Introduction
Vitamin D is an essential fat-soluble vitamin with
multiple functions. The main source of vitamin D is transformed
from 7-dehydrocholesterol after exploration with ultraviolet
irradiation and sequential hydroxylation into 25(OH)D and an active
hormone, 1,25-dihydroxy-vitamin D3
[1,25(OH)2D3], by hydroxylases in the kidney
and liver. It can also be absorbed from dietary intake or oral
supplements. It has been shown that vitamin D receptor (VDR) is
highly expressed in the intestine, kidney, thyroid and bone
(1). Previous findings showed that
several types of immune cells such as T lymphocytes, monocytes,
macrophages, and dendritic cells express VDR as well. The active
form of vitamin D exerts its effects on these tissues by binding to
VDR. In addition, some studies present that macrophages, dendritic
cells and lymphocytes also express vitamin D activating enzyme,
1-α-hydroxylase (CYP27B1) (2,3). Therefore, except for the classical
physiological function of regulation of calcium and bone
metabolism, vitamin D may also have immunomodulatory effects.
Epidemiologic evidence indicates that vitamin D
deficiency is related to autoimmune diseases including multiple
sclerosis, type 1 diabetes, systemic lupus erythematosus (SLE) and
inflammatory bowel disease (4).
Studies have found that the levels of serum 25(OH)D and
1,25(OH)2D3 are significantly lower in
patients with multiple sclerosis than those in healthy controls
(5,6).
Additionally, the effect of vitamin D on type 1 diabetes mellitus
is closely correlated with the serum concentration of 25(OH)D. An
epidemiological investigation in Britain by Staples et al
proves that the prevalence of type 1 diabetes is positively
associated with the increase of residential latitude, and inversely
associated with ultraviolet radiation, which is consistent with a
previous report for multiple sclerosis in Australia (7). Additionally, the percentage of SLE has a
close relationship with the serum concentration of 25(OH)D. In the
study of Ruiz-Irastorza et al, the percentage of vitamin D
deficiency in patients with SLE was higher than that in the healthy
population, and approximately 70% of SLE patients had a serum
concentration of 25(OH)D<30 ng/ml (8).
In addition to epidemiological data, animal
experiments have also presented relationships between vitamin D and
autoimmune diseases. In the study of Ye et al, the
percentage of CD4+ and CD8+ T lymphocytes in
guinea pigs with experimental allergic encephalomyelitis was
decreased and the CD4+/CD8+ ratio was
increased. However, when guinea pigs were supplemented with
1,25(OH)2D3, the percentage of
CD4+ and CD8+ T lymphocyte were significantly
elevated and simultaneously, CD4+/CD8+ was
reduced (9). Another study showed that
the immunological rejection of corneal allograft was inhibited by
1,25(OH)2D3 (10). Furthermore,
1,25(OH)2D3 also inhibited the expression of
inflammatory related cytokines, such as interferon-γ, interleukin-2
(IL-2) and interleukin-17A (10).
However, little is known about the immunomodulatory
effects of vitamin D in the condition of immune suppression.
Vitamin D deficiency increases the susceptibility and vulnerability
to tuberculosis. The immune response of patients with type 2
diabetes and spinal tuberculosis who receive long-term drug therapy
can be improved by supplementation with
1,25(OH)2D3 (11,12). Those
studies suggested that vitamin D may enhance the immune function
under immunocompromised condition. The aim of the present study was
to investigate the effects of vitamin D on immune function in
immunosuppressant mice induced by glucocorticoid.
Materials and methods
Reagents
Concanavalin A (Con A),
1,25(OH)2D3 and
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT)
were purchased from Sigma (St. Louis, MO, USA). A mouse
enzyme-linked immunosorbent assay (ELISA) kit for IL-2 was
purchased from R&D Systems, Inc., (Minneapolis, MN, USA).
Fluorescence-labeled anti-mouse antibody was purchased from
eBioscience, Inc. (San Diego, CA, USA).
Animal studies
A total of 40 ICR male mice (6–8 weeks old) were
purchased from the Animal Center of the Medical Laboratory in
Peking University Health Science Center. Mice were randomly divided
into five groups (8 in each group) including the control group (C
group), immunosuppression model group (M group) and three different
doses of 1,25(OH)2D3 supplemented groups. All
the mice except those in the C group were injected
intraperitoneally with dexamethasone (DEX, 25 mg/kg) for three days
to establish the immunosuppressant mouse model. Mice in the C group
were injected with the same volume of normal saline. From the first
day of DEX injection, 1,25(OH)2D3 at three
different doses of 4 IU/g (+4D group), 6 IU/g (+6D group) or 10
IU/g (+10D group) body weight was given for 7 days by intragastric
administration to mice in vitamin D-treated groups. Mice in the C
and M groups received the same volume of physiological saline via
intragastric administration.
