Introduction
Bronchopulmonary dysplasia (BPD) is one of the most
common complications observed in premature infants, approximately
12–32% newborn infants with a gestational age of <32 weeks
develop BPD (1,2). BPD was first reported and defined as a
pulmonary disease following mechanical ventilation of infants with
respiratory distress syndrome, characterized by airway injury,
inflammation and lung fibrosis by Northway et al (3,4). Hyperoxia
contributes to the development of BPD in human preterm infants and
a similar lung phenotype characterized by alveolar and pulmonary
vascular simplification in newborn rats (5).
Certain studies have demonstrated that development
of the pulmonary vasculature is a necessary factor for proper
alveolarization. Furthermore, pulmonary vasculogenesis and
angiogenesis are necessary for alveolarization during normal lung
development. Injury to the developing pulmonary circulation during
a critical period of lung growth may lead to lung hypoplasia
(6–8).
Epidermal growth factor-like domain 7 (EGFL7) is a
protein secreted from endothelial cells and is role in vascular
tubulogenesis. It has been found that EGFL7 gene expression levels
decrease significantly after hyperoxic exposure in neonatal rat
lungs. Furthermore, EGFL7 may protect endothelial cells from
hyperoxia-induced apoptosis by inhibition of the
mitochondria-dependent apoptosis pathway (9,10).
Erythropoietin (EPO) is a 30.4-kDa glycoprotein that
regulates the rate of red blood cell production, through binding to
its specific cell surface receptors (11). It was once considered to be a regulator
of erythropoiesis by controlling the apoptosis, proliferation and
differentiation of erythroid precursor cells over an extended
period of time (12). Animal
experiments have revealed that EPO exerts protective effects
against hyperoxic lung injury; these findings indicate that
treatment of premature infants with EPO might reduce the risk of
developing BPD. However, the mechanisms remain unknown, an improved
understanding of the mechanism of action of EPO is required to
translate this experimental result into clinical trials in BPD
patients (13,14).
The aim of the current study was to demonstrate
whether recombinant human (rh)EPO treatment could attenuate
hyperoxia-induced lung damage, and if so, whether this protective
effect is mediated by up-modulating the expression of EGFL7 in
newborn rats.
Materials and methods
Animal experiments
The current study was approved by the Ethics and
Research Committee of Southern Medical University (Guangzhou,
China). All research was conducted according to the Guide for the
Care and Use of Laboratory Animals of the National Institutes of
Health. Ten pregnant Sprague-Dawley rats (on gestation day 20) were
provided by the Experimental Animal Center of Southern Medical
University. A total of 109 pups were delivered spontaneously on the
following day. Among them 96 pups were selected and, within 12 h of
their birth, were randomly divided into four groups (n=24) as
follows: Room air-exposed control group, room air-exposed
rhEPO-treated group, hyperoxia-exposed group, and the
hyperoxia-exposed rhEPO-treated group. The two hyperoxia-exposed
groups were placed in an oxygen chamber and exposed to oxygen
(FiO2=0.85±0.02) continuously, the room air-exposed
rhEPO-treated and hyperoxia-exposed rhEPO-treated groups received
1,200 IU/kg rhEPO subcutaneously 30 min before oxygen exposure and
2 days after birth. Isodose saline was administered to the pups in
the room air-exposed control and hyperoxia-exposed pups according
to the same protocol. The chambers were opened for 1 h daily to
provide water and food, to change bedding and to exchange nursing
dams between the hyperoxic and room air chambers to protect the
nursing dams from oxygen toxicity. On days 3, 7 and 14, eight rats
from each group were anesthetized with 60 mg/kg pentobarbital
(intraperitoneal injection; Nanjing KeyGen Biotech Co., Ltd.,
Nanjing, China). Lungs were exposed by thoracotomy and, after
exsanguination by transecting the aorta and inferior vena cava, the
right ventricle was punctured and lungs were perfused with 3 ml
phosphate-buffered saline (PBS) at 25 cmH2O. These lung
tissue samples were collected for the subsequent experiments.
