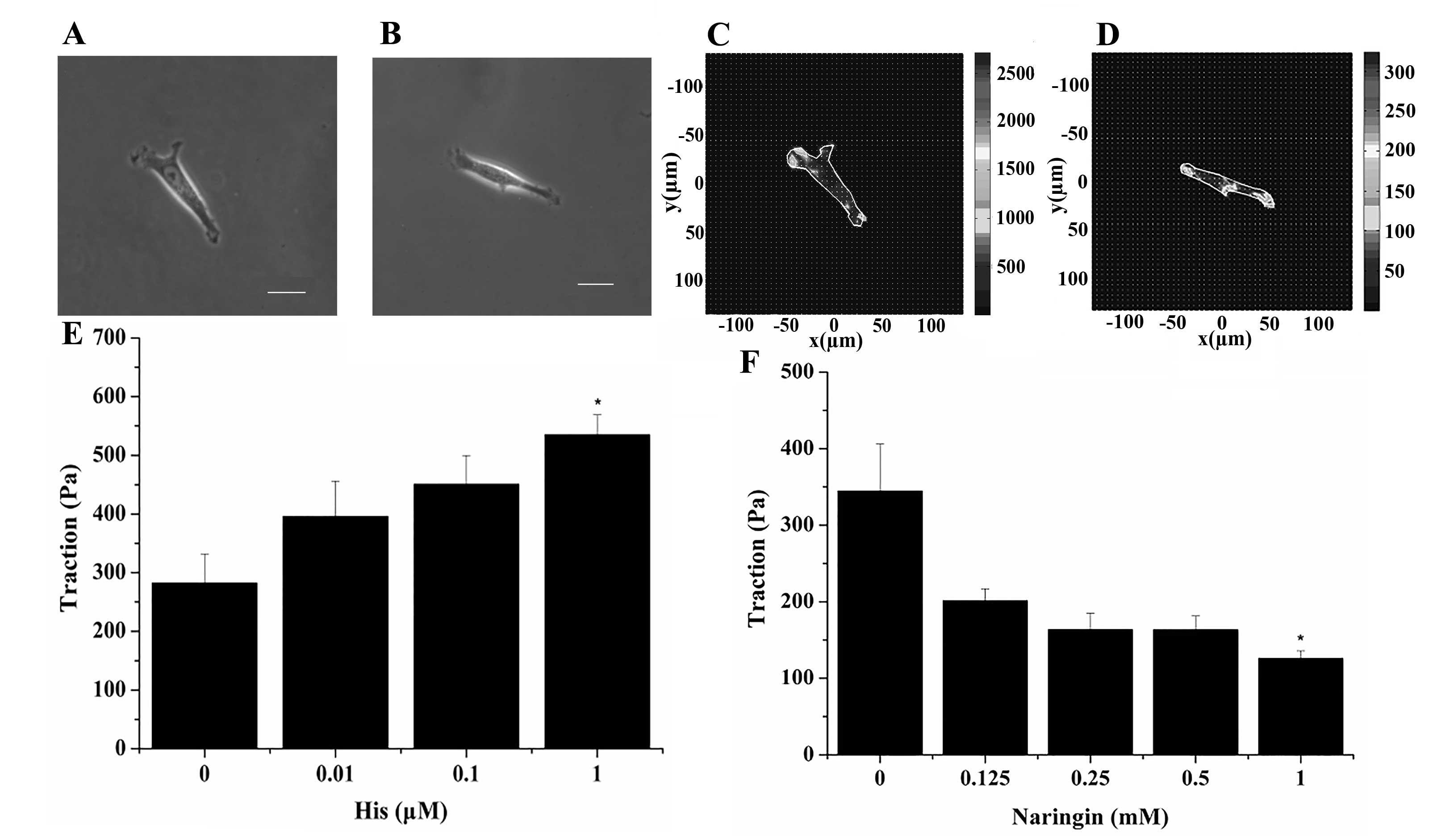
The representative phase contrast images of two
different single ASMCs attached to the surface of the flexible
polyacrylamide gel before treatment are shown in Fig. 2A and B, respectively. Upon stimulation
with histamine (a known contractile agonist of ASMCs), the two
cells exerted traction force on the gel substrate, predominantly at
the two ends of the cell and beneath the nucleus, as shown in
Fig. 2C and D, respectively. However,
the root mean square traction averaged over the entire cell
projected area either increased or decreased progressively when the
cell was exposed to further increasing doses of either histamine
(0.01, 0.1 and 1 µM) or naringin (0.125, 0.25, 0.5 and 1.0 mM),
respectively, as shown in Fig. 2E (the
lower left panel) and F (the lower right panel). Compared with the
baseline traction force at 0 µM histamine or 0 mM naringin, the
traction force generated by the ASMCs was either enhanced or
reduced significantly when the cells were treated with either 1 µM
histamine or 1 mM naringin, respectively (P<0.05).
