Introduction
Sinus histiocytosis with massive lymphadenopathy
(SHML) or Rosai-Dorfam disease is a rare self-limited and benign
disease that was first described in 1969 (1). The clinical features in the classical
form include painless enlargement of cervical lymph nodes, fever,
leukocytes, anemia, hypergammaglobulinemia and elevated erythrocyte
sedimentation (2). Frequently other
lymph nodes can be involved such as axillary, paraaortic,
mediastinal, inguinal (3) and
concurrent extranodal disease may be evident. Extranodal disease
has a particular predilection for the head and neck region (75% of
cases) (4). Involvement of ≥1
extranodal site has been identified in 43% of cases and only 23%
have extranodal disease exclusively (1,5). Documented
sites of extranodal involvement include skin, respiratory tract,
bone, genitourinary system, oral cavity, central nervous system,
eyes and ocular adnexa, salivary gland, tonsil, breast, soft tissue
and heart (6–9). This condition can occur at any age,
albeit 80% of the patients are aged 20 years or younger at onset,
with a higher prevalence in males (8,10,11).
The etiology of the disease is unknown but several
theories have been suggested. Some infectious agents have been
suspected, includig Epstein-Barr virus (12), Parvovirus B19 (13), Herpes virus type 6 and 8 (14,15) and
Polyomavirus (16). A relationship
with Klebsiella, Brucella and Cytomegalovirus was
also suggested, but any attempt to isolate the organisms
consistently failed (12). Other
proposed mechanisms include immune dysfunction or an aberrant
exaggerated immune response to an infectious agent or an antigen
that causes a proliferation of histiocytes (6). Stimulation of monocytes/macrophage via
macrophage colony-stimulating factor was also involved (17). These mechanisms suggest an immune
misrregulation (18). In addition,
10–12% of patients with SHML exhibit autoimmune phenomena (19,20).
The classical histology of this entity is
characterized by distorted nodular architecture with marked
dilation of lymphatic sinuses, partial effacement of follicles and
germinal centers, as well as capsular and pericapsular fibrosis
(1). Lymphatic sinuses are occupied by
numerous lymphocytes and histiocytes with vesicular nucleus and
abundant clear cytoplasm with phagocytized lymphocytes or plasma
cells, also known as ‘emperipolesis’ (5,6,21).
Immunohistochemical analysis revealed the cells were
positive for protein S-100, but typically negative for CD1a. These
cells also expressed α-1-antitrypsine and other pan-macrophage
antigens (CD68 and HAM56) (22). The
cytological characteristics of SHML are highly distinctive.
Consequently, fine needle aspiration (FNA) biopsy may be sufficient
to make the diagnosis in most cases thus preventing unnecessary
invasive procedures (5,6,21,22).
Case presentation
In the current study, we present a 61-year-old
Hispanic (Mexican) male patient seen on the Internal Medicine
consult with a 9-month history of low-grade fever and painless
bilateral cervical masses. On physical examination we found
bilateral cervical and right supraclavicular adenopathy accompanied
by an enlargement of the two parotid glands (Fig. 1). Laboratory exams showed anemia and
high erythrocyte sedimentation rate. As the initial suspected
diagnosis was a probable lymphoma a FNA biopsy was performed on a
cervical node and parotid gland.