Assessment of lung histological
changes
Following deep anesthesia with 60 mg/kg
pentobarbital via intraperitoneal injection, the rat chests were
opened, and the left lungs were excised and fixed overnight in 4%
paraformaldehyde at 4°C. The tissue samples were dehydrated,
transparentized and embedded with ethanol, xylene and paraffin,
respectively. Sections (4 µm) were cut from the paraffin blocks and
stained with hematoxylin and eosin to observe the histological
changes. A quantitative analysis of the radial alveolar count (RAC)
and the mean septal wall thickness was performed as previously
described (5,15). These were used to evaluate the effect
of hyperoxia on lung histological damage and the effect of rhEPO in
modulating hyperoxia-induced lung injury.
Immunohistochemistry
For CD31 immunohistochemistry, the lung tissue
samples were embedded in paraffin after fixation in 4%
paraformaldehyde, and sliced into 4-µm-thick sections. The sections
were dewaxed and hydrated in xylene and a series of graded ethanol,
and the sections were antigen retrieved in citrate buffer (pH 6.0)
using a microwave for 15 min, and incubated in 3%
H2O2 for 20 min to eliminate endogenous
peroxidase activity. The slides were incubated with a primary mouse
CD31 monoclonal antibody (cat. no. ab64543; dilution, 1:200; Abcam,
Cambridge, MA, USA) at 4°C overnight. The sections were then washed
three times with 1X PBS (pH 7.2–7.4) and incubated with a
biotinylated peroxidase-conjugated goat anti-mouse secondary
antibody (cat. no. 115225205; dilution, 1:1,000; Jackson
ImmunoResearch Laboratories, West Grove, PA, USA) at 37°C for 30
min and 0.1% 3,3′-diaminobenzidine substrate, using the standard
streptavidin–biotin-based method (10). The sections were counterstained with
hematoxylin. The negative control was incubated with 1X PBS (pH
7.2–7.4) instead of the primary antibody at 37°C for 30 min. The
slides were observed under a light microscope (Eclipse TE200; Nikon
Corporation, Tokyo, Japan) at a magnification of ×400. The
cytoplasmic brown granule indicated positive expression of CD31 and
the average integrated optical density (AIOD) values of CD31 were
measured. The vascular density was quantified by AIOD of CD31
through measuring the positive area of CD31 immunostaining relative
to the total area of lung parenchymal cells using Image-Pro Plus
6.0 (Media Cybernetics, Rockville, MD, USA) of differential
interference contrast images, as previously described by Wang and
Huang (10).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA from lung tissue samples was extracted
using RNAiso Plus (Takara Bio, Inc., Otsu, Japan) according to the
manufacturer's instructions. RT-PCR was performed with the
PrimeScript RT-PCR kit (Takara Bio, Inc.). The PCR primers for
EGFL7, Bax, Bcl-2 and β-actin were designed and synthesized by
Invitrogen (Thermo Fisher Scientific, Inc., Waltham, MA, USA). The
sequences of the primers used were as follows (10): Forward, 5′-CCGAACCATCTACCGGACTG-3′ and
reverse, 5′-GCCTGTCTGTCACCCATTCA-3′ for EGFL7; forward,
5′-AGAGGATGGCTGGGGAGAC-3′ and reverse, 5′-CGCTCAGCTTCTTGGTGGAT-3′
for Bax; forward, 5′-ACCCCTGGCATCTTCTCCT-3′ and reverse,
5′-CGACGGTAGCGACGAGAG-3′ for Bcl-2; and forward,
5′-AGGGAAATCGTGCGTGACAT-3′ and reverse, 5′-GAACCGCTCATTGCCGATAG-3′
for β-actin. The amplification reaction was conducted using an
Applied Biosystems 7500 Real-Time PCR System under the following
cycling conditions: 95°C for 30 sec, then 95°C for 5 sec, and 60°C
for 34 sec for 40 cycles. The expression level of β-actin served as
the internal control and the relative quantification of mRNA
expression was calculated using the 2−ΔΔCt method
(16).