Effect of naringin on
[Ca2+]i in the cultured ASMCs
Fig. 3 shows
representative fluorescence microscopy images (Fig. 3A) and fluorescence intensity
time-course traces (Fig. 3B) of the
cultured AMSCs in response to treatment with naringin. In the
fluorescence microscopy images, a single cell can be seen
responding to the addition of naringin (1 mM) as the fluorescence
intensity of the calcium ion specific dye (Fluo-4 AM) inside the
cell increased following treatment. Prior to naringin addition (0
sec), the fluorescence was almost invisible, whereas a bright level
of fluorescence was observed at 33 sec after adding naringin
(Fig. 3A). The time-course traces of
the changing overall fluorescence intensity inside the cell further
confirmed the response and the dose-dependent effect of the ASMCs
exposure to naringin (Fig. 3B). In the
absence of naringin, the cell exhibited a stable baseline level
(F0) of the [Ca2+]i, as determined by the
cell's overall fluorescence intensity. When naringin (0, 0.25, 0.5
or 1.0 mM) was added to the culture medium at the time point of 20
sec, the [Ca2+]i of the cell increased rapidly, reaching
a peak value at ~30 sec and then decreasing towards the baseline.
Although the pattern of the transient response was similar in all
cases, the magnitude of the response seemed to be dependent on the
dose of naringin at which the cell was exposed to. After
normalizing to the baseline value (F0) to eliminate
uncertainty due to differences between individual cells and dyeing
processes, naringin at the concentration as low as 0.25 mM was
found to already induce a marked response on the [Ca2+]i
in the cell. As the concentration of naringin increased further to
0.5 and 1.0 mM, the peak value of the [Ca2+]i response
increased progressively and reached ~4-fold of the baseline level
at 1.0 mM.
Characterization of the OVA-induced
asthma model of Balb/c mice
Fig. 4 displays the
test results of Rrs increase (as the % of the baseline value) in
response to Mch stimulation in the normal (control) and OVA-induced
asthma model (OVA) of Balb/c mice. When challenged with increasing
concentration of Mch (0, 2, 8, 32 and 64 mg/ml), the Rrs measured
by the invasive FlexiVent system increased progressively compared
with the corresponding baseline values. However, the increase was
greater in OVA-treated mice than in the control mice, and the
difference of Rrs between the OVA and control groups was
statistically significant (P<0.01; Fig.
4) when the Mch concentration was increased to 8 mg/ml and
higher. The threshold of 4- to 5-fold increase in Rrs is usually
required for the maximum bronchial constriction induced by Mch in
the study of AHR (10). In the present
study, the maximum dose of Mch to reach this threshold was 64 mg/ml
in the control group and 32 mg/ml in the OVA group, as shown in
Fig. 4. The OVA-treated mice also
exhibited other hallmarks of asthma, including airway inflammation
and remodeling (data not shown). These test results verified that
the OVA-treated mice presented the characteristics, particularly
the occurrence of AHR, that are observed in allergic asthma, thus
it was suitable to be used in the evaluation of naringin's efficacy
in the relaxation of ASMCs.
Effect of naringin on Rrs of normal or
OVA-induced asthma Balb/c mice
To evaluate the in vivo effect of naringin on
bronchodilation in asthmatic mice, the Rrs of normal (control) or
OVA-treated (OVA) Balb/c mice was measured. Mice were first
challenged with Mch to induce maximum airway contraction (4–5-fold
increase of Rrs over the baseline value), and then atomized with
naringin (15, 30 or 60 µg) via inhalation. As a positive control,
the mice were atomized with albuterol (3 µg), a common
β2-adrenergic agonist used as a bronchodilator for
treating asthma.