The patient's samples were stained to describe
morphologic differences by Papanicolaou (Pap) and Diff-Quik stain
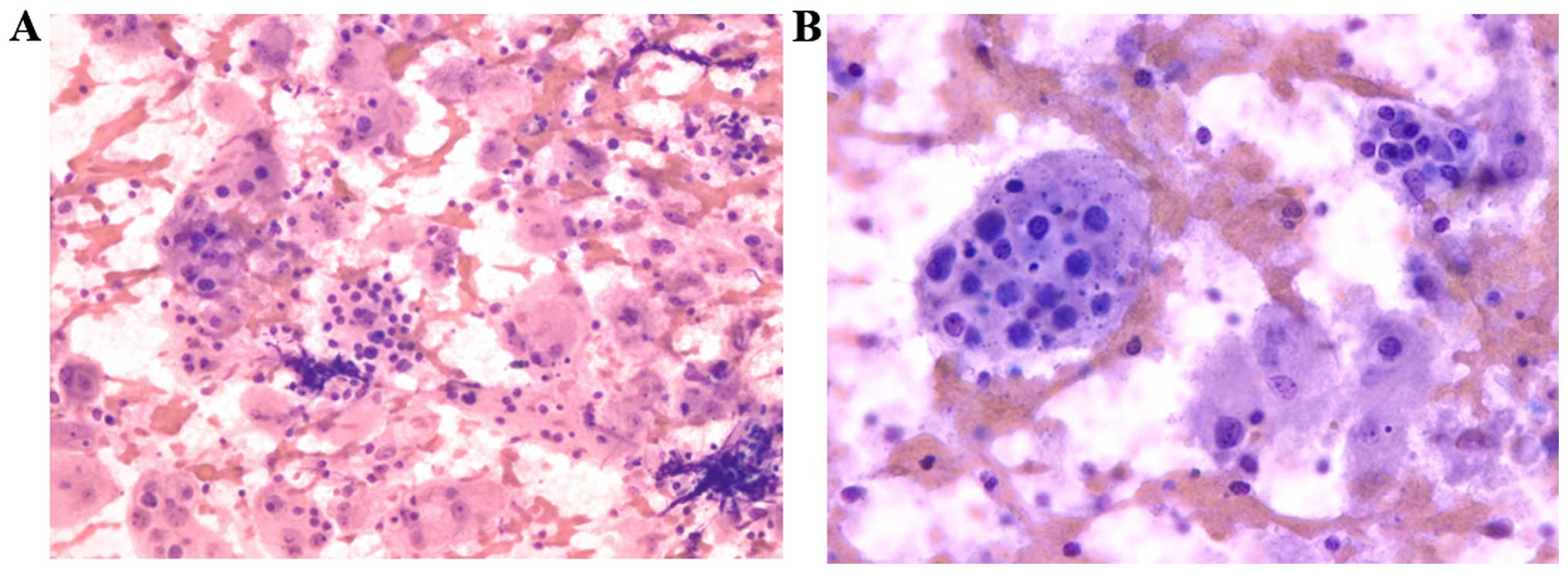
techniques. The microscopic examination revealed a highly cellular
sample with abundant histiocytes with large eosinophilic cytoplasm,
in a reactive lymphocytic background, made up of lymphocytes,
plasma cells, and few eosinophils and neutrophils. The cytoplasm of
these histiocytes has numerous intact lymphocytes and plasma cells
(Figs. 2 and 3). These findings were constant on the
parotid gland and node. Finally, we evaluated a cellular block
stained with hematoxylin and eosin and observed the classic
histopathological charateristics of this disease as, distorted node
architecture with marked dilation of sinuses and partial effacement
of follicles and germinal centers. The sinuses are occupied by
numerous histiocytes with a vesicular nucleus and abundant clear
cytoplasm with phagocytized intact lymphocytes, known as
‘emperopolesis’ (Fig. 4). Following
immunohistochemical analysis, the cells were found to be positive
for CD68 and negative for CD1a.
Discussion
As in histology, cytology from FNA biopsy is usually
highly cellular, with numerous histiocytes with vesicular nucleus
and abundant clear cytoplasm with fine vacuoles and
lymphophagocytosis in a reactive background of lymphocytes, plasma
cells and occasionally neutrophils (5,10,23). Lymphatic sinuses are occupied by
numerous lymphocytes and histiocytes with vesicular nucleus and
abundant clear cytoplasm with phagocytized lymphocytes, neutrophils
or plasma cells, also known as ‘emperopolesis’ (5,6).