Western blot analysis
Total proteins were collected from the lung tissue
samples of the pups, using lysis buffer and the Total Protein
Extraction Reagent kit (Nanjing KeyGen Biotech Co., Ltd.), and the
protein concentration was determined using a BCA protein assay kit
(Nanjing KeyGen Biotech Co., Ltd.). Equal loading protein (30
µg/lane) were dissolved in 12% SDS-PAGE gel (Guangzhou TianJun
Biotech Co., Ltd., Guangzhou, China) for protein separation and
transferred to polyvinylidene fluoride membranes for 60 min at 200
mA. The membranes were blocked with 5% skimmed milk in
Tris-buffered saline and 0.1% Tween-20 (Guangzhou TianJun Biotech
Co., Ltd.) for 1 h at room temperature. The membranes were
subsequently incubated with primary rabbit polyclonal antibodies
against EGFL7 (cat. no. 19291-1-AP; dilution, 1:500; ProteinTech
Group, Inc., Chicago, IL, USA), Bax (cat. no. ab182733; dilution,
1:1,000; Epitomics; Abcam, Cambridge, USA), Bcl-2 (cat. no. BS1511;
dilution, 1:500; Bioworld Technology, Inc., St. Louis Park, MN,
USA) and β-actin (cat. no. BS1002; dilution, 1:2,000; Bioworld
Technology, Inc.) overnight at 4°C. The next day, the membranes
were incubated with anti-rabbit (H+L) HRP secondary antibody (cat.
no. AP0032M; dilution, 1:3,000; Bioworld Technology, Inc.) at room
temperature for 60 min. The protein bands were visualized with an
enhanced chemiluminescence reaction kit (EMD Millipore, Billerica,
MA, USA) on a Chemi Imager 5500 image analysis instrument (Alpha
Innotech Corp., San Leandro, CA, USA). Subsequently, the density
values of the bands were analyzed using Quantity One (version 4.6.2
PC) software (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Relative protein expression levels of EGFL7, Bax and Bcl-2 were
normalized to β-actin.
Statistical analysis
All data are provided as means ± standard deviation.
Comparison among the groups was performed by one-way analysis of
variance and Student's t-test. P<0.05 was considered to indicate
a statistically significant difference.
Results
Lung histology and morphometric
analyses
Lung histology images obtained at a magnification of
×400 are presented in Fig. 1. In the
room air-exposed control group and room air-exposed rhEPO-treated
group, no histological damage or inflammatory infiltrates, or
septal wall thickening were observed. Furthermore, the terminal
alveoli were well formed. By contrast, in the hyperoxia-exposed
group rats, a large number of inflammatory cells infiltrating the
interstitial lung was observed, as well as marked alveolar
simplification and the septal walls were noticeably thicker.
Treatment with rhEPO attenuated septal wall thickening and markedly
increased the RAC. In addition, the morphological characteristics
were similar to the room air-exposed control group. Compared with
the hyperoxia-exposed group, the RAC was significantly increased
and the septal wall thickness was significantly decreased in the
hyperoxia-exposed rhEPO-treated group (P<0.05; Fig. 1 and Table
I).
 | Table I.RAC and mean ST in each group. |
Table I.
RAC and mean ST in each group.
|
| RAC | ST (µm) |
|---|
|
|
|
|
|---|
| Group | Day 7 | Day 14 | Day 7 | Day 14 |
|---|
| Control | 6.05±0.22 | 8.43±0.44 | 7.02±0.22 | 6.25±0.59 |
| Control + EPO | 6.23±0.14 | 8.42±0.27 | 6.78±0.43 | 6.04±0.52 |
| Hyperoxia |
5.23±0.12a |
5.52±0.18a |
9.73±0.35a |
10.74±0.32a |
| Hyperoxia + EPO |
5.84±0.17b,c |
7.55±0.43b,c |
7.65±0.33b,c |
6.47±0.25c |
Immunohistochemical analysis of CD31
expression levels and vascular density
Cytoplasmic brown granules indicated the positive
expression of CD31. Vascular density was quantified by measuring
the positive area of CD31 immunostaining relative to the total area
of the lung parenchymal cells. CD31and vascular density in the
hyperoxia-exposed group were decreased compared with the control
group at each time-point. The CD31 and vascular density in the
hyperoxia-exposed rhEPO-treated group were markedly increased when
compared with the hyperoxia-exposed group on postnatal days 7 and
14 (P<0.05; Fig. 2 and Table II).
 | Table II.Relative protein levels of CD31 and
vascular density of rat lung tissue samples treated with or without
EPO. |
Table II.
