Introduction
Myopia is becoming more prevalent in China. High
myopia has many serious complications that may lead to severe
vision impairment. During the development of myopia, there is a
loss of extracellular matrix (ECM), which may cause sclera
remolding and axial elongation (1).
Despite the changes in scleral ECM that occur during mammalian
myopia development, there is relatively little understanding of the
cellular and signaling factors that drive such changes. Increasing
evidence has indicated that the retina and other relevant ocular
tissues may synthesize and secrete transforming growth factor-β2
(TGF-β2) to regulate the remodeling of the sclera (2–5), which may
result in myopia development. TGF-β has been demonstrated in
vitro to induce matrix metalloproteinases (MMPs) production
from fibroblasts by interfering with the Smad and mitogen-activated
protein kinases (MAPK) signaling pathways (6). MMPs are a family of enzymes that are
capable of triggering the decomposition of scleral ECM components,
the activities of which are regulated by physiological inhibitors,
known as tissue inhibitors of matrix metalloproteinases (TIMPs)
(7–13). TGF-β2, MMPs and TIMPs are key in the
progression of ECM degradation during the pathological processes of
myopia (14–17). To the best of our knowledge, the
correlation between TGF-β2 and MMPs/TIMPs in human aqueous humor in
myopic patients has not previously been reported. Recently, TGF-β2
and MMP/TIMP levels in the aqueous humor were evaluated in 65
patients with high myopia or cataract and it was identified that
the TGF-β2 and MMP/TIMP levels in the aqueous humor of patients
with high myopia were significantly different from patients with
cataract (17,18). As a follow up study of our published
studies (17,18), the aim of the present study was to use
the data (17,18) to analyze the correlation between
TGF-β2 and MMP/TIMP levels in the aqueous humor from these
patients.
Materials and methods
Patients and samples
The subjects and methods used for the measurement of
multiple factors in the current study have been reported previously
(17,18). The previous studies included two
groups of patients as follows: High myopia (35 cases) and cataract
(30 cases). High myopia was defined as patients (with or without
cataract) with refraction <-6 D (35 cases, 35 eyes). Cataract
cases included cataract patients with a range of different
refractive statuses; including emmetropia, hyperopia and myopia,
with the exception of high myopia (30 cases, 30 eyes).
Specimens were obtained at the beginning of the
clear lens extraction or cataract extraction surgery to avoid the
breakdown of the blood-aqueous barrier associated with surgical
manipulation. A 30-gauge needle attached to a tuberculin
microsyringe was used to aspirate the aqueous humor from the
central pupillary area without touching the iris, lens, or corneal
endothelium with the needle to avoid trauma and the contamination
of the aqueous humor specimens by various tissue components or
blood. Specimens were stored immediately below −80°C until analysis
(17,18).
All samples were assayed for total protein levels of
TGF-β2, MMP-1, −2, −3, TIMP-1, −2, and −3 using a Luminex system
(Luminex xMAP Technology, Bio-Rad Laboratories, Inc., Hercules, CA,
USA) and commercially available Milliplex xMAP kits (TGFB-64K-03,
HMMP2MAG-55K-02, HMMP1MAG-55K-01, HTMP2MAG-54K-03; EMD Millipore,
Billerica, MA, USA) (17–20).
All patients signed informed consent and the study
was approved by the Institutional Review Board at the Shanghai
Ninth People's Hospital, Shanghai Jiaotong University School of
Medicine (Shanghai, China) (17,18).
Analysis of the association between
TGF-β2 and TIMPs
The association between the levels of TGF-β2 and
various MMPs/TIMPs (MMP-2, MMP-3, TIMP-1, TIMP-2 and TIMP-3) was
analyzed separately by evaluating the significance of the
correlation coefficient between various groups.
Statistical analysis
The original data were not normally distributed
according to the Kolmogorov-Smirnov test. Therefore, the results
were expressed as medians and ranges (25th and 75th percentiles)
using continuous variables that are not normally distributed, such
as the levels of TGF-β2 and TIMPs, or as a mean (standard
deviation) for normally distributed continuous variables, such as
age. The association between TGF-β2 and TIMPs was analyzed using
Spearmans correlation test. SPSS 22.0 software for Windows (IBM
Corp., Armonk, NY, USA) was used to perform these analyses. A
two-tailed P<0.05 was considered to indicate a statistically
significant difference.
