Introduction
Bacterial keratitis is a major cause of blindness
worldwide with a high incidence (1).
This is due to low hygiene standards and water quality with a
greater risk of infection in poor countries. In addition, the
widespread use of contact lenses and associated complications in
wealthier countries contributes to severe keratitis (2,3).
Staphylococcus aureus and Pseudomonas aeruginosa are
two predominant gram-positive and gram-negative bacterial strains
responsible for causing this disease (1,4,5). The resistance of bacteria to antibiotics
is reducing the efficacy of bacterial keratitis treatment.
Therefore, research into the development of novel therapeutic
approaches is essential. In this regard photodynamic inactivation
has been in experimental and clinical use throughout the last
decade (6–8). Research on cystic fibrosis established an
antimicrobial effect of gaseous nitric oxide (gNO) in infections
with P. aeruginosa using a rat model (9). Furthermore, a study in white rabbits
demonstrated that a treatment using 200 ppm gNO significantly
reduced the bacterial load in wounds of the dorsal skin surface
(10). Thus, in view of the
possibility of a surface treatment for keratitis, this therapeutic
strategy demonstrated a feasible approach.
The aim of the current study was to evaluate gNO
application as a potential treatment of experimental keratitis
in vivo for the first time. However, this series of
experiments did not serve as proof of the anti-inflammatory effect
of gNO.
Materials and methods
Animals and anaesthesia
Young (age, 9–10.5 weeks) female C57BL/6 mice [n=19;
standard housing conditions (3–5 animals per cage) with 12-h
light/dark cycle, minimum 70% humidity and chow/water were provided
ad libitum; Janvier Labs, Saint-Berthevin Cedex, France]
were used in the present study. All animal care and experimental
procedures were approved by the local governmental animal care
committee (permit no. 58/2013) and were conducted in accordance
with European legislation on the protection of animals (Directive
2010/63/EU) and National Institutes of Health (NIH) guidelines on
the care and use of laboratory animals (NIH publication no. 85–23
Rev. 1985). Animals were anesthetized via i.p. injection of
ketamine (75 mg kg-1 body weight; Pharmacia GmbH, Erlangen,
Germany) and xylazine (15 mg kg-1 body weight; Rompun, Bayer,
Leverkusen, Germany).
Infection and treatment
Three scratches (length, 2 mm) were made in the
centre of the cornea using a 27-gauge syringe, subsequently 5 µl of
the inoculum was pipetted onto the cornea [according to (4)]. Carprofen (Rimadyl®; Zoetis
Schweiz GmbH, Zürich, Switzerland) was injected during anaesthesia.
For the inoculum, the multi drug resistant P. aeruginosa
strain 54 (clinical isolate, collected in 2009 at the Institute of
Medical Microbiology and Hygiene, Saarland University Medical
Center, Homburg, Germany. The resistance profile of PA54 was
determined using the automated VITEK 2 system (BioMérieux GmbH,
Nürtingen, Germany) was cultured on blood agar plates (Trypticase
Soy Agar II, 5% Sheep Blood; BD GmbH, Heidelberg, Germany) for 24
h, and suspended in 10 ml 2% Luria Bertani (LB) broth (LB Broth;
Difco, Saint Egrève, France) for an overnight culture (37°C). The
overnight culture (100 µl) was diluted in 10 ml 2% LB for another 3
h of culturing (37°C) and finally the suspension was adjusted using
a photometer to an optical density of 10 at a wavelength of 600 nm
and then used for inoculation. Only one eye from each animal was
infected, with certain non-infected eyes analyzed to serve as
controls. Eyes were either not infected or infected, and the
infected eyes were either treated or not treated
(uninfected/untreated, n=6 eyes; infected/treated, n=11 eyes;
infected/untreated, n=8 eyes).