Relative protein levels of CD31 and
vascular density of rat lung tissue samples treated with or without
EPO.
|
| AIOD of CD31 | Vascular density (%
area) |
|---|
|
|
|
|
|---|
| Group | Day 7 | Day 14 | Day 7 | Day 14 |
|---|
| Control | 42.60±3.22 | 123.73±10.12 | 6.02±0.25 | 10.88±0.53 |
| Control + EPO | 43.33±3.61 | 125.98±4.79 | 6.17±0.21 | 11.00±0.63 |
| Hyperoxia |
18.10±0.39a |
49.20±5.44a |
3.78±0.18a |
6.43±0.44a |
| Hyperoxia + EPO |
40.10±2.43b |
115.40±8.34b |
5.57±0.46b |
9.95±0.66b |
mRNA expression levels of EGFL7, Bax
and Bcl-2 in lung tissue samples by RT-qPCR
The EGFL7 and Bcl-2 mRNA expression levels from the
lung tissue samples of the hyperoxia-exposed group pups were lower,
although the Bax mRNA expression level was higher when compared
with the room air-exposed control group on postnatal days 3, 7 and
14 (P<0.01). In the hyperoxia-exposed rhEPO-treated group, the
mRNA expression levels of EGFL7 and Bcl-2 were upregulated, while
the Bax mRNA expression level was downregulated significantly when
compared with the hyperoxia-exposed group at each time-point
(P<0.05). No significant differences were identified among the
room air-exposed control group, room air-exposed rhEPO-treated
group and the hyperoxia-exposed rhEPO-treated group (Fig. 3).
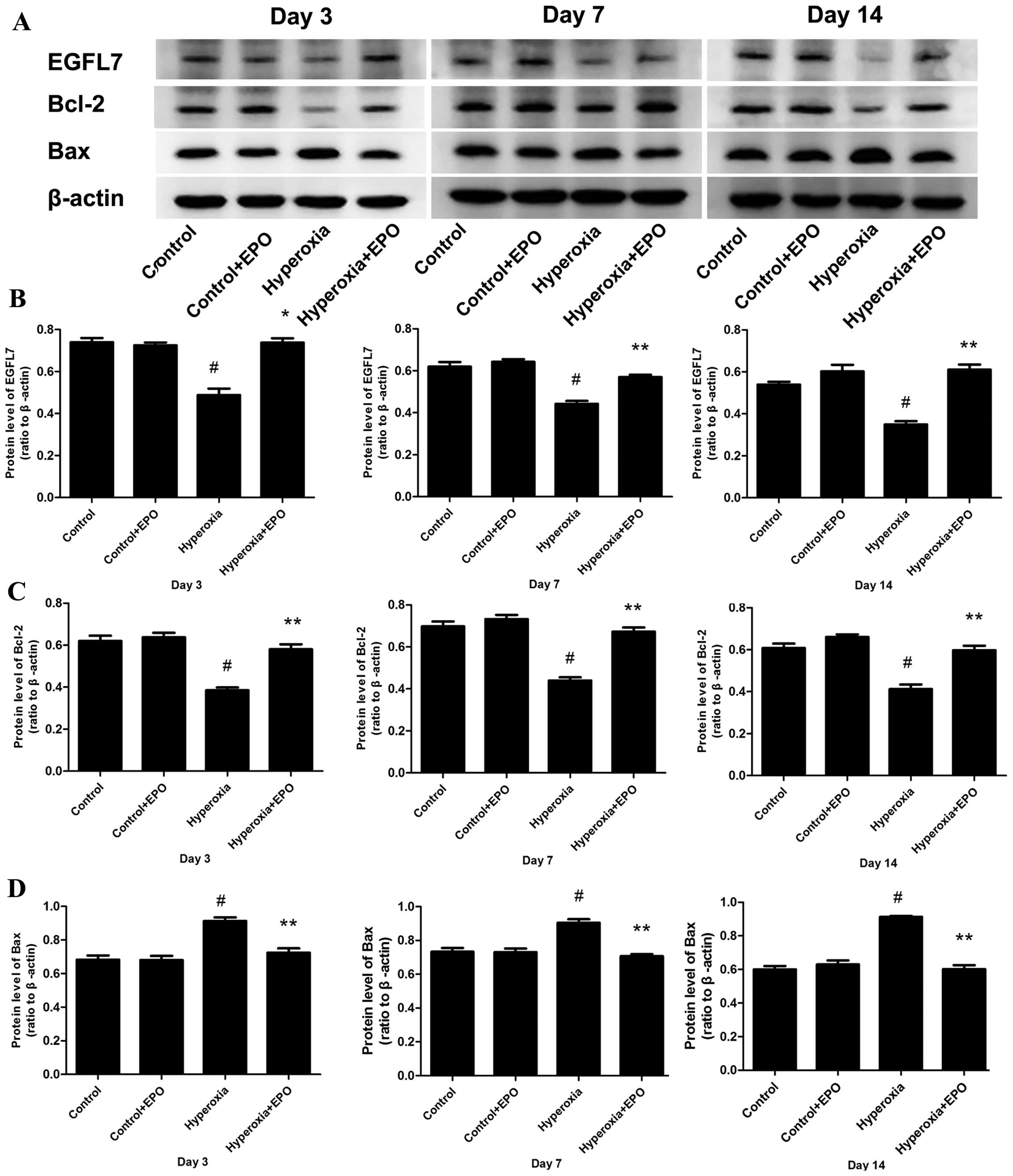
Protein expression levels of EGFL7,
Bax and Bcl-2 in lung tissue samples by western blotting
The protein expression levels of EGFL7, Bax, and
Bcl-2 in lung tissue samples obtained from each group were examined
by western blotting. The protein expression levels of EGFL7 and
Bcl-2 in the hyperoxia-exposed group were decreased, while the
protein expression level of Bax was elevated when compared with the
room air-exposed control group on postnatal days 3, 7 and 14
(P<0.01). In the hyperoxia-exposed rhEPO-treated group,
significantly higher expression levels of EGFL7 and Bcl-2 protein
were identified, while the expression level of Bax protein was
decreased when compared with the hyperoxia-exposed group
(P<0.05; Fig. 4).
Discussion
BPD is the most common complication of prematurity,
despite its high incidence on preterm newborns, its pathogenesis is
not yet clearly understood and there are no effective therapeutic
strategies. BPD is a multifactorial disease characterized by
impaired alveolar and vascular development (17). Therapy with hyperoxia is often used to
treat newborns with respiratory disorders; however, high
concentrations and extended durations of oxygen exposure in a
newborn may lead to oxidative stress injury and lung epithelial
cell death in the immature lung, which is crucial in the
development of BPD (18,19). Previous studies have demonstrated that
pulmonary vasculogenesis and angiogenesis are necessary for
