Introduction
Vitamin D deficiency is a global health problem; an
estimated 1 billion people across all ethnicities and age groups
have deficient or inadequate levels of vitamin D worldwide
(1). Vitamin D deficiency is mainly a
result of reduced exposure to sunlight, which is essential for
ultraviolet-B (UVB)-induced vitamin D production in the skin
(2). Previous studies have indicated
that vitamin D deficiency may be associated with increased
incidence rates of cardiovascular disease, cancer, diabetes and
cognitive impairment (3–6). It is considered that vitamin D
deficiency is unlikely to occur in regions with adequate sunshine,
and as such vitamin D deficiency may be overlooked in the
neighboring countries of Nepal, including India and China (7,8). While
different boundaries have been proposed to define vitamin D
deficiency, Holick (9) defined
vitamin D insufficiency as <30 ng/ml serum 25-hydroxyvitamin D
[25(OH)D], and vitamin D deficiency as a serum 25(OH)D level <20
ng/ml. Vitamin D is considered the major sun-induced vitamin, and
thus the traditional fashions in Nepal and India, religious or
otherwise, may be responsible for vitamin D deficiency (8). In particular, indoor workers are
probably unable to achieve normal serum levels of 25(OH)D from sun
exposure alone. Though vitamin D3 is naturally present in small
quantities in foodstuffs including oily fish, eggs and fortified
foods, vitamin D supplementation is generally required to achieve
the desired threshold concentration of serum 25(OH)D among
deficient individuals (10).
There is no universally accepted threshold at which
initiating vitamin D supplementation would achieve the greatest
impact. Therapeutic strategies that aid adherence to treatment with
the aim of providing long-term vitamin D supplement are of clinical
interest. Similar to the trend in India (11), at present, it is common practice by
physicians in Nepal to prescribe cholecalciferol at a dose of
60,000 IU/week for two months, then at 60,000 IU/month for six
months for occult vitamin D deficiency. However, the short- and
long-term effects on serum 25(OH)D levels in Nepalese individuals
have not been systematically studied. Therefore, the present study
reports on 19 healthy laboratory personnel working at the National
Center for Rheumatic Diseases, Kathmandu, Nepal, with severe
vitamin D deficiency who received oral cholecalciferol at 60,000
IU/week for a duration of 2 months, with the aim of assessing
changes in the serum concentration of 25(OH)D induced by
cholecalciferol supplementation.
Materials and methods
Location
Kathmandu is the capital and largest metropolitan
city of Nepal in terms of population, located centrally at an
elevation of ~1,400 m (4,600 feet) above sea level in the Kathmandu
Valley. At high altitude UVB travels over a shorter distance to the
earth's surface and thus the skin is exposed to higher levels,
enabling it to produce more vitamin D3; according to Holick et
al (12), there is direct
correlation of increased previtamin D3 production with increased
altitude. Notably, they observed in the Indian city, Agra (169 m
altitude), that there was a 5-fold decrease in previtamin D3
production compared with that at Mount Everest base camp (5,300 m
altitude) (12).
Subjects
Between January 2016 and March 2016, a prospective
open-label 2-month study was performed on 19 healthy laboratory
volunteers (mean age 23.47±3.18 years) working at the National
Center for Rheumatic Diseases. Serum 25(OH)D level was measured at
baseline and after a 2-month regimen with oral cholecalciferol
supplement at a dose of 60,000 IU (1,500 µg)/week (Cholirol-60K;
Vega Pharmaceuticals Pvt., Ltd., Nepal) in tablet form. The
majority of the volunteers (n=19) had insufficient sunlight
exposure as they generally worked in the laboratory from 8 a.m. to
6 p.m.