All the mice were weighed and sacrificed by cervical
dislocation at day 8. Spleens and thymuses were collected and
weighed. Indexes for thymus and spleen were calculated according to
the formulae: Spleen index (mg/g) = weight of spleen (mg)/body
weight (g) and thymus index = weight of thymuses (mg)/body weight
(g). Animal experiments performed in the study were approved by the
Ethics Committee (ref no. LUNSHEN 2014012) of the 306th Hospital of
PLA (Beijing, China).
Proliferation assay of
splenocytes
Cell proliferation was assessed by MTT assay. The
splenocyte suspension was obtained by grinding the spleen tissue in
RPMI-1640 and passing tissue through a fine-mesh cell strainer.
Erythrocytes were removed by hemolytic solution. Cells were washed,
centrifuged and suspended in RPMI-1640 with 10% fetal calf serum
(FCS). Cells were counted, and resuspended in culture medium at a
concentration of 1×107 cells/ml. Cells (100 µl/well)
were seeded in 96-well plates. Culture medium (100 µl/well)
containing Con A (10 µg/ml) or without Con A (as control) was added
to each well and cultured for 48 h. Cell proliferation was detected
by MTT assay. MTT reagent (20 µl, 5 mg/ml) was added to each well
and cultured for an additional 4 h. Culture medium was discarded
and 100 µl dmethyl sulfoxide was added to each well. Optical
density (OD) value was read at 570 nm using a spectrophotometer
(Bio-Rad Laboratories, Hercules, CA, USA). Stimulated proliferation
index was calculated according to the formula: Proliferation index
= ODConA/ODcontrol.
Quantification for IL-2
production
The concentration of IL-2 in cultures of splenocytes
was determined using a mouse ELISA kit. Cells from spleen were
prepared as described in the proliferation assay. Cells
(107/ml; 350 µl/well) were plated on 24-well plates and
350 µl/well Con A (10 µg/ml) was added to each well and cultured
for 48 h. The cell culture medium was collected. The concentration
of IL-2 in cell culture medium was detected by ELISA following the
manufacturer's instructions.
Flow cytometric analysis of cell
markers of CD4 and CD8
To determine the ratio of CD4+ and
CD8+ T lymphocytes (CD4+/CD8+) in
peripheral blood, the anticoagulated blood was collected from all
the mice and treated with haemolysin. Cells were washed and cell
suspension was prepared for immunofluorescent staining.
Fluorescein-labeled rat anti-mouse monoclonal antibodies (1:200
diluted anti-CD4 FITC, cat. no. 11-0041; and 1:200 diluted anti-CD8
PE, cat. no. 12-0081) were added to the cells and incubated for 30
min in dark. The cells were washed and analyzed by FACSCalibur and
Cell Quest software (BD Biosciences, Franklin Lakes, NJ, USA).
Statistical analysis
Statistical analysis was performed using Statistical
Product and Service Solutions (SPSS, Armonk, NY, USA) software
version 16.0. Data were expressed as mean ± standard deviation
(mean ± SD). One-way ANOVA analysis was carried out to compare
means variability among all the groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
1,25(OH)2D3
improves immune organ recovery
As shown in Table I,
the thymus and spleen indexes in mice in the M group were
significantly lower than those in mice in the C group. When mice
were given 1,25(OH)2D3 for 7 days, the thymus
indexes increased compared with the mice in the M group
(P<0.05). Among the three vitamin D-treated groups, the recovery
of the thymus index in mice of the +6D group was the most
significant. However, for the spleen indexes, there was no
difference between vitamin D-treated mice and the mice in M group
(Table I).
 | Table I.The effect of
1,25(OH)2D3 on thymus index and spleen
indexes (mean ± SD). |
Table I.