alveolarization during normal lung development. Alveolar
development and pulmonary vascular development are interlocked
processes, as evidenced by the finding that experimental inhibition
of angiogenesis during fetal lung development results in the arrest
of distal airspace development, and that the disruption of normal
lung vascular growth is vital in the pathogenesis of BPD (20,21).
EPO is a 30.4-kDa glycoprotein that regulates the
rate of red blood cell production, through binding to its specific
cell surface receptors; EPO is an angiogenic factor, as well as an
antioxidant due to decreasing the plasma iron concentration and
increasing the ability of plasma to inhibit lipid peroxidation
(11,22). As abnormal vascular growth are proposed
to be major contributing factors in the pathogenesis of BPD, the
benefits of EPO treatment in this disease have previously been
investigated (11). Studies have
demonstrated that rhEPO treatment attenuated hyperoxia-induced lung
injury by down-modulating the inflammatory responses and decreasing
apoptosis in neonatal rats (14,23).
CD31 is a 130-kDa molecular weight protein and a
member of the immunoglobulin gene superfamily (24). CD31 is expressed in newly formed, small
blood vessels and pre-existing vessels, and CD31 is commonly used
as an endothelial cell marker in vessels (25,26). The
vascular density is quantified by measuring the positive area of
CD31 immunostaining relative to the total area of lung parenchymal
cells. In the current study, the alveolar and capillary damage were
identified to be milder in the hyperoxia-exposed rhEPO-treated
group when compared with the hyperoxia-exposed group. In addition,
the vascular density significantly increased in the
hyperoxia-exposed rhEPO-treated group when compared with the
hyperoxia-exposed group. In the present study, EPO treatment
reversed the negative effect of hyperoxia that was observed in the
hyperoxia-exposed group resulting in increased alveolar and
pulmonary vascular injury.
In the present study, the hyperoxia-exposed group
exhibited markedly decreased expression levels of EGFL7 mRNA and
protein when compared with the control. However, the mRNA and
protein expression levels of EGFL7 were significantly increased at
each time-point in the hyperoxia-exposed rhEPO-treated group when
compared with the hyperoxia-exposed group. EGFL7, also termed
vascular endothelial statin, is an endothelial cell-derived
secreted factor that is important in vascular tubulogenesis;
diminished expression levels of EGFL7 may be associated with
hyperoxia-induced endothelial cell death and lung injury, and
hEGFL7 may protect endothelial cells from hyperoxia-induced
apoptosis by inhibition of the mitochondria-dependent apoptosis
pathway (9).