Subjects included in the study were aged 18 years
and older, working in the laboratory and available for follow-up
after 2 months. Subjects excluded from the study were part time
laboratory workers, not available for follow-up and/or taking
vitamin D ≥400 IU/day prior to the study. All subjects were
counseled to confirm the weekly intake of cholecalciferol and the
2-month follow-up after completion of treatment. Volunteers were
advised to retain the same sun exposure pattern during the study
period. Drug compliance was also assessed by counting empty
sachets. As the study was a pilot, the trial was not registered.
The study was conducted according to the guidelines provided in the
Declaration of Helsinki (13).
Additionally, the study was approved by the Ethics Committee of the
National Center for Rheumatic Diseases, and informed written
consent was obtained from all patients prior to their enrollment in
the study.
Data collection
Vitamin D estimation was performed in the Diagno
Labs Pvt., Ltd., Gurgaon, India using an ELISA kit provided by
Roche Diagnostics GmbH (06506780160; Mannheim, Germany). Serum
25(OH)D levels were measured by electrochemiluminescence using a
fully automated system (Cobas e411; Roche Diagnostics GmbH,
Mannheim, Germany). Vitamin D deficiency, insufficiency and
sufficiency was defined based on serum 25(OH)D concentrations of
<20, 20–30 and 30–100 ng/ml, respectively (9).
Data analysis
Statistical analysis was performed using SPSS 20.0
software (IBM Corp., Armonk, NY, USA). Data are presented as means
± standard deviation. A dependent samples t-test was used to
compare the means of serum 25(OH)D levels at baseline and after the
2-month regimen with oral cholecalciferol supplement. The
statistical testing was two-tailed, and P<0.05 was considered to
indicate statistical significance.
Results