The effect of
1,25(OH)2D3 on thymus index and spleen
indexes (mean ± SD).
| Groups | Thymus (mg) | Spleen (mg) | Thymus index
(mg/g) | Spleen index
(mg/g) |
|---|
| C | 73.38±11.53 | 109.09±22.42 | 2.83±0.42 | 4.20±0.73 |
| M |
32.43±8.07a | 79.08±17.09 |
1.23±0.30a |
3.01±0.62a |
| +4D |
41.79±6.27a | 78.48±17.75 |
1.77±0.21a,b | 3.3±0.72 |
| +6D |
66.99±15.36b | 78.06±13.41 |
2.58±0.62b |
2.99±0.46a |
| +10D |
55.75±15.95b |
76.99±10.3a |
2.11±0.53a,b |
2.93±0.37a |
1,25(OH)2D3
promotes proliferation of lymphocytes in spleen
We evaluated the effects of
1,25(OH)2D3 on the proliferation of T
lymphocytes in spleen. As shown in Fig.
1, the proliferation index of lymphocytes was obviously
inhibited by the glucocorticoid hormone. The proliferation index
was much lower in the M group (1.5±0.35) compared with the C group
(7.49±2.16, P=0.01). However, the proliferation index was higher in
the +6D group (2.55±0.66) compared to the M group (1.50±0.35,
P=0.021). Differences of proliferation indexes of mice were not
significant between M group and the other two
1,25(OH)2D3-treated groups.
1,25(OH)2D3
promotes the production of IL-2 in spleen lymphocytes
To investigate the effects of
1,25(OH)2D3 on the function of T lymphocytes
in spleen, the production of IL-2 in cultures of lymphocytes was
detected. As expected, the secretion of IL-2 in spleen lymphocytes
was significantly inhibited by glucocorticoid hormone injection in
mice of the M group (P<0.01). However, mice supplemented with
1,25(OH)2D3 (6 IU/g) had much higher levels
of IL-2 (0.58±0.22mg/l) compared to the M group (0.35±0.10mg/l,
P=0.03). The level of IL-2 in mice of the +6D group was similar to
that in the control group. However, IL-2 production of spleen
lymphocytes in mice of the +4D and +10D groups was not different
from that in mice of the M group (Fig.
2).
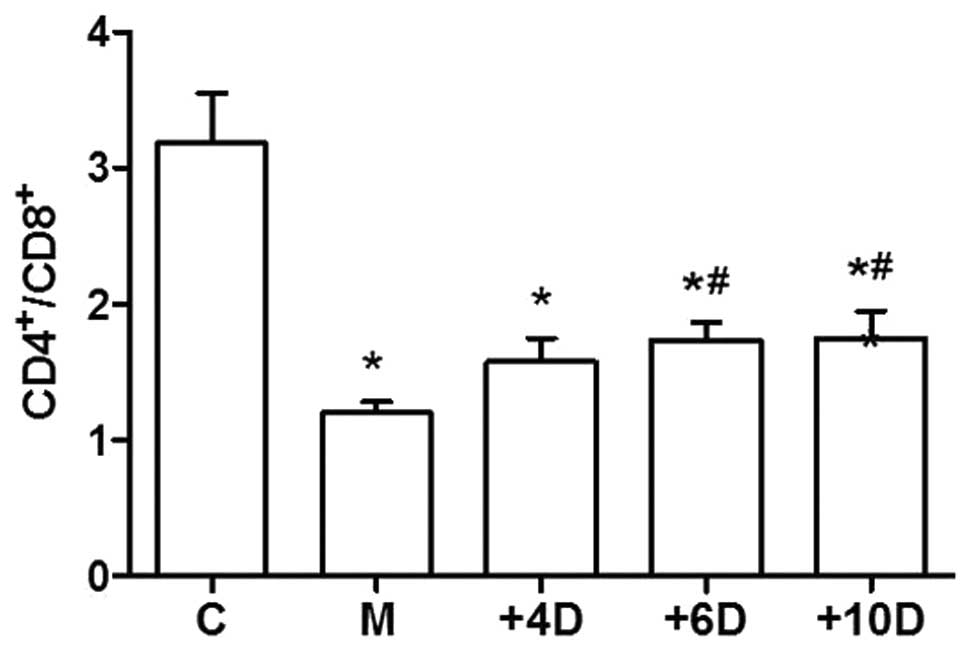
1,25(OH)2D3
upregulates the ratio of CD4+ to CD8+ T
lymphocytes
To investigate the effects of
1,25(OH)2D3 on the immune functions, we
examined the ratio of CD4+ to CD8+ T cells in
peripheral blood. As the data show in Fig.