A previous study demonstrated that the
overexpression of EGFL7 reduced the expression level of the
pro-apoptotic protein, Bax, and increased the expression level of
the anti-apoptotic protein, Bcl-2, which prevents hyperoxia-induced
endothelial cell death and promotes lung vascular development
(9). It is known that Bax promotes
apoptosis through binding Bcl-2 and it inhibits the anti-apoptotic
function during mitochondria-regulated programmed cell death
(27). In the current study, the
expression level of Bax was significantly increased in the
hyperoxia-exposed group when compared with the air-exposed group,
and it was decreased in the hyperoxia-exposed rhEPO-treated group
when compared with the hyperoxia-exposed group; an opposite trend
was observed in the expression level of Bcl-2. This suggests that
rhEPO attenuates hyperoxia-induced lung injury and that this effect
of rhEPO may occur through upregulating EGFL7 expression.
Subsequently, the increased EGFL7 expression reduces the expression
level of Bax and increases the expression level of Bcl-2, which
prevents hyperoxia-induced endothelial cell death and promotes lung
vascular development.
In conclusion, rhEPO treatment significantly
attenuated hyperoxia-induced lung injury (such as decreased
alveolization and increased cell apoptosis) by upregulating EGFL7
expression in newborn rats. These findings support the potential
use of rhEPO as a therapeutic agent in the prevention of BPD.
Further studies regarding dosage and safety are required for the
translation of EPO treatment into clinical trials. Thus, rhEPO may
have potential for use as a therapeutic strategy for BPD in
neonates.
Acknowledgements
The present study was supported by the Guangdong
Province Science and Technology Plan Project (grant no.
2013B051000049 awarded to Professor Weimin Huang).
References
|
1
|
Li C, Fu J, Liu H, Yang H, Yao L, You K
and Xue X: Hyperoxia arrests pulmonary development in newborn rats
via disruption of endothelial tight junctions and downregulation of
Cx40. Mol Med Rep. 10:61–67. 2014.PubMed/NCBI
|
|
2
|
Trembath A and Laughon MM: Predictors of
bronchopulmonary dysplasia. Clin Perinatol. 39:585–601. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lal CV and Ambalavanan N: Genetic
predisposition to bronchopulmonary dysplasia. Semin Perinatol.
39:584–591. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Northway WH Jr, Rosan RC and Porter DY:
Pulmonary disease following respirator therapy of hyaline-membrane
disease. Bronchopulmonary dysplasia. N Engl J Med. 276:357–368.
1967. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shivanna B, Zhang S, Patel A, Jiang W,
Wang L, Welty SE and Moorthy B: Omeprazole attenuates pulmonary
aryl hydrocarbon receptor activation and potentiates
hyperoxia-induced developmental lung injury in newborn mice.
Toxicol Sci. 148:276–287. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jakkula M, Le Cras TD, Gebb S, Hirth KP,
Tuder RM, Voelkel NF and Abman SH: Inhibition of angiogenesis
decreases alveolarization in the developing rat lung. Am J Physiol
Lung Cell Mol Physiol. 279:L600–L607. 2000.PubMed/NCBI
|
|
7
|
Remesal A, Pedraz C, San Feliciano L and
Ludeña D: Pulmonary expression of vascular endothelial growth
factor (VEGF) and alveolar septation in a newborn rat model exposed
to acute hypoxia and recovered under conditions of air or
hyperoxia. Histol Histopathol. 24:325–330. 2009.PubMed/NCBI
|
|
8
|
Chang M, Bany-Mohammed F, Kenney MC and
Beharry KD: Effects of a superoxide dismutase mimetic on biomarkers
of lung angiogenesis and alveolarization during hyperoxia with
intermittent hypoxia. Am J Transl Res. 5:594–607. 2013.PubMed/NCBI
|
|
9
|
Xu D, Perez RE, Ekekezie II, Navarro A and
Truog WE: Epidermal growth factor-like domain 7 protects
endothelial cells from hyperoxia-induced cell death. Am J Physiol
Lung Cell Mol Physiol. 294:L17–L23. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang XH and Huang WM: Astragalus
polysaccharides exert protective effects in newborn rats with
bronchopulmonary dysplasia by upregulating the expression of EGFL7
in lung tissue. Int J Mol Med. 34:1529–1536. 2014.PubMed/NCBI
|
|
11
|
Ozer EA, Kumral A, Ozer E, Yilmaz O, Duman
N, Ozkal S, Koroglu T and Ozkan H: Effects of erythropoietin on
hyperoxic lung injury in neonatal rats. Pediatr Res. 58:38–41.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Luo W, Hu L and Wang F: The protective
effect of erythropoietin on the retina. Ophthalmic Res. 53:74–81.