As shown in Fig. 5,
when challenged with Mch at a concentration of 64 mg/ml in the
control and 32 mg/ml in the OVA mice, Rrs in the two groups
increased significantly (P=3.39×10−6 vs. baseline in the
control group; P=8.38×10−7 vs. baseline in the OVA
group) to up to ~5-fold of the baseline value. Following the Mch
challenge, atomization inhalation of albuterol (3 µg) effectively
reduced Rrs (P=3.38×10−5 vs. Mch in the control group;
P=1.65×10−5 vs. Mch in the OVA group) to a considerably
lower level in the control and OVA groups, which was expected since
albuterol is a well-known bronchodilator. More importantly,
atomization inhalation of naringin also caused a reduction of Rrs
in the control and OVA groups in a progressive manner as the
concentration of naringin increased from 15 to 60 µg. Specifically,
inhalation of 3 µg albuterol resulted in a decrease of the Rrs by
35±3.8% in the normal mice and 45±1.8% in the OVA-treated mice,
while inhalation of 60 µg naringin led to a decrease in the Rrs by
38±4.4% in the normal mice and 36±1.4% in the OVA-treated mice.
Further increase of naringin concentration to 60 µg caused
additional decrease of Rrs in the OVA-treated mice, however this
was not observed in the normal group.
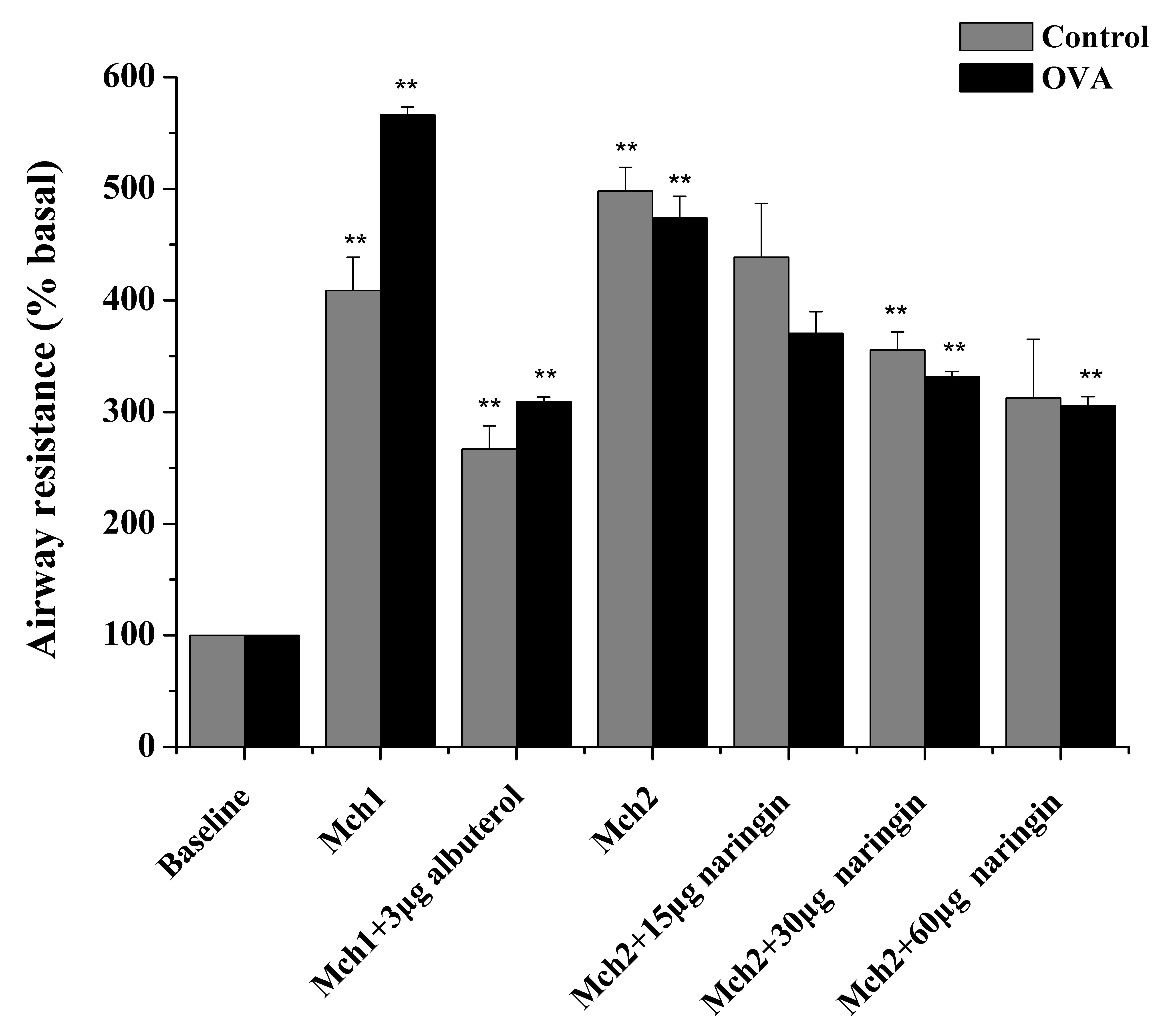 | Figure 5.Effect of naringin on attenuation of
Rrs in Mch-challenged Balb/c mice. Rrs was measured in the normal
(control) or OVA-induced asthma Balb/c mice prior to challenge
(baseline) and following challenge with Mch. To ensure
comparability, the normal and OVA-treated mice were challenged with
64 and 32 mg/ml Mch, respectively, to induce a 4–5-fold increase in
Rrs. Two different groups of Mch-challenged mice, Mch1 and Mch2,
were further treated with albuterol (3 µg) or naringin (15, 30 and
60 µg), respectively. The Rrs was normalized against the baseline
value in each group, and the normalized Rrs of albuterol-treated
group (Mch1 + 3 µg albuterol, control or OVA) was compared to that
of the corresponding Mch1 group. The normalized Rrs of
naringin-treated group (Mch2 + 15/30/60 µg naringin, control and
OVA) was compared to that of the corresponding Mch2 group,
respectively. Data are presented as the mean ± standard error
(n=5–6). **P<0.01 vs. Mch1/Mch2 group. Mch, methacholine; OVA,
ovalbumin; Rrs, airway resistance. |
Discussion
The primary finding of the present study was that