The chromatin of the histiocytes was satisfactory
and evenly distributed, although the nuclear shapes varied from
round to extremely bizarre configurations. The nucleoli are usually
not prominent (24). Occasionally,
atypical morphology may be seen and, when present, it can lead to a
misdiagnosis of malignancy (25).
Large binucleated and multinucleated forms were also present
(21). Immunohistochemistry revealed
the cells were positive for protein S-100, α-1-antitrypsine and
pan-macrophage antigens (CD68 and HAM56), but typically negative
for CD1a (6,22).
Although the cytomorphological features may be well
defined, diagnosis of SHML can be difficult to make, particularly
in extranodal sites (23). Shi et
al (5) reviewed 49 cases of
Rosai-Dorfman disease diagnosed with FNA cytology, and found a
significant misdiagnosis of SHML by FNA more often in extranodal
rather than in nodal disease: 12% (3 out of 25) misdiagnosed cases
in lymph node aspirations vs. 50% (6 out of 12) misdiagnosed or
inconclusive cases in extranodal aspirations. In addition,
diagnosis requires correlation with an appropriate clinical
history.
In our case SHML is likely to be mistaken for
lymphoma because of its typical presentation as a painless and
bilateral cervical lymphadenopathy accompanied by non-specific
signs of immune activation, occasional fever, neutrophilia and a
high erythrocyte sedimentation rate. Therefore, SHML should be
considered in the differential diagnosis of painless, unilateral or
bilateral cervical lymphadenopathy, usually of marked size
(10).
The differential cytological diagnosis includes
reactive lymph node hyperplasia, infectious lymphadenitis,
hemophagocytic syndrome, Langerhans cell histiocytosis,
tuberculosis, and lymphoma (23–26). The
main differential diagnoses are summarized in Table I. In the lymph node reactive
hyperplasia there are sinusoidal hyperplasia with loose clusters of
histiocytes, accompanying lymphocytes, germinal center cells,
immunoblasts, and tingible body macrophages; however cytology
usually does not show extensive emperipolesis while protein S-100
is negative. Techniques such as Pap and Diff-Quik staining, allow
us to observe cellular differentiation between normal and squamous
cells, as well as various features. In the case of SHML, techniques
last mentioned help improve the cytological characterization.
 | Table I.Common differential diagnoses for
Rosai-Dorfman disease and their cytologic characteristics compared
against other similar diseases. |
Table I.
Common differential diagnoses for
Rosai-Dorfman disease and their cytologic characteristics compared
against other similar diseases.
| Entity/Disease | Cytology |
Immunohistochemistry | Clinical
features |
|---|
| Rosai Dorfman
disease | Histiocytes with
vesicular nucleus and abundant clear cytoplasm with fine vacuoles
and phagocytosed lymphocytes and a reactive background with
abundant lymphocytes, plasma cells and occasionally
neutrophils | S-100 and CD68
expression Negative for CD1a | Affects children and
young adults. More common in males. Big but painless adenopathies.
They can present in extranodal sites, usually head and neck |
| Sinusal hyperplasia
of lymph node | Extended neutrophils,
histiocytes can or cannot be present Phagocytosed lymphhocytes or
emperipolesis are not common | Sinusoidal
histiocytes negative for S-100 | Malaise and general
symptoms, painless adenopathies Self-limited disease |
| Langerhans cell
histiocytosis | Polymorphic
infiltrate with eosinophils and histiocytes with cleaved
nucleus | CD1a positive | Variable clinical
picture with single or multiple lesions or disseminated disease.
Nodal involvement may be the sole manifestation of disease or it
can be associated with systemic disease |
| Hemophagocytic
lymphohistiocytosis | Benign histiocytes
engulfing erythrocytes and platelets | CD68 positive | Associated to
malignacy, mainly of hemathological origin and to infections.