2015.PubMed/NCBI
|
|
13
|
Wang XL, Fu JH and Xue XD: Effects of
hyperoxia on erythropoietin receptor expression in lung development
of neonatal rats. Zhonghua Er Ke Za Zhi. 49:361–366. 2011.(In
Chinese). PubMed/NCBI
|
|
14
|
Lee JH, Sung DK, Koo SH, Shin BK, Hong YS,
Son CS, Lee JW, Chang YS and Park WS: Erythropoietin attenuates
hyperoxia-induced lung injury by down-modulating inflammation in
neonatal rats. J Korean Med Sci. 22:1042–1047. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Martin CR, Zaman MM, Gilkey C, Salguero
MV, Hasturk H, Kantarci A, Van Dyke TE and Freedman SD: Resolvin D1
and lipoxin A4 improve alveolarization and normalize septal wall
thickness in a neonatal murine model of hyperoxia-induced lung
injury. PLoS One. 9:e987732014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Carrera P, Di Resta C, Volonteri C,
Castiglioni E, Bonfiglio S, Lazarevic D, Cittaro D, Stupka E,
Ferrari M and Somaschini M: BPD and Genetics Study Group: Exome
sequencing and pathway analysis for identification of genetic
variability relevant for bronchopulmonary dysplasia (BPD) in
preterm newborns: A pilot study. Clin Chim Acta 451 (Pt A). 39–45.
2015. View Article : Google Scholar
|
|
18
|
Spiteller G: The important role of lipid
peroxidation processes in aging and age dependent diseases. Mol
Biotechnol. 37:5–12. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dang H, Wang S, Yang L, Fang F and Xu F:
Upregulation of Shh and Ptc1 in hyperoxia induced acute lung injury
in neonatal rats. Mol Med Rep. 6:297–302. 2012.PubMed/NCBI
|
|
20
|
D'Angio CT and Maniscalco WM: The role of
vascular growth factors in hyperoxia-induced injury to the
developing lung. Front Biosci. 7:d1609–d1623. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Asikainen TM, Ahmad A, Schneider BK, Ho
WB, Arend M, Brenner M, Günzler V and White CW: Stimulation of
HIF-1alpha, HIF-2alpha, and VEGF by prolyl 4-hydroxylase inhibition
in human lung endothelial and epithelial cells. Free Radic Biol
Med. 38:1002–1013. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bany-Mohammed FM, Slivka S and Hallman M:
Recombinant human erythropoietin: Possible role as an antioxidant
in premature rabbits. Pediatr Res. 40:381–387. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ding L, Wu BQ, Huang JJ, Liu ZP and Chen
L: Effect of erythropoietin on apoptosis following hyperoxic lung
injury in neonatal rats. Zhongguo Dang Dai Er Ke Za Zhi.
12:576–579. 2010.(In Chinese). PubMed/NCBI
|
|
24
|
Piedboeuf B, Gamache M, Frenette J,
Horowitz S, Baldwin HS and Petrov P: Increased endothelial cell
expression of platelet-endothelial cell adhesion molecule-1 during
hyperoxic lung injury. Am J Respir Cell Mol Biol. 19:543–553. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Privratsky JR and Newman PJ: PECAM-1:
Regulator of endothelial junctional integrity. Cell Tissue Res.
355:607–619. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Matsuda Y, Hagio M and Ishiwata T: Nestin:
A novel angiogenesis marker and possible target for tumor
angiogenesis. World J Gastroenterol. 19:42–48. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Oltvai ZN, Milliman CL and Korsmeyer SJ:
Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that
accelerates programmed cell death. Cell. 74:609–619. 1993.
View Article : Google Scholar : PubMed/NCBI
|