Twenty-four hours post infection the animals were
treated under deep anesthesia as described above. For NO treatment,
the infected eyes were exposed to gas (Praxair Deutschland GmbH,
Düsseldorf, Deutschland) consisting of nitrogen and NO (200 ppm)
for 30 min under anesthesia. A gNO stream with a velocity of 5
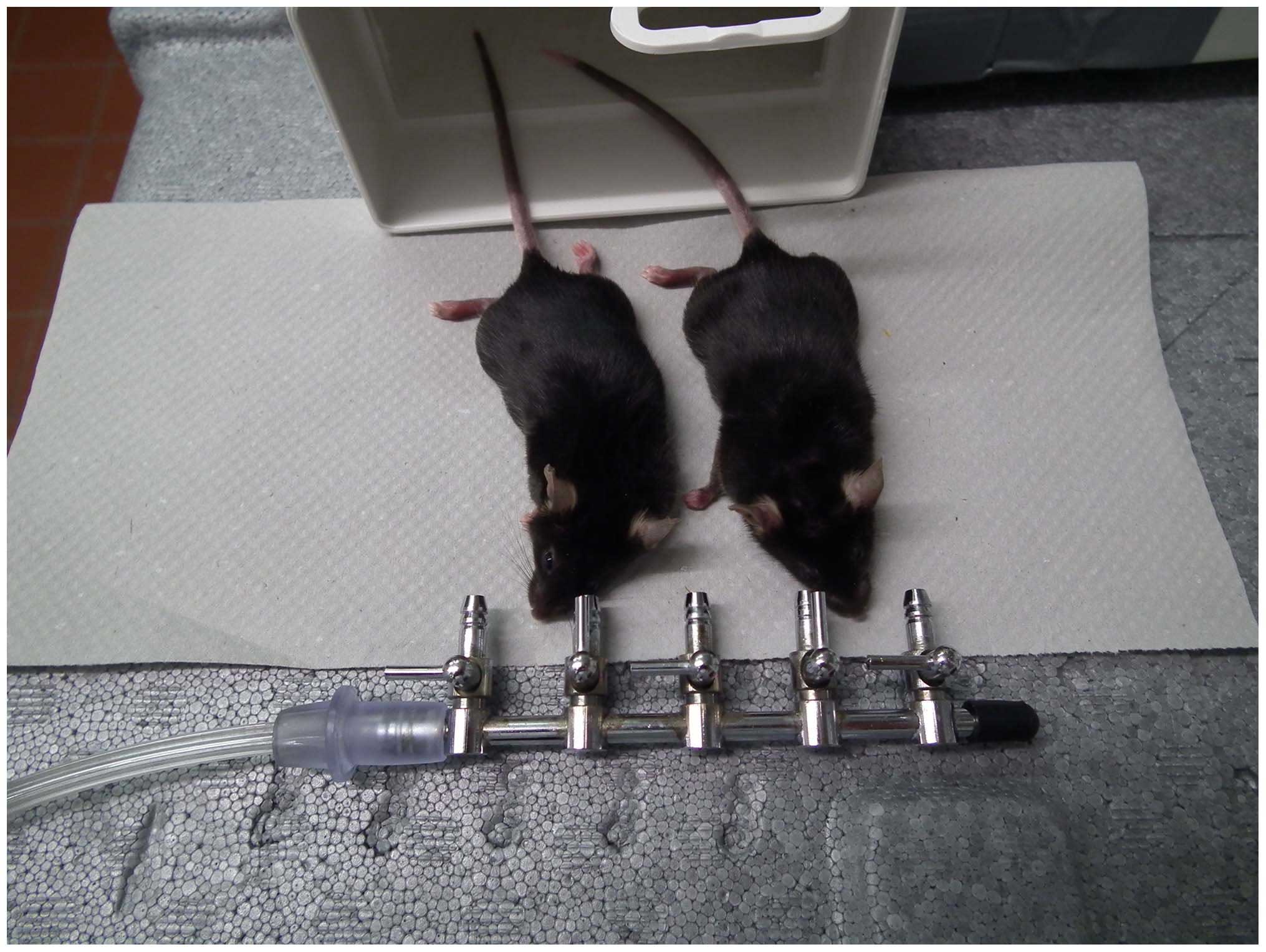
m/sec was directed to each eye using a nozzle. This nozzle was
placed at a distance of 1 cm from the eye (Fig. 1). Three days after the infection (in
two cases, 7 days after the infection to assess whether treatment
required >3 days to develop an effect) the animals were
sacrificed by cervical dislocation.