Characteristics of study
participants
As presented in Table
I, relevant data were available for all 19 subjects included in
the study. Participants ranged in age from 19 to 30 years (mean age
23.47±3.18 years), and there was a greater proportion of females
(n=15, 79.0%) than males (n=4, 21.0%). At baseline, 17 subjects
(89.4%) exhibited vitamin D deficiency, 1 (5.3%) exhibited
insufficiency and 1 (5.3%) exhibited sufficiency. The mean 25(OH)D
level of females was 10.15±8.45 ng/ml while the mean 25(OH)D level
of males was 17.80±13.42 ng/ml.
 | Table I.General characteristics of study
population. |
Table I.
General characteristics of study
population.
| Variable | Subjects, n=19 |
|---|
| Mean age ± SD, years
(range) | 23.47±3.18
(19–31) |
| Female, n (%) | 15 (79) |
| Male, n (%) | 4 (21) |
| Mean 25(OH)D level ±
SD, ng/ml |
|
|
Females | 10.15±8.45 |
|
Males | 17.80±13.42 |
| Vitamin D status, n
(%) |
|
|
Deficiency | 17 (89.4) |
|
Insufficiency | 1 (5.3) |
|
Sufficiency | 1 (5.3) |
Effect of oral cholecalciferol on
serum 25(OH)D concentration
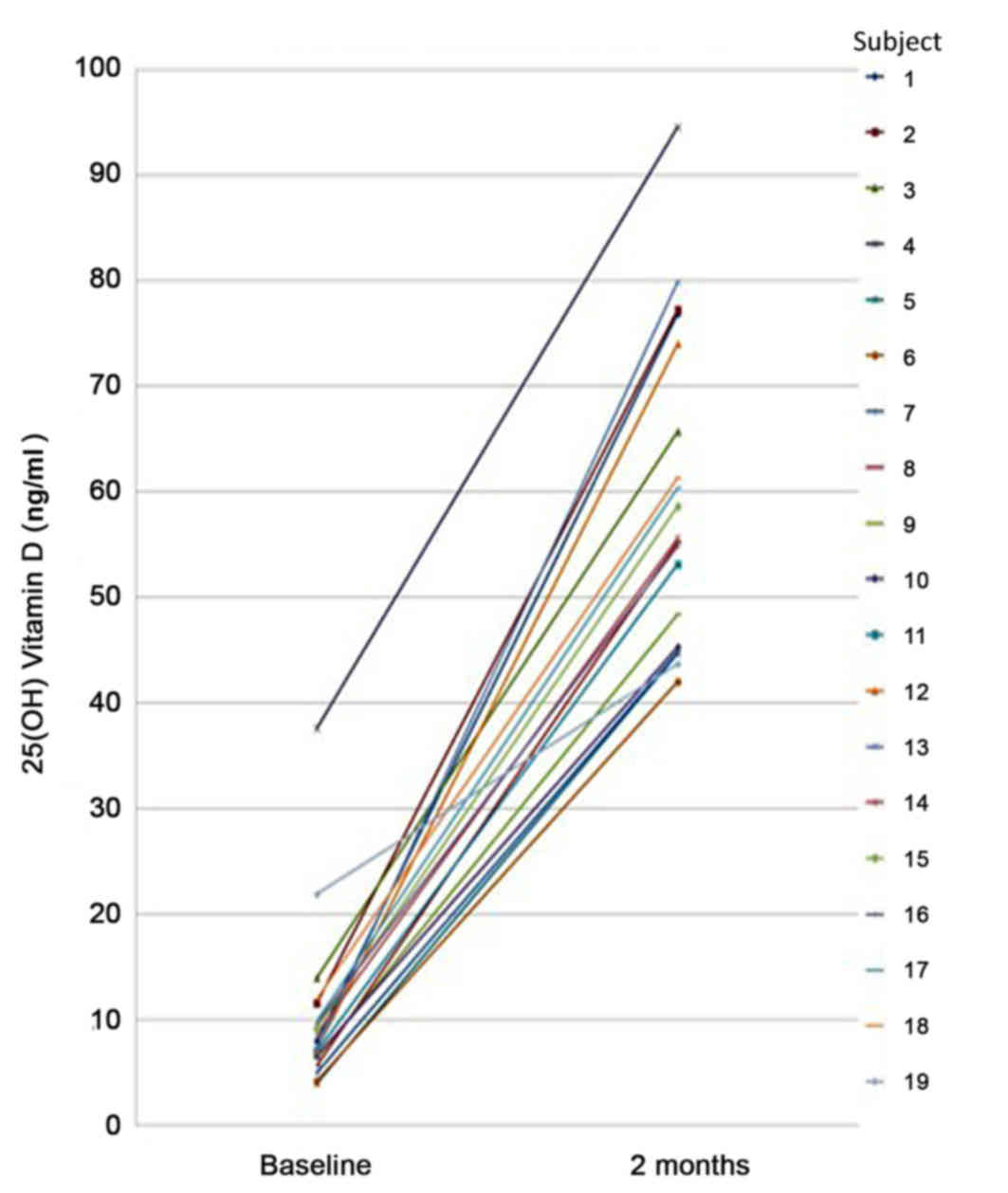
At baseline, the mean 25(OH)D level was 10.31±7.78
ng/ml, with a minimum of 4 ng/ml and maximum of 37.6 ng/ml.
Following completion of the course of oral cholecalciferol, the
serum concentration of 25(OH)D in all volunteers was in the
sufficient range (30–100 ng/ml). The mean serum level of 25(OH)D
following the supplement course was 59.78±14.74 ng/ml, with a
minimum of 42 ng/ml and maximum of 94.50 ng/ml (Fig. 1 and Table
I). Notably, following the weekly supplement of cholecalciferol
for two months, the increase in the mean serum level of 25(OH)D
compared with baseline was significant to P<0.001 (Table II).
 | Table II.Mean 25(OH)D levels of laboratory
personnel at baseline and following cholecalciferol supplement
therapy. |
Table II.