naringin, as a widely available bitter substance and flavanone, was
able to effectively relax ASMCs both in vitro and in
vivo. This effect was manifested by the reduction of traction
force and induction of [Ca2+]i in cultured ASMCs due to
exposure to naringin in solution, and by the attenuation of the
Mch-induced Rrs increase in normal mice and in an OVA-induced
asthma model of Balb/c mice upon inhalation of naringin in aerosol
form. However, the extent of naringin's effect on the relaxation of
the cultured ASMCs or bronchial airways in mice was dependent on
the dose of naringin to which the cultured cells or mice were
exposed. In the cultured ASMCs, the presence of naringin in the
culture medium at increasing concentration up to 1.0 mM caused the
traction force to decrease, and [Ca2+]i to increase
progressively. In the normal and OVA-treated mice, inhalation of
naringin at an increasing dose up to 60 mg caused the
Mch-contracted bronchial airways to dilate progressively, as
measured by the progressive reduction of Rrs. However, the
bronchodilatory effect of naringin appeared to be marginally
greater in the OVA-treated mice compared with that in the normal
mice, suggesting differential efficacy of naringin for dilation of
bronchial airways with or without AHR.
In the present study, Fourier transform traction
cytometry was used to quantify the ability of ASMCs to generate
traction force (equivalent to cell contraction), which has been
widely used as an assay to evaluate contraction/relaxation of ASMCs
during stimulation with a contractile agonist, such as histamine,
or a relaxing agonist, such as isoproterenol (17,18).
Regarding targeting the receptor TAS2R of ASMCs for relaxation, it
has been reported previously that a prototypic TAS2R agonist,
namely quinine hydrochloride, can reduce the average traction force
generated by ASMCs in a dose-dependent manner, which is mediated by
the activation of the four most highly expressing TAS2Rs in human
ASMCs (which are TAS2R4/10/14/31) (10,27). The
current study demonstrated that naringin, a naturally abundant
bitter substance, was also able to increasingly reduce the traction
force generated by ASMCs when the cells were treated with an
increasing dose of naringin (0.125, 0.25, 0.5 and 1.0 mM), thus to
the best of our knowledge, providing for the first time, evidence
that naringin exerts a relaxation effect on airway smooth muscle at
the cell level.