Histology and evaluation
Their eyes were enucleated, histologically
processed, and stained with hematoxylin and eosin. From each eye,
five subsequent sections (thickness, 5 µm, with a distance of 50 µm
from each other) from the center of the cornea (middle of the
pupil) were obtained for evaluation (Fig.
2). The maximum corneal thickness was measured (CellSens
Standard 1.8.1; Olympus Deutschland GmbH, Hamburg, Germany) and the
mean value was taken. The severity of hypopyon was evaluated with a
self-defined score, from 0 to 3 (0, no hypopyon; 1, mild hypopyon;
2, moderate hypopyon; 3, severe/massive hypopyon; Fig. 3).
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 5 (GraphPad Software, Inc., La Jolla, CA, USA). The
Mann-Whitney U test or the χ2 test were used for
inferential statistics. Mean values and standard errors of the mean
(SEM) were calculated and two-sided P<0.05 was considered to
indicate a statistically significant difference.
Results
Maximum corneal thickness as an
indication of corneal inflammation
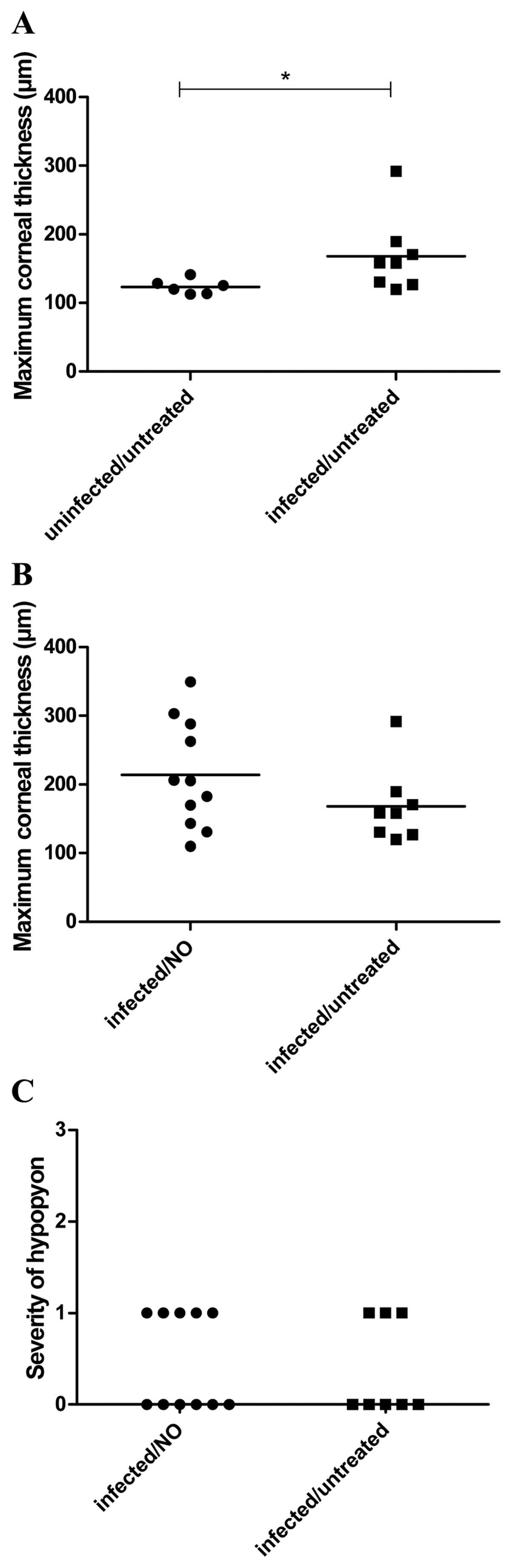
Concerning the maximum corneal thickness, the
experiments resulted in mean values of 124±4µm (mean ± SEM) for
uninfected/untreated, 168±20 µm (mean ± SEM) for infected/untreated
and 214±23 µm (mean ± SEM) for infected/treated animals (Fig. 4A and B). The infection led to a
significant increase in the maximum corneal thickness. A
significant influence of the gNO treatment was not identified for
maximum corneal thickness.