3, the CD4+/CD8+ ratio in the M group
(1.20±0.24) was significantly decreased compared to that in the C
group (3.18±1.04; P<0.05). The ratios of
CD4+/CD8+ T cells were 1.58±0.49, 1.74±0.35
and 1.75±0.57 in the +4D, +6D and +10D groups, respectively. The
ratio of CD4+/CD8+ T cells was significantly
higher in mice treated with 1,25(OH)2D3 at
doses of 6 and 10 IU/g body weights compared with that in mice of
the M group (Fig. 3).
Discussion
Previous reports regarding the effects of
1,25(OH)2D3 on the regulation of immune
function were inconsistent. Most evidence of epidemiological
studies, animal and in vitro experiments suggest a role for
1,25(OH)2D3 in negatively influencing the T
cells in autoimmune disorders (9,13). The
results of an in vivo study suggest that
1,25(OH)2D3 supplementation inhibits the
proliferation of T lymphocytes, reduces the immune organ indexes
and decreases the ratio of CD4+/CD8+ T cells
in a model of adjuvant arthritis (14). In mice stimulated with Bacillus
Calmette-Guerin, vitamin D deficiency may result in increasing the
percentage of CD4+ and CD8+ T cells and
reducing the CD4+/CD8+ ratio, while
1,25(OH)2D3 supplementation leads to a
reduction of CD4+ and CD8+ T lymphocytes and
elevation of CD4+/CD8+ ratio. These results
suggest that vitamin D exerts immunosuppressive effects in
autoimmune diseases. By contrast, data from a clinical study show
that the immunity of patients with long-term type 2 diabetes may be
improved by vitamin D supplementation (11). Furthermore, the study of Gao et
al suggests that the concentrations of 25(OH)D in patients with
tuberculosis is lower than that in healthy adults, and 25(OH)D has
a positive correlation with the CD4+/CD8+
ratio (15). A study by Panda et
al, an animal model of 1-α hydroxylase deficiency was used to
investigate the effects of 1,25(OH)2D3 on
immune function. Their result showed that vitamin D deficiency
resulted in the inhibition of the proliferation of spleen
lymphocyte and a reduction in CD4+ and CD8+
peripheral T cells (16). The results
presented in our study showed that
1,25(OH)2D3 may improve the thymus index
recovery, promote the IL-2 production and proliferation of T cells
and elevate CD4+/CD8+ ratio in
glucocorticoid-induced immunosuppressant mice. The data indicates
that 1,25(OH)2D3 has a positive influence on
immune function under the state of hypoimmunity. The
immunomodulatory effects of 1,25(OH)2D3 may
be dependent on the immune status.
How vitamin D affects the immune system has not been
fully elucidated. Previous studies have demonstrated that T cells
express VDR (17). In addition,
activated T cells express Cyp27B1 (vitamin D activating enzyme)
(18,19). Those studies suggest that T cells are
not only the targets of 1,25(OH)2D3 but are
also able to produce 1,25(OH)2D3 locally.
IL-2, secreted by type 1 helper T cells, mediates the cellular
immune response and can induce the proliferation of T, B and
natural killer cells (20). IL-2 may
participate in the regulatory effects of vitamin D on immune
system. A study showed that IL-2 secreted by CD4+ T
cells was enhanced by 1,25(OH)2D3 treatment
(21). Additionally, in vitro
treatment with 1,25(OH)2D3 may upregulate the
expression of IL-2 receptor in T lymphocytes and monocytes
(22). The current study showed that
vitamin D enhanced IL-2 production of T cells in spleen. Thus, the
changes of IL-2 production may participate in the immune regulatory
effects of vitamin D.
T cells mainly include CD4+ T cells and
CD8+ T cells. The CD4+/CD8+ ratio
has been recognized as an important indicator for evaluating the
state of immunomodulation and response to homeostasis of the
intrinsic immune system (23).
Previous studies have shown that the increased
CD4+/CD8+ ratio has been observed in many
autoimmune diseases such as SLE (24),
inflammatory bowel disease (25) and
multiple sclerosis (26). A low
CD4+/CD8+ ratio was considered as a marker of
decreased immune function (27), which
could result in increasing the risk of HIV-infected, pulmonary
tuberculosis and even some tumors (28,29). The
current study demonstrated that the CD4/CD8 ratio significantly
decreased by glucocorticoid injection and partially recovered by
vitamin D supplementation. These results further prove that vitamin
D may improve the immune status in immunosuppressant
conditions.
The present findings have demonstrated that vitamin
D can partially improve the immune recovery in immunosuppressant
mice by stimulating T-cell proliferation and elevating IL-2
production.