In comparison to other bitter taste compounds,
naringin appears to be remarkably potent in inducing ASMC
relaxation. For instance, at the same concentration of 1 mM,
naringin caused a reduction of ASMC traction force by ~64% (from
350 to 225 Pa), whereas quinine reduced it by only ~31% (from 325
to 225 Pa) (28), while saccharin and
chloroquine reduced cell stiffness (another estimate of ASMC
relaxation) by 40–50% (10). A
previous study observed that bitter substances, such as saccharin
or quinine, induce relaxation of the ASMC by mediating transient
increase of [Ca2+]i in the cell, which can be observed
by fluorescent laser scanning confocal microscopy while using
calcium ion specific dye Fluo-4 AM to label the intracellular
calcium ions (10). In the present
study, we used the same method to elucidate whether naringin also
induces relaxation of ASMCs via mediation of [Ca2+]i in
the cell. Naringin was found to mediate the increase of
[Ca2+]i in the cell as the concentration of naringin
increased from 0.25 to 1.0 mM. The general trend of the
[Ca2+]i response to naringin stimulation was consistent
with the effect induced by other well-known bitter tastants, such
as quinine (28), saccharin and
chloroquine (10). Nevertheless, the
magnitude of [Ca2+]i response to naringin treatment
(2.75-fold change at a concentration of 1 mM) was greater as
compared with that observed for other bitter tastants (1 mM),
including saccharin (1.87-fold) (10),
chloroquine (1.75-fold) (10) and
quinine (0.75-fold) (28), further
demonstrating the potency of naringin as an ASMC relaxant among the
tested bitter taste compounds. Furthermore, the change in
[Ca2+]i was also closely associated with the change in
traction force generation (relaxation) of the ASMCs in response to
naringin stimulation, similar as in the case of ASMCs stimulated by
quinine (2).
TAS2Rs and agonists are known to be coupled to evoke
[Ca2+]i signal in specialized taste cells of the tongue,
and this signal is also found in the functioning of known
bronchoconstrictive G protein coupled receptors (GPCRs), such as
those for histamine, acetylcholine and bradykinin (29). According to this effect, it can be
assumed that bitter agonists would cause bronchoconstriction, as
opposed to the experimental results so far indicating that all
bitter agonists are bronchodilators. This seeming contradiction is
resolved as further studies have revealed the difference between
the [Ca2+]i signaling in response to GPCR agonist or
TAS2R agonist. GPCR agonists, such as histamine, mediate
[Ca2+]i increase throughout the entire cell by membrane
depolarization and thus induce contraction of the ASMCs. By
contrast, TAS2R agonists, such as quinine hydrochloride, mediate a
localized [Ca2+]i response at the cell membrane, which
expands large-conductance Ca2+-activated K+
channels, and thus leads to membrane hyperpolarization and
relaxation of the ASMC (10,30–33). Based
on these previous studies and the similarity between the bitter
tastants, it may be reasonable to attribute the naringin-induced
relaxation of ASMCs in the current study to the cell membrane
hyperpolarization due to localized [Ca2+]i response,
although direct evidence of this underlying mechanism is yet to be
seen in future studies.
In addition to the relaxation effect of naringin on
ASMCs cultured in vitro, we further evaluated the
bronchodilatory effect of naringin on mice since the in vivo
results would be much more relevant to the requirement of clinical
treatment. For this purpose, normal or OVA-induced asthma Balb/c
mice were used. The establishment of an asthma model in the mice
was characterized and verified by presentation of the cardinal
hallmarks associated with allergic asthma, including AHR, as
measured by the excessive increase in Rrs upon Mch stimulation. The
results indicated that atomization inhalation of naringin (15, 30
and 60 µg) attenuated the Mch-induced increase of Rrs in a
dose-dependent manner in the normal and OVA-treated mice.
Furthermore, the naringin-induced bronchodilation in the
OVA-treated mice continued to increase until the dose reached 60
mg, whereas that in the normal mice had approached the maximum
effect at the dose of 30 mg. A previous study by Deshpande et
al (10) identified that inhaled
aerosolized quinine (150 µg) decreased Rrs from the Mch-challenged
level in normal and OVA-treated mice by 53 and 50%, respectively.