Results
Analysis of the levels of MMPs, TIMPs
and TGF-β2
Briefly, the average age of the 65 patients was
67.0±11.7 years, comprising of 29 males and 36 females (17). Among the 65 subjects, 30 eyes had
cataracts and 35 eyes were highly myopic (all in the stationary
stage). The results of the levels of TGF-β2 and TIMPs were
published in our previous papers (17,18), which
revealed that MMP-1 was not detected and TGF-β2, MMP-2, MMP-3,
TIMP-1, TIMP-2 and TIMP-3 were detected in the aqueous humor. The
levels of TGF-β2, MMP-2, TIMP-1, TIMP-2, TIMP-3 in the high myopia
group were significantly higher than those in the cataract eyes
group, while MMP-3 levels in the high myopia group were not
statistically higher than that in the cataract eyes group.
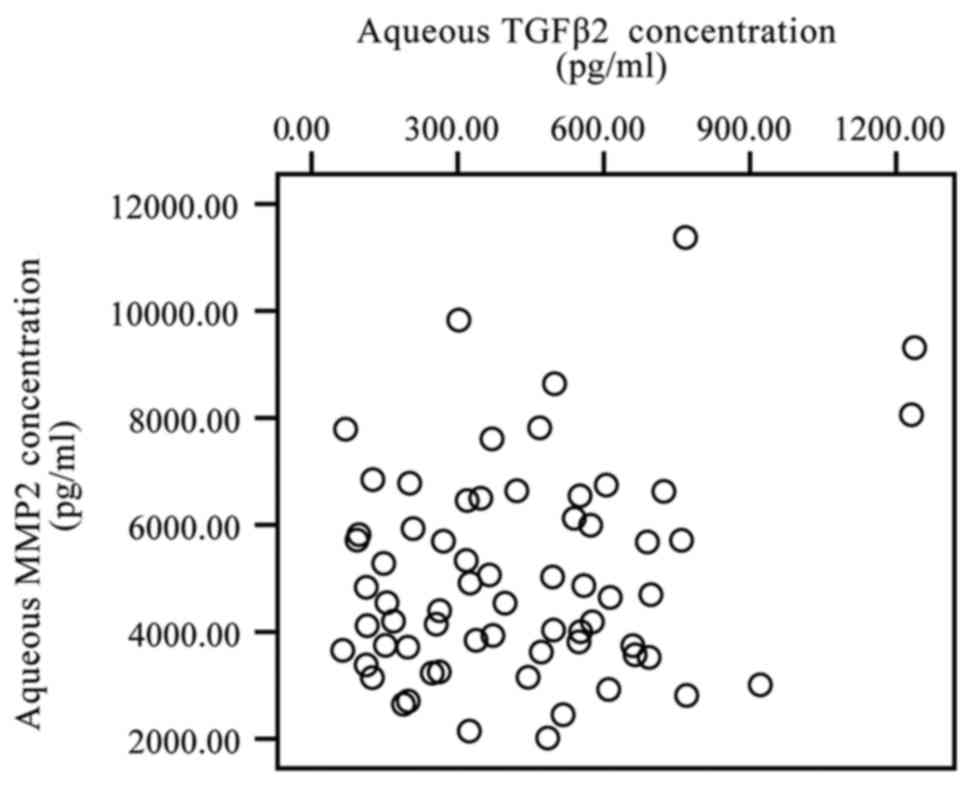
Correlation of TGF-β2 with MMPs
In the present study, the correlation between the
levels of TGF-β2 and different MMPs/TIMPs in the aqueous humor
specimens was analyzed by Spearmans correlation test. The TGF-β2
level was not identified to be significantly correlated with the
MMP-2 level (P>0.05), although the levels of the two increased
in the aqueous humor (Fig. 1).