Manifested by multiple organ failure, pancytopenia, and
hepatosplenomegaly |
| Non-Hodgkin
lymphoma | Monomorphic
population of lymphoid cells | Variable depending on
cell line | Adenopathies. Weight
loss and general symptoms can or cannot be present may or may not
have general symptoms |
| Hodgkin lymphoma | Polymorphic
background with small lymphocytes and eosinophils with the presence
of Reed-Sternberg cells | Reed-Sternberg cells
positive for CD15 and CD30 | Adenopathies and B
symptoms |
Mallick et al (24) carried out a cytomorphological and
morphometric analysis of 22 cases of SHML, and the authors
quantified and compared the cell dimensions and nuclear dimensions
of SHML histiocytes with those in the reactive lymph nodes.
Morphometric parameters show the mean nuclear diameter of the SHML
histiocytes was 16.7 µm compared with the diameter of reactive
histiocytes at 10.1 µm, which was statistically significant
(P<0.01). The median nuclear area in SHML histiocytes was 163.4
µm2 and in reactive histiocytes it was 66.14
µm2, which was statistically significant (P<0.001).
SHML histiocytes were also significantly greater in size
(P<0.001) than those in the reactive lymph nodes (24).
Hemophagocytic syndromes should be differentiated
from Rosai-Dorfman disease on the basis of the presence of
hemophagocytosis and absence of emperipolesis. This syndrome has a
high association with hemathopoyetic malignancy and infectious
processes and it is presented as systemic failure, frequently with
pancytopenia and hepatosplenomegaly. Under microscopic examination,
the most relevant, is the phagocytosis of red cells by histiocytes
(6,27).
In Langerhans cell histiocytosis, Langerhans cells have grooved and
twisted nuclei and the background has eosinophilic microabscess.
Langerhans cells also express CD1a (28).
Following microscopy, we identified tuberculous
lymphadenitis and other granulomatous lymphadenitis with cohesive
aggregates of epithelioid histiocytes, frequently with associated
necrosis but with a lack of phagocytized lymphocytes. Aggregates of
epithelioid histiocytes were absent in Rosai-Dorfman disease
(26,29). Smears from patients with Hodgkin
lymphoma show lymphocytes, plasma cells, histiocytes, eosinophils,
and Reed-Sternberg cells (30). In
non-Hodgkin lymphoma the most important cytological feature is the
monomorphic population of lymphoid cells (31). Additionally, none of the previously
described conditions is characterized by a prominent emperipolesis,
as was observed in SHML.
Most patients with SHML have spontaneous remission
and some can recur or have persistent disease with asymptomatic but
persistent lymphadenopathy. In very few cases it progresses to an
aggressive tumor and can be fatal. Involvement of extranodal sites
generally carries a poorer prognosis, and disease tends to be
chronic and relapsing (2,10). A poorer prognosis has been associated
with dissemination and involvement of kidney, upper respiratory
airway, liver and patients with underlying immune abnormalities or
anemia (20). Death occurs in
approximately 7% of patients, linked to a possible defect in the
immune system (32).
An ideal treatment for SHML remains to be
identified; nevertheless, approximately 50% of the patients require
treatment. The management options range from observation for those
patients with mild manifestations with no functional or cosmetic
abnormalities to surgical resection, systemic steroids and in some
cases chemo- or radiotherapy for patients with severe symptoms, as
well as compromise of vital organs (33,34).
In conclusion, the cytological features of SHML are
distinctive in the correct clinical context, whereby biopsy with
FNA may be sufficient for diagnosis in most cases, thus preventing
unnecessary invasive approaches. Surgical resection for
histological diagnosis should be considered in cases with
inconclusive cytological findings, or unusual clinical
presentation.
Acknowledgements
The authors acknowledge the critical reading of the
manuscript by Dr Sergio Lozano.