In the present study, inhaled aerosolized naringin (60 µg)
decreased Rrs from the Mch-challenged level in normal and
OVA-treated mice by 38 and 36%, respectively (Fig. 5). In both the present and previous
study, the bitter tastant (quinine or naringin) caused similar
extents of airway relaxation in normal and sensitized mice,
suggesting that the expression of TAS2Rs may be regulated during
disease progression.
It should be noted that the maximum dose of naringin
used in the current study was limited to 60 µg due to its
solubility. Therefore, the difference in airway relaxation extent
of the mice exposed to quinine (28)
and naringin does not mean that quinine was more potent compared
with naringin, since the two compounds were delivered at different
doses (150 vs. 60 µg, respectively). On the contrary, it was
demonstrated that in vitro and at the same dose of 1 mM,
naringin was more potent in inducing relaxation of cultured ASMCs
when compared with other known bitter tastants, including quinine,
saccharin and chloroquine, as discussed earlier. It is also noted
that naringin caused in vivo airway relaxation with an
extent that was 68% of that caused by 150 µg quinine, although the
naringin dose was only 40% of the 150 µg quinine (28). This indicates that naringin may be more
potent than quinine in inducing in vivo airway relaxation if
delivered at the same dose.
It is also important to note that naringin and
quinine are two bitter taste compounds with similar features of the
chemical structure. Specifically, they both have aromatic rings and
hydroxyl groups, which may act on the same type of TAS2Rs and the
associated signal pathways (27,34).
Therefore, it is not surprising that inhalation of naringin at the
dose of 60 µg and quinine at 150 µg (10) caused bronchodilation to a similar
extent. However, such level of bronchodilation by the bitter
tastants (60 mg naringin; 150 mg quinine) was equivalent to that
caused by 3 µg albuterol, which is a compound commonly used in
clinical treatment. This comparison may suggest that naringin, as
well as quinine, may be one order of magnitude less potent compared
to albuterol as bronchodilators. However, β2-adrenergic
agonists, like albuterol, are known to be associated with several
side effects, such as tachyphylaxis, individual variations in
responsiveness and safety concerns, which makes them less effective
for long-term treatment. One should also consider that there are
thousands of known bitter substances readily available for
screening as potential bronchodilators. Among them, there are
possibly certain compounds that can stimulate TAS2Rs at extremely
low concentrations, and therefore it is likely that highly potent
bronchodilators will be identified in the future by following the
same strategy as in the present study. At the same time, two recent
publications have investigated the therapeutic potential of
naringin in treating asthma (35,36). These
two studies have mainly evaluated the effect of naringin on the
alleviation of asthma symptoms, including airway inflammation,
coughing and AHR, from the phenomenological point of view using
in vivo animal studies (35,36). While
confirming the effect of naringin on airway inflammation and AHR,
the present study further elucidated the underlying mechanisms
through which naringin reduces AHR particularly from ASMC
biomechanics aspect, using both an in vivo animal model and
in vitro cultured cells. Finally, it is worth mentioning
that the current study was limited to the assessment of only the
traction force in cultured ASMCs and Rrs in Balb/c mice. In
addition, small numbers of sample populations, unoptimized drug
formulation and delivery, and a selective animal model of asthma
were used in the present study. These limitations may raise
questions regarding the real potential and value of naringin in the
clinical treatment of human asthma, and therefore the current
findings need to be further investigated in vitro and in
vivo with human cells and tissues in the future.
In conclusion, the present study demonstrated that
naringin, one of the most common TAS2R agonists, was able to in
vitro relax ASMCs in culture and in vivo dilate
contracted bronchial airways in mice. These results confirm that
this TAS2R may be a novel target for bronchodilation in obstructive
airway diseases, such as asthma. More importantly, it was verified
that naringin is a promising drug candidate of effective, safe and
inexpensive bronchodilator for asthma therapy, at least in the
current experimental settings. Finally, such evaluation can also be
extended to screen the vast bank of natural bitter substances,
which may lead to the discovery of more potent bronchodilators.
Acknowledgements
The authors would like to thank Dr Wenxian Gu
(Changzhou No. 2 People's Hospital, Changzhou, China) for their
assistance in examination of the mouse lung pathology and
histology. This study was supported by the National Natural Science
Foundation of China (grant nos. 11532003, 11402037 and 11172340),
the Office for Talent Recruitment of Jiangsu Province, China
(Double Talent Plan grant no. SRCB-2012-39), and the Bureau of
Science and Technology of Changzhou Municipality, Jiangsu Province,
China (Key Laboratory grant no. CM20133005).