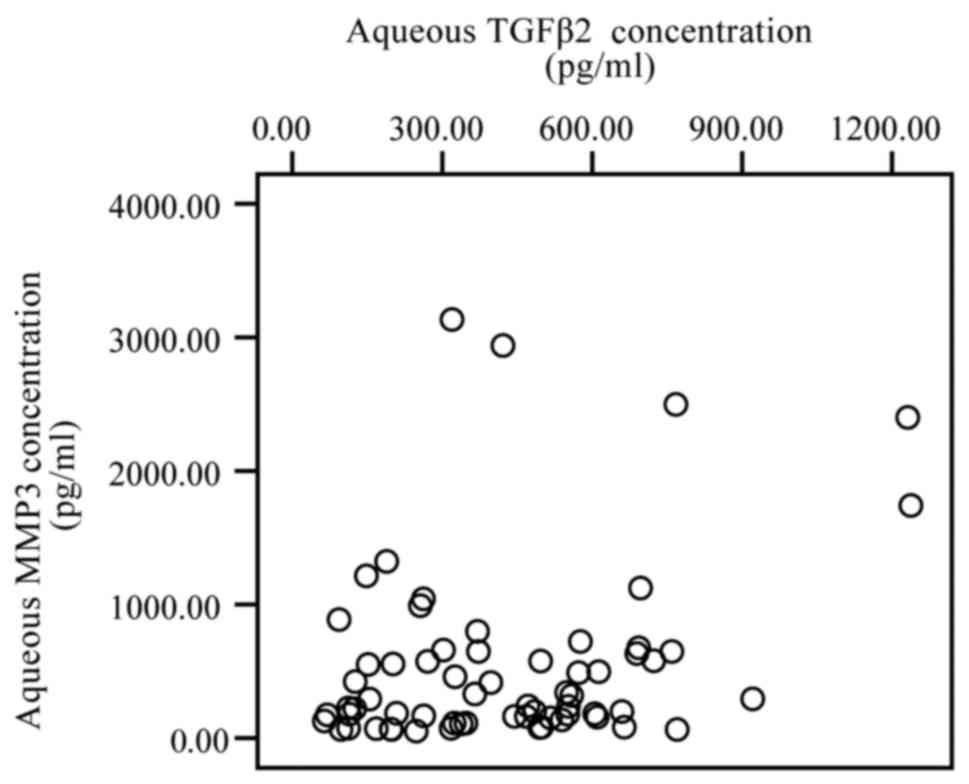
Furthermore, the levels of TGF-β2 were also not significantly
correlated with the levels of MMP-3 (P>0.05; Fig. 2).
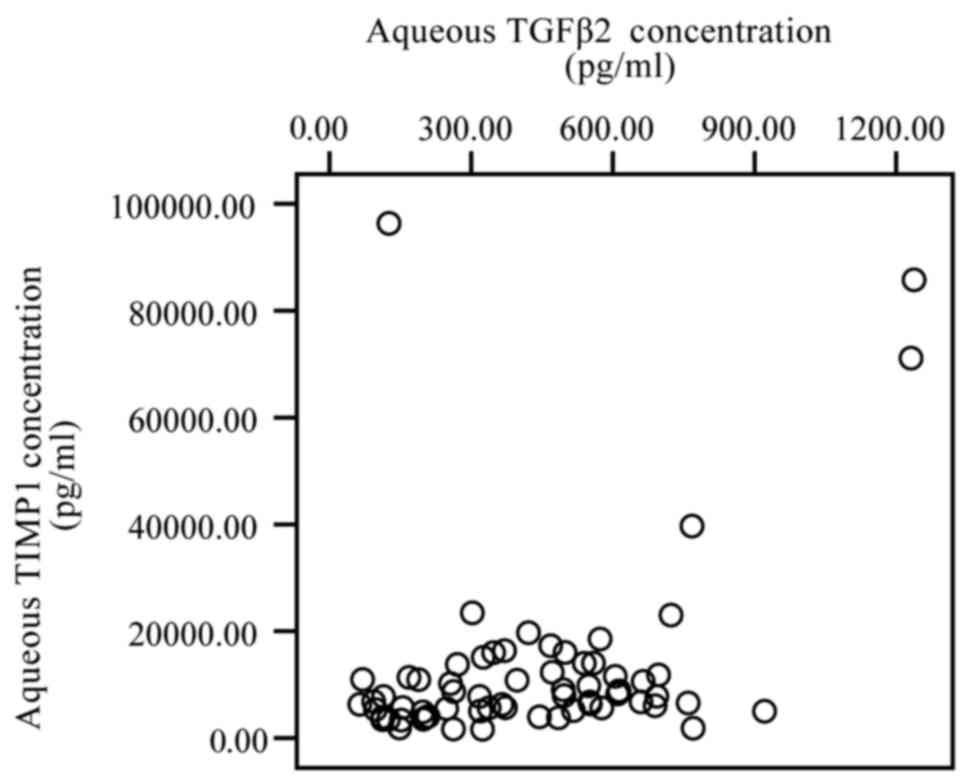
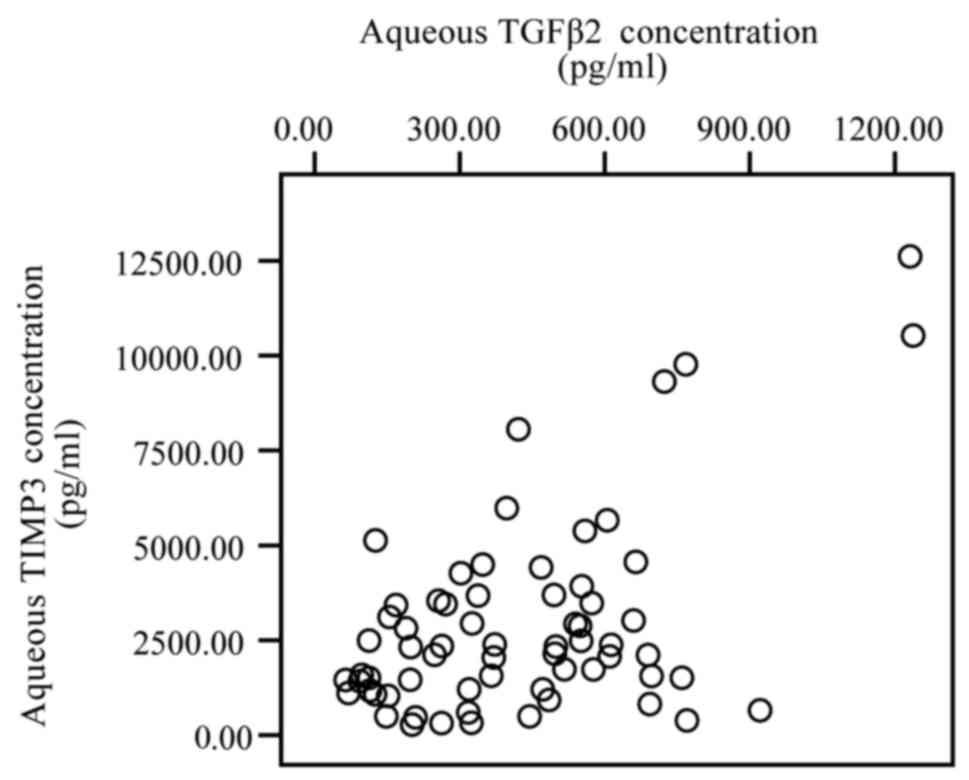
Correlation of TGF-β2 with TIMPs
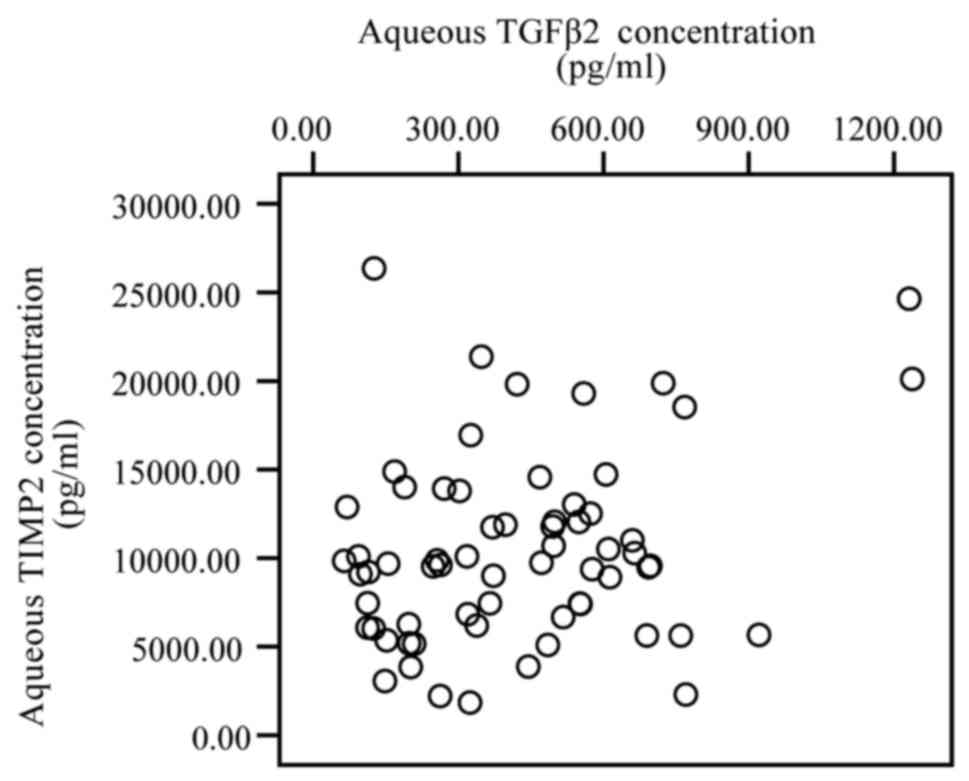
A significantly positive correlation was identified
between the levels of TGF-β2 and TIMP-1 (r=0.334, P=0.007; Fig. 3), and with TGF-β2 and TIMP-3 (r=0.309,
P=0.012; Fig. 4). However, no
significant correlation was observed between the levels of TGF-β2
and TIMP-2 (P>0.05; Fig. 5).