Acknowledgements
The present study was supported by The Medical
Research Grant of The 306th Hospital of PLA, Beijing, China.
Glossary
Abbreviations
Abbreviations:
|
OH
|
hydroxide
|
|
1,25(OH)2D3
|
1,25-dihydroxy vitamin D3
|
|
Con A
|
concanavalin A
|
|
MTT
|
3-(4,5-dimethyl
thiazol-2-yl)-2,5-diphenyl-tetrazolium bromide
|
|
IL-2
|
interleukin-2
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
DEX
|
dexamethasone
|
|
FCS
|
fetal calf serum
|
|
OD
|
optical density
|
References
|
1
|
Wang Y, Zhu J and DeLuca HF: Where is the
vitamin D receptor? Arch Biochem Biophys. 2012 Jul 1;523(1):
123–133. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Overbergh L, Decallonne B, Valckx D,
Verstuyf A, Depovere J, Laureys J, Rutgeerts O, Saint-Arnaud R,
Bouillon R and Mathieu C: Identification and immune regulation of
25-hydroxyvitamin D-1-alpha-hydroxylase in murine macrophages. Clin
Exp Immunol. 120:139–146. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hewison M, Freeman L, Hughes SV, Evans KN,
Bland R, Eliopoulos AG, Kilby MD, Moss PA and Chakraverty R:
Differential regulation of vitamin D receptor and its ligand in
human monocyte-derived dendritic cells. J Immunol. 170:5382–5390.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Prietl B, Treiber G, Pieber TR and Amrein
K: Vitamin D and immune function. Nutrients. 5:2502–2521. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Soilu-Hänninen M, Airas L, Mononen I,
Heikkilä A, Viljanen M and Hänninen A: 25-Hydroxyvitamin D levels
in serum at the onset of multiple sclerosis. Mult Scler.
11:266–271. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Correale J, Ysrraelit MC and Gaitán MI:
Immunomodulatory effects of vitamin D in multiple sclerosis. Brain.
132:1146–1160. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Staples JA, Ponsonby AL, Lim LL and
McMichael AJ: Ecologic analysis of some immune-related disorders,
including type 1 diabetes, in Australia: Latitude, regional
ultraviolet radiation, and disease prevalence. Environ Health
Perspect. 111:518–523. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ruiz-Irastorza G, Egurbide MV, Olivares N,
Martinez-Berriotxoa A and Aguirre C: Vitamin D deficiency in
systemic lupus erythematosus: Prevalence, predictors and clinical
consequences. Rheumatology (Oxford). 47:920–923. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ye YL, Li ZX and Ye XM: Influence of 1,
25-dihydroxyvitamin D3 on variation of peripheral blood lymphocyte
subsets in guinea pigs with experimental autoimmune
encephalomyelitis. J Chin Gen Pract. 11:391–393. 2008.(In
Chinese).
|
|
10
|
Wu J, Zhang J, Hou GH, Cui YB, Wang C and
Chen J: Effect of 1α,25-dihydroxyvitamin D3 on T helper cell 17 and
expression of related cytokines in penetrating keratoplasty in
mice. Chin J Pathophysiol. 30:2226–2231. 2014.(In Chinese).
|
|
11
|
Luwen C, Liqing T and Ruiping Y: Study of
impact of vitamin D in immune function and infections in patients
with type 2 diabetes. Chin J Nosocomiol. 25:1106–1109. 2015.
|
|
12
|
Dezhi L, Zehua Z, Litao L and Zhengqi C:
Effection of vitamin D deficiency to T lymphocyte subgroups in
spinal tuberculosis Chin. J Orthop. 20:448–450. 2012.
|
|
13
|
Cantorna MT, Snyder L, Lin YD and Yang L:
Vitamin D and 1,25(OH)2D regulation of T cells. Nutrients.
7:3011–3021. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang C, Wu J, Hou GH, Chen J, Qi WJ, Cui
YB and Zhang J: The immuno-regulation effect of
1,25(OH)2D3 on T lymphocytes. J Guangdong
Med. 34:3114–3116. 2013.
|
|
15
|
Gao WW, Wang Y, Zhang XR, Yin CY, Hu CM,
Tian M, Wang HW and Zhang X: Levels of
1,25(OH)2D3 for patients with pulmonary
tuberculosis and correlations of 1,25(OH)2D3
with the clinical features of TB. J Thorac Dis. 6:760–764.