References
|
1
|
Foucar E, Rosai J and Dorfman R: Sinus
histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease):
Review of the entity. Semin Diagn Pathol. 7:19–73. 1990.PubMed/NCBI
|
|
2
|
Gaitonde S: Multifocal, extranodal sinus
histiocytosis with massive lymphadenopathy: An overview. Arch
Pathol Lab Med. 131:1117–1121. 2007.PubMed/NCBI
|
|
3
|
Najafi-Sani M, Saneian H and Mahjoub F:
Rosai-Dorfman disease with nodal and extranodal involvements: A
case report. J Res Med Sci. 16:1251–1256. 2011.PubMed/NCBI
|
|
4
|
Cocker RS, Kang J and Kahn LB:
Rosai-Dorfman disease. Report of a case presenting as a midline
thyroid mass. Arch Pathol Lab Med. 127:e197–e200. 2003.PubMed/NCBI
|
|
5
|
Shi Y, Griffin AC, Zhang PJ, Palmer JN and
Gupta P: Sinus histiocytosis with massive lymphadenopathy
(Rosai-Dorfman Disease): A case report and review of 49 cases with
fine needle aspiration cytology. Cytojournal. 8:32011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kumar B, Karki S and Paudyal P: Diagnosis
of sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman
disease) by fine needle aspiration cytology. Diagn Cytopathol.
36:691–695. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vemuganti GK, Naik MN and Honavar SG:
Rosai Dorfman disease of the orbit. J Hematol Oncol. 1:72008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kala C, Agarwal A and Kala S: Extranodal
manifestation of Rosai-Dorfman disease with bilateral ocular
involvement. J Cytol. 28:131–133. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sandoval-Sus JD, Sandoval-Leon AC, Chapman
JR, Velazquez-Vega J, Borja MJ, Rosenberg S, Lossos A and Lossos
IS: Rosai-Dorfman disease of the central nervous system: Report of
6 cases and review of the literature. Medicine (Baltimore).
93:165–175. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ruggiero A, Attinà G, Maurizi P, Mulè A,
Tarquini E, Barone G, Lazzareschi I and Riccardi R: Rosai-Dorfman
disease: Two case reports and diagnostic role of fine-needle
aspiration cytology. J Pediatr Hematol Oncol. 28:103–106. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jani PA and Banjan D: A case of sinus
histiocytosis with massive lymphadenopathy (Rosai-Dorfman syndrome)
from western India. Mcgill J Med. 11:156–159. 2008.PubMed/NCBI
|
|
12
|
Tsang WY, Yip TT and Chan JK: The
Rosai-Dorfman disease histiocytes are not infected by Epstein-Barr
virus. Histopathology. 25:88–90. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mehraein Y, Wagner M, Remberger K, Füzesi
L, Middel P, Kaptur S, Schmitt K and Meese E: Parvovirus B19
detected in Rosai-Dorfman disease in nodal and extranodal
manifestations. J Clin Pathol. 59:1320–1326. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Arakaki N, Gallo G, Majluf R, Diez B,
Arias E, Riudavets MA and Sevlever G: Extranodal rosai-dorfman
disease presenting as a solitary mass with human herpesvirus 6
detection in a pediatric patient. Pediatr Dev Pathol. 15:324–328.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ortonne N, Fillet AM, Kosuge H, Bagot M,
Frances C and Wechsler J: Cutaneous Destombes-Rosai-Dorfman
disease: Absence of detection of HHV-6 and HHV-8 in skin. J Cutan
Pathol. 29:113–118. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Al-Daraji W, Anandan A, Klassen-Fischer M,
Auerbach A, Marwaha JS and Fanburg-Smith JC: Soft tissue
Rosai-Dorfman disease: 29 new lesions in 18 patients, with
detection of polyomavirus antigen in 3 abdominal cases. Ann Diagn
Pathol. 14:309–316. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Middel P, Hemmerlein B, Fayyazi A, Kaboth
U and Radzun HJ: Sinus histiocytosis with massive lymphadenopathy:
Evidence for its relationship to macrophages and for a
cytokine-related disorder. Histopathology. 35:525–533. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang Q, Chang KL and Weiss LM: Extranodal
Rosai-Dorfman disease involving the bone marrow: A case report. Am
J Surg Pathol. 30:1189–1192. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Grabczynska SA, Toh CT, Francis N,
Costello C and Bunker CB: Rosai-Dorfman disease complicated by
autoimmune haemolytic anaemia: Case report and review of a
multisystem disease with cutaneous infiltrates. Br J Dermatol.