Discussion
In the current study, the associations between
TGF-β2 and MMPs/TIMPs in the aqueous humor of myopia and cataract
patients were analyzed, with the aim of highlighting the role of
TGF-β2 and MMPs/TIMPs in the development of myopia.
TGF-β2 is an important factor in the modulation of
growth and development of the eyeball (21). In four-week-old guinea pigs, it was
demonstrated that the retinal levels of the TGF-β2 protein are
highly correlated with ocular refraction and axial length (22). TGF-β2 is a key factor in the
progression of myopia development and axial elongation; however,
reports of its expression in experimental myopia have been
controversial as animal studies have demonstrated increases and
decreases (22–26). It appears that the changes in TGF-β2
expression during the development of myopia are species- and
tissue-specific (22–24,25,27). Our
previous study (18) and the study by
Zhuang et al (28) found that
TGF-β2 levels were increased in the aqueous or vitreous humor in
stationary high myopia patients with axial elongation (18,28).
TIMPs are a group of endogenous specific inhibitors
of the activity of various MMPs, and the balance between MMPs/TIMPs
regulates ECM turnover and remodeling during normal development and
pathogenesis (11). Animal studies
have revealed that the levels of MMPs increased during the
development period of myopia (29,30). Our
previous study (17) and the study by
Zhuang et al (28) identified
that aqueous MMP-2 and −3 levels increased in ocular specimens
during the stationary stage of high myopia patients (17,28). TIMPs
inhibit the degradation of ECM, which is caused by MMPs. Therefore,
it is expected that during various physiological or pathological
processes that involve the degradation of the ECM, TIMP levels
decrease and are associated with increased levels of MMPs; these
changes lead to degradation of the ECM (8,10–12). However, under certain circumstances
the levels of TIMPs do not decrease, they increase (8,10–13,31–45). These
contradictory changes of TIMP have been explained by the
homeostasis hypothesis, that is, the elevation of TIMP levels
reflects a cellular compensatory reaction to counteract and limit
the intensive degradation of ECM by MMPs. In this case, the TIMP
expression levels should increase rather than decrease (11–13,40,41,46,47).
In animal studies of myopia, the changes of TIMP
levels during the development period of myopia are complicated; it
has been reported that the levels of TIMP mRNA in the scleral
increase (14,48), decrease (49,50) or do
not change significantly (49,50).
During the recovery stage of experimental myopia, the MMP-2 levels
invariably decrease, and the TIMP levels usually increase (29,30,50,51).
Our previous studies revealed that the levels of
TIMPs in the aqueous humor increased during the stationary stage of
high myopia patients (17). This may
be explained by the homeostasis hypothesis; the elevation in TIMP
levels reflects a cellular compensatory reaction to counteract the
degradation of ECM by MMPs.
Little is known regarding the molecular mechanism of
this compensatory response of increased levels of TIMPs in myopia.
The present study has demonstrated that the increase of TIMP-1 and
TIMP-3 in the aqueous humor of high myopia patients was positively
correlated with the increase of TGF-β2 levels, indicating that
TGF-β2 may be the molecule that causes the increase in TIMP
expression levels in high myopia. This is consistent with previous
reports demonstrating that TGF-β2 increased the levels of TIMP-1 in
human RPE cells (52), and the
upregulation of TIMP by TGF-βs in human, rat or bovine chondrocytes
and fibroblasts (53–58).