References
|
1
|
Alotaibi, GhaziAbdulrahman: Asthma control
and self-management: The role of asthma education. Saudi J Health
Sci. 4:16–22. 2015. View Article : Google Scholar
|
|
2
|
Al Frayh AR, Shakoor Z, Gad El Rab MO and
Hasnain SM: Increased prevalence of asthma in Saudi Arabia. Ann
Allergy Asthma Immunol. 86:292–296. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Centers for Disease Control and Prevention
(CDC), . 2012 National Health Interview Survey (NHIS) Data.
http://www.cdc.gov/asthma/nhis/2012/data.htm
|
|
4
|
Albazzaz MK and Patel KR: Effect of
azelastine on bronchoconstriction induced by histamine and
leukotriene C4 in patients with extrinsic asthma. Thorax.
43:306–311. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fredberg JJ: Bronchospasm and its
biophysical basis in airway smooth muscle. Respir Res. 5:22004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hershenson MB, Brown M, Camoretti-Mercado
B and Solway J: Airway smooth muscle in asthma. Annu Rev Pathol.
3:523–555. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
King GG, Paré PD and Seow CY: The
mechanics of exaggerated airway narrowing in asthma: The role of
smooth muscle. Respir Physiol. 118:1–13. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Weaver J: How bitter medicine could clear
up asthma. PLoS Biol. 11:e10015002013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Grassin-Delyle S, Abrial C, Fayad-Kobeissi
S, Brollo M, Faisy C, Alvarez JC, Naline E and Devillier P: The
expression and relaxant effect of bitter taste receptors in human
bronchi. Respir Res. 14:1342013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Deshpande DA, Wang WC, McIlmoyle EL,
Robinett KS, Schillinger RM, An SS, Sham JS and Liggett SB: Bitter
taste receptors on airway smooth muscle bronchodilate by localized
calcium signaling and reverse obstruction. Nat Med. 16:1299–1304.
2010. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xie R, Wen S, Li Y, Zuo C and Zhang J:
Study on the antiinflammation and analgesia of naringin. J Hunan
Normal Univ (Med Sci). 85–8. (12)2011.
|
|
12
|
Luo YL, Zhang CC, Li PB, Nie YC, Wu H,
Shen JG and Su WW: Naringin attenuates enhanced cough, airway
hyperresponsiveness and airway inflammation in a guinea pig model
of chronic bronchitis induced by cigarette smoke. Int
Immunopharmacol. 13:301–307. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hirst SJ: Airway smooth muscle cell
culture: Application to studies of airway wall remodelling and
phenotype plasticity in asthma. Eur Respir J. 9:808–820. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Durand-Arczynska W, Marmy N and Durand J:
Caldesmon, calponin and alpha-smooth muscle actin expression in
subcultured smooth muscle cells from human airways. Histochemistry.
100:465–471. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dembo M and Wang YL: Stresses at the
cell-to-substrate interface during locomotion of fibroblasts.
Biophys J. 76:2307–2316. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pelham RJ Jr and Wang Y: Cell locomotion
and focal adhesions are regulated by substrate flexibility. Proc
Natl Acad Sci USA. 94:13661–13665. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang N, Tolić-Nørrelykke IM, Chen J,
Mijailovich SM, Butler JP, Fredberg JJ and Stamenović D: Cell
prestress. I. Stiffness and prestress are closely associated in
adherent contractile cells. Am J Physiol Cell Physiol.