2014.PubMed/NCBI
|
|
16
|
Panda DK, Miao D, Tremblay ML, Sirois J,
Farookhi R, Hendy GN and Goltzman D: Targeted ablation of the
25-hydroxyvitamin D 1alpha -hydroxylase enzyme: Evidence for
skeletal, reproductive, and immune dysfunction. Proc Natl Acad Sci
USA. 98:7498–7503. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schedel M, Jia Y, Michel S, Takeda K,
Domenico J, Joetham A, Ning F, Strand M, Han J, Wang M, et al:
1,25D3 prevents CD8(+)Tc2 skewing and asthma development through
VDR binding changes to the Cyp11a1 promoter. Nat Commun.
7:102132016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ooi JH, McDaniel KL, Weaver V and Cantorna
MT: Murine CD8+ T cells but not macrophages express the vitamin D
1α-hydroxylase. J Nutr Biochem. 25:58–65. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kongsbak M, von Essen MR, Levring TB,
Schjerling P, Woetmann A, Ødum N, Bonefeld CM and Geisler C:
Vitamin D-binding protein controls T cell responses to vitamin D.
BMC Immunol. 15:352014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lu Z, Jin M, Huang M and Wang Y and Wang
Y: Bioactivity of selenium-enriched exopolysaccharides produced by
Enterobacter cloacae Z0206 in broilers. Carbohydr Polym.
96:131–136. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yan G, Xi Y, Xu S, Chen J, Lin Y, Dai H,
Cheng P, Xiao H, Liu Z and Qi Z: Inhibiting accelerated rejection
mediated by alloreactive CD4(+) memory T cells and prolonging
allograft survival by 1α,25-dihydroxyvitamin D(3) in nude mice.
Immunol Lett. 149:54–61. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Prehn JL and Jordan SC: Incubation of T
cell or monocytic cell lines with 1,25-dihydroxyvitamin D3 before
mitogen stimulation potentiates IL-2 and IL-1 beta mRNA levels.
Transplant Proc. 21:90–91. 1989.PubMed/NCBI
|
|
23
|
Dhur A, Galan P, Preziosi P and Hercberg
S: Lymphocyte subpopulations in the thymus, lymph nodes and spleen
of iron-deficient and rehabilitated mice. J Nutr. 121:1418–1424.
1991.PubMed/NCBI
|
|
24
|
Zhao L, Jiang Z, Jiang Y, Ma N, Wang K and
Zhang Y: Changes in immune cell frequencies after cyclophosphamide
or mycophenolate mofetil treatments in patients with systemic lupus
erythematosus. Clin Rheumatol. 31:951–959. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang FY, Su M, Zheng YQ, Wang XG, Kang N,
Chen T, Zhu EL, Bian ZX and Tang XD: Herbal prescription Chang'an
II repairs intestinal mucosal barrier in rats with
post-inflammation irritable bowel syndrome. Acta Pharmacol Sin.
36:708–715. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Harrer A, Pilz G, Wipfler P, Oppermann K,
Sellner J, Hitzl W, Haschke-Becher E, Afazel S, Rispens T, van der
Kleij D, et al: High interindividual variability in the CD4/CD8 T
cell ratio and natalizumab concentration levels in the
cerebrospinal fluid of patients with multiple sclerosis. Clin Exp
Immunol. 180:383–392. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hadrup SR, Strindhall J, Køllgaard T,
Seremet T, Johansson B, Pawelec G, Straten P thor and Wikby A:
Longitudinal studies of clonally expanded CD8 T cells reveal a
repertoire shrinkage predicting mortality and an increased number
of dysfunctional cytomegalovirus-specific T cells in the very
elderly. J Immunol. 176:2645–2653. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yin Y, Qin J, Dai Y, Zeng F, Pei H and
Wang J: The CD4+/CD8+ ratio in pulmonary
tuberculosis: Systematic and meta-analysis article. Iran J Public
Health. 44:185–193. 2015.PubMed/NCBI
|
|
29
|
Serrano-Villar S, Sainz T, Lee SA, Hunt
PW, Sinclair E, Shacklett BL, Ferre AL, Hayes TL, Somsouk M, Hsue
PY, et al: HIV-infected individuals with low CD4/CD8 ratio despite
effective antiretroviral therapy exhibit altered T cell subsets,
heightened CD8+ T cell activation, and increased risk of non-AIDS
morbidity and mortality. PLoS Pathog. 10:e10040782014. View Article : Google Scholar : PubMed/NCBI
|