The signaling pathways of TGF-β-induced TIMP
expression in myopia have not been investigated systematically;
however, they has been evaluated in human and experimental animal
fibroblasts and chondrocytes (53,54,59).
Morris et al (59) demonstrated that TGF-β increased the
levels of the TIMP-3 protein in human cartilage, but did not
significantly affect the expression levels of TIMP-3 mRNA (59). Wang et al (53) observed that TGF-β induces TIMP-3
expression in rat chondrocytes via activation of the extracellular
signal-regulated kinase (ERK)1/2 and Smad2/3 signaling pathways.
Leivonen et al (54)
identified that TGF-β induced TIMP-3 mRNA expression in mouse and
human fibroblasts. This effect was abolished by the inhibition of
ERK1/2 activation and p38 mitogen-activated protein kinase (p38
MAPK), indicating that ERK1/2 and p38 MAPK mediate the effect of
TGF-β on TIMP-3 expression levels. Furthermore, Smad3 co-operated
with p38 MAPK and ERK1/2 in the induction of TIMP-3 expression. The
study demonstrated that TGF-β induces TIMP-3 expression via a
complex interplay between Smad3, p38, and ERK1/2 signaling
(54).
The present analysis revealed that the levels of
TGF-β2 were positively correlated with the levels of TIMP-1 and
TIMP-3, but were not associated with the levels of TIMP-2. This may
be relevant to the different effects of various TIMPs on the
expression levels of MMP; TIMP-1 and −3 are inhibitors of various
MMPs. TIMP-2 is an inhibitor of MMPs, as well as an activator for
pro-MMP-2. Furthermore, TIMP-2 binds to latent MMP-2 and MT1-MMP at
the cell surface, resulting in proteolytic activation of the latent
MMP-2 by adjacent MT1-MMP (7–9,11). It has
been reported that at high concentrations, TIMP-2 causes
inhibition; however, at low concentrations it increases the
activities of MMP-2 (8,11). This may provide an explanation for the
different associations between TGF-β and various TIMPs.
It has been reported that TGF-βs may influence the
expression level of MMPs (6).
However, in the present study, the levels of MMPs in the aqueous
humor in cataract or high myopia eyes were not identified to be
correlated with TGF-β2 levels. Therefore, the results of the
present analysis indicate that in human high myopia, the effects of
TGF-β2 on the pathogenesis of myopia may be via the modulation of
TIMP expression levels rather than by MMP expression levels.
However, the present study was based on the analysis of factors in
the aqueous humor during the stationary stage of myopia, therefore,
the results should be interpreted cautiously.
In conclusion, the present analysis has revealed
that an increase in the levels of TIMPs in the aqueous humor in the
stationary stage of human high myopia patients was positively
correlated with the increase in TGF-β2 levels. The elevation of
TIMP expression levels most likely reflects a cellular compensatory
reaction to counteract and limit the intense degradation of the ECM
by MMPs. TGF-β2 is possibly one of the molecules that is involved
in the modulation of this process. However, the cause-effect
association between the increase in TGF-β and TIMP levels in the
development of myopia and its mechanism requires further
investigation.
Acknowledgements
The current study was supported by the National
Nature Science Foundation of China (grant no. 81371050) and the
Shanghai Municipal Commission of Health and Family Planning Fund
(grant no. 201440354).
References
|
1
|
Chen BY, Wang CY, Chen WY and Ma JX:
Altered TGF-β2 and bFGF expression in scleral desmocytes from an
experimentally-induced myopia guinea pig model. Graefes Arch Clin
Exp Ophthalmol. 251:1133–1144. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hodos W and Kuenzel WJ: Retinal-image
degradation produces ocular enlargement in chicks. Invest
Ophthalmol Vis Sci. 25:652–659. 1984.PubMed/NCBI
|
|
3
|
Nagineni CN, Cherukuri KS, Kutty V,
Detrick B and Hooks JJ: Interferonamma differentially regulates
TGF-β1 and TGF-β2 expression in human retinal pigment epithelial
cells through JAK-STAT pathway. J Cell Physiol. 210:192–200. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Seko Y, Tanaka Y and Tokoro T: Scleral
cell growth is influenced by retinal pigment epithelium in vitro.