Mean 25(OH)D levels of laboratory
personnel at baseline and following cholecalciferol supplement
therapy.
| Serum 25(OH)D level,
ng/ml | Mean ± SD | P-value |
|---|
| Baseline | 10.31±7.78 |
|
| After
supplementation | 59.78±14.74 | <0.001 |
Discussion
Few studies have been conducted on oral vitamin D3
supplementation in Nepal. However, low levels of vitamin D have
been associated with reduced health, particularly by contributing
to increased risk of fractures, functional limitations and chronic
diseases (3–5). To the best of our knowledge, the present
study was the first prospective study in Nepal to evaluate the
effect of high dose oral vitamin D3 supplementation (60,000 IU
cholecalciferol) on mean serum 25(OH)D concentration among
laboratory personnel.
Consistent with previous studies (14–16), the
results of the present study indicated that females had lower
25(OH)D levels than males. Traditional and religious clothing
habits and lifestyle are considered to be responsible for vitamin D
deficiency, particularly in women (17,18). In
Nepal, the majority of individuals, particularly females, wear
clothes that shield the majority of the skin from sunlight, due to
religious beliefs and/or cosmetic concerns, which thus reduces the
synthesis of vitamin D.
The major source of vitamin D in humans is sunlight.
In the present study, at baseline, the majority of volunteers
exhibited deficient 25(OH)D levels. Exposure to sunlight for ~20
min per day is considered to maintain sufficient levels of vitamin
D (15,16). However, all participants in the
present study worked in a laboratory during typical sunlight hours
(~8 a.m. to 6 p.m.), and thus their sunlight exposure was minimal,
which was the probable cause for their vitamin D deficiency, and is
in accordance with previous studies (19,20). Roomi
et al (18) concluded that
indoor occupations were associated with hypovitaminosis D, and a
later study on coal mine workers identified vitamin D deficiency
and insufficiency among the participants (21). Furthermore, a study in Korea (22) demonstrated that occupational factors
including shift work (shifts from 2 pm to midnight, 9 pm to 8 am
the following day and 24-hour shifts) and office work were
associated with an increased risk of vitamin D deficiency.
Similarly, a British cohort study (23) indicated that individuals working at
night and for longer hours may be vulnerable to deficits in vitamin
D.
As further factors to consider, Wacker et al
(24) observed that vitamin D
deficiency was associated with weather, time of day, altitude, air
pollution and blockage of sunlight by high-rise buildings.
Kathmandu is at high altitude, which may be beneficial for the skin
regarding its production level of vitamin D3; however, being among
the most polluted cities globally, the blockage of sunlight by air
pollution may be a reason for deficits in vitamin D (18).
Few studies have indicated the effect of high dose
vitamin D on serum 25(OH)D. However, in the present study,
following the 2-month regimen of weekly oral cholecalciferol, the
mean serum level of 25(OH)D was significantly increased. This is
similar to the findings of Gowda et al (25) and Garrett-Mayer et al (26). Notably, following the oral
supplementation regimen, the present study observed an almost
5-fold increase in serum 25(OH)D level compared with that at
baseline. This trend of a rise in serum 25(OH)D following 2 months
of supplementation with cholecalciferol is similar to observations
in Indian (11) and Caucasian
(27) subjects. Additionally,
findings of the present study demonstrated that supplementation
with high dose vitamin D3 (60,000 IU/week cholecalciferol) restored
serum levels to sufficient levels (>30 ng/ml) among laboratory
personnel, even for those with lower baseline levels.
Although the US Endocrine Society Clinical Practice
Guidelines (28) recommends
1,500–2,000 IU/day vitamin D for adults aged 19 years and over,
there is currently no recommendation for vitamin D supplementation
in Nepal. Thus, health policy-makers should formulate a uniform
guideline for the supplementation of vitamin D in Nepal.
Furthermore, health professionals working indoors and office
workers should be educated on the benefits of vitamin D
supplementation.
There were a number of limitations to the present
study. Firstly, the majority of study subjects were women, and thus
the results may not be generalizable to general populations.