Occurrence and severity of
hypopyon
Similarly, a significant influence of the gNO
treatment was not identified with regard to the severity of
hypopyon (Fig. 4C).
Discussion
Treatment with gNO is a novel and innovative
approach as no resistance has yet been identified for this
bactericidal mechanism. This initial in vivo study did not indicate
an anti-inflammatory effect using gNO. In a previous study
regarding bacterial pneumonia that was induced by P.
aeruginosa, no influence of NO treatment was observed on the
inflammation parameters, which was consistent with the present
results (9). By contrast, the authors
identified a marked reduction in the bacterial load of
pseudomonades. The present study hypothesized that i) bacteria were
killed and ii) corneal inflammation was reduced following gNO
treatment. It might be a similar situation in our keratitis model
as in the pneumonia model described in the study by Miller et
al (9) where inflammation was not
reduced in contrast to the bacterial load. The bacterial load was
not determined in the present study, as bacterial load can only be
determined in the whole globes, but not separately within the
cornea alone, due to the small structure of the murine cornea.
However, the hypothesis remains that gNO application kills bacteria
within the cornea, but alternative experimental approaches are
required. Another aspect of the study was the technical
administration of gNO treatment. This treatment strategy was
established specifically for the current study. The gas application
could be improved in future to ensure that a constant gNO
concentration is applied to the surface of the cornea, for example,
using an eye chamber, although this may be difficult due to the
size of the mouse eye.
In conclusion, the aim of the current study was to
evaluate gNO application as a treatment for experimental keratitis;
this is the first study, to the best of our knowledge, to attempt
to treat keratitis using this method. No anti-inflammatory effects
were observed; however, this method should be investigated further
to evaluate the bactericidal effects in vivo.
Acknowledgements
The authors would like to thank Ms. Ann Soether for
language editing and Ms. Tina Wiesen-Philipps for help with the
manuscript.
References
|
1
|
Ong HS and Corbett MC: Corneal infections
in the 21st century. Postgrad Med J. 91:565–571. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lorenzo-Morales J, Khan NA and Walochnik
J: An update on Acanthamoeba keratitis: Diagnosis, pathogenesis and
treatment. Parasite. 22:102015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Young G, Young AG and Lakkis C: Review of
complications associated with contact lenses from unregulated
sources of supply. Eye Contact Lens. 40:58–64. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Girgis DO, Sloop GD, Reed JM and
O'Callaghan RJ: Susceptibility of aged mice to Staphylococcus
aureus keratitis. Curr Eye Res. 29:269–275. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Marquart ME: Animal models of bacterial
keratitis. J Biomed Biotechnol. 2011:6806422011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Makdoumi K, Mortensen J, Sorkhabi O,
Malmvall BE and Crafoord S: UVA-riboflavin photochemical therapy of
bacterial keratitis: A pilot study. Graefes Arch Clin Exp
Ophthalmol. 250:95–102. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stachon T, Wang J, Song X, Langenbucher A,
Seitz B and Szentmáry N: Impact of
crosslinking/riboflavin-UVA-photodynamic inactivation on viability,
apoptosis and activation of human keratocytes in vitro. J Biomed
Res. 29:321–325. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu MF, Stachon T, Wang J, Song X, Colanesi
S, Seitz B, Wagenpfeil S, Langenbucher A and Szentmáry N: Effect of
keratocyte supernatant on epithelial cell migration and
proliferation after corneal crosslinking (CXL). Curr Eye Res.
41:466–473. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Miller CC, Hergott CA, Rohan M,
Arsenault-Mehta K, Döring G and Mehta S: Inhaled nitric oxide
decreases the bacterial load in a rat model of Pseudomonas
aeruginosa pneumonia. J Cyst Fibros. 12:817–820. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ghaffari A, Jalili R, Ghaffari M, Miller C
and Ghahary A: Efficacy of gaseous nitric oxide in the treatment of
skin and soft tissue infections. Wound Repair Regen. 15:368–377.
2007. View Article : Google Scholar : PubMed/NCBI
|