145:323–326. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Maric I, Pittaluga S, Dale JK, Niemela JE,
Delsol G, Diment J, Rosai J, Raffeld M, Puck JM, Straus SE, et al:
Histologic features of sinus histiocytosis with massive
lymphadenopathy in patients with autoimmune lymphoproliferative
syndrome. Am J Surg Pathol. 29:903–911. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Juskevicius R and Finley JL: Rosai-Dorfman
disease of the parotid gland: Cytologic and histopathologic
findings with immunohistochemical correlation. Arch Pathol Lab Med.
125:1348–1350. 2001.PubMed/NCBI
|
|
22
|
Panikar N and Agarwal S: Salivary gland
manifestations of sinus histiocytosis with massive lymphadenopathy:
Fine-needle aspiration cytology findings. A case report. Diagn
Cytopathol. 33:187–190. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pettinato G, Manivel JC, d'Amore ES and
Petrella G: Fine needle aspiration cytology and immunocytochemical
characterization of the histiocytes in sinus histiocytosis with
massive lymphadenopathy (Rosai-Dorfman syndrome). Acta Cytol.
34:771–777. 1990.PubMed/NCBI
|
|
24
|
Mallick S, Ghosh R, Iyer VK, Jain D and
Mathur SR: Cytomorphological and morphometric analysis of 22 cases
of Rosai-Dorfman disease: A large series from a tertiary care
centre. Acta Cytol. 57:625–632. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Deshpande V and Verma K: Fine needle
aspiration (FNA) cytology of Rosai Dorfman disease. Cytopathology.
9:329–335. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kushwaha R, Ahluwalia C and Sipayya V:
Diagnosis of sinus histiocytosis with massive lymphadenopathy
(Rosai-Dorfman Disease) by fine needle aspiration cytology. J
Cytol. 26:83–85. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zeppa P, Vetrani A, Ciancia G, Cuccuru A
and Palombini L: Hemophagocytic histiocytosis diagnosed by fine
needle aspiration cytology of the spleen. A case report. Acta
Cytol. 48:415–419. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sachdev R and Shyama J: Co-existent
Langerhans cell histiocytosis and Rosai-Dorfman disease: A
diagnostic rarity. Cytopathology. 19:55–58. 2008.PubMed/NCBI
|
|
29
|
Cozzolino I, Scognamiglio G, Fernandez LV
Sosa and Zeppa P: Lymph nodes Fine Needle Cytology in the diagnosis
of infectious diseases: Cytological and histological correlations.
Infez Med. 20:(Suppl 3). 16–20. 2012.PubMed/NCBI
|
|
30
|
Chhieng DC, Cangiarella JF, Symmans WF and
Cohen JM: Fine-needle aspiration cytology of Hodgkin disease: A
study of 89 cases with emphasis on false-negative cases. Cancer.
93:52–59. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bangerter M, Brudler O, Heinrich B and
Griesshamnuer M: Fine needle aspiration cytology and flow cytometry
in the diagnosis and subclassification of non-Hodgkin's lymphoma
based on the World Health Organization classification. Acta Cytol.
51:390–398. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhu F, Zhang JT, Xing XW, Wang DJ, Zhu RY,
Zhang Q, Wang HT and Lang SY: Rosai-Dorfman disease: A
retrospective analysis of 13 cases. Am J Med Sci. 345:200–210.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Córdova Ramos G, González V Machin and
Benítez Tang SM: Rosai-Dorfman's disease: A propos of an
interesting case study. Acta Otorrinolaringol Esp. 59:311–313.
2008.(In Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen J, Tang H, Li B and Xiu Q:
Rosai-Dorfman disease of multiple organs, including the epicardium:
An unusual case with poor prognosis. Heart Lung. 40:168–171. 2011.
View Article : Google Scholar : PubMed/NCBI
|