Secondly, the sample size was relatively small and there was a lack
of consideration of exposure to sunlight, sunscreen application,
clothing coverage, dietary information and other vitamin D
supplementation, which may have influenced the change in serum
25(OH)D during the course of the study. Finally, the study was
conducted during winter (January to March), and a previous study
has demonstrated that there is seasonal variation in sunlight
exposure (29). Therefore, the data
was not adjusted for seasonal factors, which may have influenced
serum 25(OH)D levels. Despite these limitations, the study had
several strengths. The effect of high dose, oral vitamin D3
supplementation on mean serum 25(OH)D concentration was
investigated among laboratory personnel, which have rarely been
studied despite having clinical importance. Thus, the present
results may be useful in guiding early interventions that prevent
vitamin D deficiency, and for the formulation of guidelines to
implement vitamin D supplementation among indoor and office workers
in Nepal.
In conclusion, to the best of our knowledge, the
present study was the first to investigate vitamin D levels in
laboratory personnel at baseline and following an oral
supplementation regimen with cholecalciferol in Nepal. Vitamin D
deficiency is an important but modifiable public health risk in
Nepal, though often remains undiagnosed or insufficiently treated.
Adequate sun exposure and vitamin D supplementation are key to
achieving the vitamin D requirement of the body. The present
findings highlight that vitamin D deficiency is common among
laboratory workers. Further prospective studies with larger sample
sizes are now required in this direction to examine the correlation
between vitamin D deficiency and working conditions.
Acknowledgements
The tests and medicines in the present study were
funded by the Nepal Rheumatic Disease Relief Foundation.
References
|
1
|
Holick MF and Chen TC: Vitamin D
deficiency: A worldwide problem with health consequences. Am J Clin
Nutr. 87:1080–1086. 2008.
|
|
2
|
Nair R and Maseeh A: Vitamin D: The
‘sunshine’ vitamin. J Pharmacol Pharmacother. 3:118–126.
2012.PubMed/NCBI
|
|
3
|
Mozos I and Marginean O: Links between
vitamin D deficiency and cardiovascular diseases. BioMed Res Int.
2015:1092752015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ames BN and Wakimoto P: Are vitamin and
mineral deficiencies a major cancer risk? Nat Rev Cancer.
2:694–704. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
González-Molero I, Rojo-Martínez G,
Morcillo S, Gutiérrez-Repiso C, Rubio-Martín E, Almaraz MC, Olveira
G and Soriguer F: Vitamin D and incidence of diabetes: A
prospective cohort study. Clin Nutr. 31:571–573. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Balion C, Griffith LE, Strifler L,
Henderson M, Patterson C, Heckman G, Llewellyn DJ and Raina P:
Vitamin D, cognition, and dementia: A systematic review and
meta-analysis. Neurology. 79:1397–1405. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Babu US and Calvo MS: Modern India and the
vitamin D dilemma: Evidence for the need of a national food
fortification program. Mol Nutr Food Res. 54:1134–1147.
2010.PubMed/NCBI
|
|
8
|
Yu S, Fang H, Han J, Cheng X, Xia L, Li S,
Liu M, Tao Z, Wang L, Hou L, et al: The high prevalence of
hypovitaminosis D in China: A multicenter vitamin D status survey.