282:C606–C616. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tolić-Nørrelykke IM, Butler JP, Chen J and
Wang N: Spatial and temporal traction response in human airway
smooth muscle cells. Am J Physiol Cell Physiol. 283:C1254–C1266.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang XR, Lin MJ, Yip KP, Jeyakumar LH,
Fleischer S, Leung GP and Sham JS: Multiple ryanodine receptor
subtypes and heterogeneous ryanodine receptor-gated Ca2+ stores in
pulmonary arterial smooth muscle cells. Am J Physiol Lung Cell Mol
Physiol. 289:L338–L348. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Remillard CV, Zhang WM, Shimoda LA and
Sham JSK: Physiological properties and functions of Ca(2+) sparks
in rat intrapulmonary arterial smooth muscle cells. Am J Physiol
Lung Cell Mol Physiol. 283:L433–L444. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen H, Cheng S, Wang A, Bunjhoo H, Cao Y,
Xie J, Wang C, Xu Y and Xiong W: IL-21 does not involve in
OVA-induced airway remodeling and chronic airway inflammation. Int
J Clin Exp Med. 8:10640–10645. 2015.PubMed/NCBI
|
|
22
|
Zhang CH, Li Y, Zhao W, Lifshitz LM, Li H,
Harfe BD, Zhu MS and ZhuGe R: The transmembrane protein 16A
Ca(2+)-activated Cl- channel in airway smooth muscle contributes to
airway hyperresponsiveness. Am J Respir Crit Care Med. 187:374–381.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jie Z, Jin M, Cai Y, Bai C, Shen Y, Yuan
Z, Hu Y and Holgate S: The effects of Th2 cytokines on the
expression of ADAM33 in allergen-induced chronic airway
inflammation. Respir Physiol Neurobiol. 168:289–294. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Song A, Liao Q, Li J, Lin F, Liu E, Jiang
X and Deng L: Chronic exposure to sulfur dioxide enhances airway
hyperresponsiveness only in ovalbumin-sensitized rats. Toxicol
Lett. 214:320–327. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang YJ, Wang YN, Ding YJ, He LY, Liu X
and Kang QZ: Establishment of bronchial asthma model induced with
OVA. Henan Medical Research. 21:268–270. 2012.
|
|
26
|
Jonasson S, Hjoberg J, Hedenstierna G and
Basu S: Allergen-induced formation of F2-isoprostanes in a murine
asthma model identifies oxidative stress in acute airway
inflammation in vivo. Prostaglandins Leukot Essent Fatty Acids.
80:1–7. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Meyerhof W, Batram C, Kuhn C, Brockhoff A,
Chudoba E, Bufe B, Appendino G and Behrens M: The molecular
receptive ranges of human TAS2R bitter taste receptors. Chem
Senses. 35:157–170. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zeng HL, Wang Y, Luo MZ, Shi XH, Lu Y, Pan
Y and Deng LH: Bitter taste receptor agonist (Quinine) induces
traction force reduction and calcium flux increase in airway smooth
muscle cells from ovalbumin-sensitized and challenged rats. J Adv
Biomed Eng Technol. 2:20–27. 2015. View Article : Google Scholar
|
|
29
|
Billington CK and Penn RB: Signaling and
regulation of G protein-coupled receptors in airway smooth muscle.
Respir Res. 4:22003. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pulkkinen V, Manson ML, Säfholm J, Adner M
and Dahlén SE: The bitter taste receptor (TAS2R) agonists
denatonium and chloroquine display distinct patterns of relaxation
of the guinea pig trachea. Am J Physiol Lung Cell Mol Physiol.
303:L956–L966. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
An SS, Wang WC, Koziol-White CJ, Ahn K,
Lee DY, Kurten RC, Panettieri RA Jr and Liggett SB: TAS2R
activation promotes airway smooth muscle relaxation despite
β(2)-adrenergic receptor tachyphylaxis. Am J Physiol Lung Cell Mol
Physiol. 303:L304–L311. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Townsend EA, Yim PD, Gallos G and Emala
CW: Can we find better bronchodilators to relieve asthma symptoms?
J Allergy (Cairo). 2012.3219492012.PubMed/NCBI
|
|
33
|
Clifford RL and Knox AJ: Future
bronchodilator therapy: A bitter pill to swallow? Am J Physiol Lung
Cell Mol Physiol. 303:L953–L955. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Behrens M, Brockhoff A, Kuhn C, Bufe B,
Winnig M and Meyerhof W: The human taste receptor hTAS2R14 responds
to a variety of different bitter compounds. Biochem Biophys Res
Commun. 319:479–485. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Guihua X, Shuyin L, Jinliang G and Wang S:
Naringin protects ovalbumin-induced airway inflammation in a mouse
model of asthma. Inflammation. 39:891–899. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jiao HY, Su WW, Li PB, Liao Y, Zhou Q, Zhu
N and He LL: Therapeutic effects of naringin in a guinea pig model
of ovalbumin-induced cough-variant asthma. Pulm Pharmacol Ther.
33:59–65. 2015. View Article : Google Scholar : PubMed/NCBI
|