Medicine (Baltimore). 94:e5852015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Holick MF: Vitamin D deficiency. N Engl J
Med. 357:266–281. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Boyages S and Bilinski K: Seasonal
reduction in vitamin D level persists into spring in NSW Australia:
Implications for monitoring and replacement therapy. Clin
Endocrinol (Oxf). 77:515–523. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Goswami R, Gupta N, Ray D, Singh N and
Tomar N: Pattern of 25-hydroxy vitamin D response at short (2
month) and long (1 year) interval after 8 weeks of oral
supplementation with cholecalciferol in Asian Indians with chronic
hypovitaminosis D. Br J Nutr. 100:526–529. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Holick MF, Chen TC, Lu Z and Sauter E:
Vitamin D and skin physiology: A D-lightful story. J Bone Miner
Res. 22:28–33. 2007. View Article : Google Scholar
|
|
13
|
World Medical Association, . Declaration
of Helsinki-Ethical Principles of Medical Research Involving Human
SubjectsAdopted by the 18th WMA General Assembly, Helsinki,
Finland, June 1964 and amended by the 55th WMA Assembly. Tokyo,
Japan: October. 2004
|
|
14
|
Hammami MM and Yusuf A: Differential
effects of vitamin D2 and D3 supplements on 25-hydroxyvitamin D
level are dose, sex, and time dependent: A randomized controlled
trial. BMC Endocr Disord. 17:122017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Holick MF: Vitamin D: Importance in the
prevention of cancers, type 1 diabetes, heart disease, and
osteoporosis. Am J Clin Nutr. 79:362–371. 2004.PubMed/NCBI
|
|
16
|
Zittermann A: Vitamin D and disease
prevention with special reference to cardiovascular disease. Prog
Biophys Mol Biol. 92:39–48. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Buyukuslu N, Esin K, Hizli H, Sunal N,
Yigit P and Garipagaoglu M: Clothing preference affects vitamin D
status of young women. Nutr Res. 34:688–693. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Roomi MA, Farooq A, Ullah E and Lone KP:
Hypovitaminosis D and its association with lifestyle factors. Pak J
Med Sci. 31:1236–1240. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Erden G, Ozdemir S, Ozturk G, Erden A,
Kara D, Isik S, Ergil J, Vural C and Arzuhal AE: Vitamin D levels
of anesthesia personnel, office workers and outdoor workers in
Ankara, Turkey. Clin Lab. 62:931–937. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cinar N, Harmanci A, Yildiz BO and
Bayraktar M: Vitamin D status and seasonal changes in plasma
concentrations of 25-hydroxyvitamin D in office workers in Ankara,
Turkey. Eur J Intern Med. 25:197–201. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Peng M, Chen S, Jiang X, Zhang W, Wang Y
and Wu S: Cardiovascular Survey Group OB: Dissociation between low
vitamin D level and hypertension in coal mine workers: Evidence
from the Kailuan Study. Intern Med. 55:1255–1260. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jeong H, Hong S, Heo Y, Chun H, Kim D,
Park J and Kang MY: Vitamin D status and associated occupational
factors in Korean wage workers: Data from the 5th Korea national
health and nutrition examination survey (KNHANES 2010–2012). Ann
Occup Environ Med. 26:282014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ward M, Berry DJ, Power C and Hyppönen E:
Working patterns and vitamin D status in mid-life: A
cross-sectional study of the 1958 British birth cohort. Occup
Environ Med. 68:902–907. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wacker M and Holick MF: Sunlight and
Vitamin D: A global perspective for health. Dermatoendocrinol.
5:51–108. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gowda U, Ruwanpathirana T, Fong DPS, Kaur
A and Renzaho AMN: Efficacy of high dose vitamin D supplementation
in improving serum 25(OH)D among migrant and non migrant
population: A retrospective study. BMC Health Serv Res. 16:5792016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Garrett-Mayer E, Wagner CL, Hollis BW,
Kindy MS and Gattoni-Celli S: Vitamin D3 supplementation (4000 IU/d
for 1 year) eliminates differences in circulating 25-hydroxyvitamin
D between African American and white men. Am J Clin Nutr.
96:332–336. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Malabanan A, Veronikis IE and Holick MF:
Redefining vitamin D insufficiency. Lancet. 351:805–806. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Holick MF, Binkley NC, Bischoff-Ferrari
HA, Gordon CM, Hanley DA, Heaney RP, Murad MH and Weaver CM:
Endocrine Society: Evaluation, treatment, and prevention of vitamin
D deficiency: an Endocrine Society clinical practice guideline. J
Clin Endocrinol Metab. 96:1911–1930. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hollis BW and Wagner CL: Normal serum
vitamin D levels. N Engl J Med. 352:515–516; author reply 515–516.
2005. View Article : Google Scholar : PubMed/NCBI
|