Graefes Arch Clin Exp Ophthalmol. 232:545–552. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Troilo D, Nickla DL, Mertz JR and Summers
Rada JA: Change in the synthesis rates of ocular retinoic acid and
scleral glycos-aminoglycan during experimentally altered eye growth
in marmosets. Invest Ophthalmol Vis Sci. 47:1768–1777. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Asano K, Shikama Y, Shoji N, Hirano K,
Suzaki H and Nakajima H: Tiotropium bromide inhibits TGF-β-induced
MMP production from lung fibroblasts by interfering with smad and
MAPK pathways in vitro. Int J Chron Obstruct Pulmon Dis. 5:277–286.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Murphy G and Nagase H: Progress in matrix
metalloproteinase research. Mol Aspects Med. 29:290–308. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kahari V and Saarialho-Kere U: Matrix
metalloproteinases and their inhibitors in tumour growth and
invasion. Ann Med. 31:34–45. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brew K, Dinakarpandian D and Nagase H:
Tissue inhibitors of metalloproteinases: evolution, structure and
function. Biochim Biophys Acta. 1477:267–283. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Groblewska M, Siewko M, Mroczko B and
Szmitkowski M: The role of matrix metalloproteinases (MMPs) and
their inhibitors (TIMPs) in the development of esophageal cancer.
Folia Histochem Cytobiol. 50:12–19. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chirco R, Liu XW, Jung KK and Kim HR:
Novel functions of TIMPs in cell signaling. Cancer Metastasis Rev.
25:99–113. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Egeblad M and Werb Z: New functions for
the matrix metalloproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sternlicht MD and Werb Z: How matrix
metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol.
17:463–516. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
McBrien NA and Gentle A: Role of the
sclera in the development and pathological complications of myopia.
Prog Retin Eye Res. 22:307–338. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gao H, Frost MR, Siegwart JT Jr and Norton
TT: Patterns of mRNA and protein expression during minus-lens
compensation and recovery in tree shrew sclera. Mol Vis.
17:903–919. 2011.PubMed/NCBI
|
|
16
|
Shelton L and Rada JS: Effects of cyclic
mechanical stretch on extracellular matrix synthesis by human
scleral fibroblasts. Exp Eye Res. 84:314–322. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jia Y, Hu DN, Zhu D, Zhang L, Gu P, Fan X
and Zhou J: MMP-2, MMP-3, TIMP-1, TIMP-2, and TIMP-3 protein levels
in human aqueous humor: relationship with axial length. Invest
Ophthalmol Vis Sci. 55:3922–3928. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jia Y, Hu DN and Zhou J: Human aqueous
humor levels of TGF- β2: relationship with axial length. Biomed Res
Int. 2014:2585912014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Manise M, Holtappels G, Van Crombruggen K,
Schleich F, Bachert C and Louis R: Sputum IgE and cytokines in
asthma: relationship with sputum cellular profile. PLoS One.
8:e583882013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Codices V, Martins C, Novo C, de Sousa B,
Lopes Â, Borrego M and Matos O: Dynamics of cytokines and
immunoglobulins serum profiles in primary and secondary
Cryptosporidium parvum infection: usefulness of Luminext xMAP
technology. Exp Parasitol. 133:106–113. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bajracharya D, Shrestha B, Kamath A, Menon
A and Radhakrishnan R: Immunohistochemical correlation of matrix
metalloproteinase-2 and tissue inhibitors of metalloproteinase-2 in
tobacco associated epithelial dysplasia. Dis Marker.
2014:1978132014. View Article : Google Scholar
|
|
22
|
Seko Y, Shimokawa H and Tokoro T:
Expression of bFGF and TGF-beta 2 in experimental myopia in chicks.
Invest Ophthalmol Vis Sci. 36:1183–1187. 1995.PubMed/NCBI
|
|
23
|
Honda S, Fujii S, Sekiya Y and Yamamoto M:
Retinal control on the axial length mediated by transforming growth
factor-beta in chick eye. Invest Ophthalmol Vis Sci. 37:2519–2526.
1996.PubMed/NCBI
|
|
24
|
McBrien NA: Regulation of scleral
metabolism in myopia and the role of transforming growth
factor-beta. Exp Eye Res. 114:128–140. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jobling AI, Nguyen M, Gentle A and McBrien
NA: Isoform-specific changes in scleral transforming growth
factor-β expression and the regulation of collagen synthesis during
myopia progression. J Biol Chem. 279:18121–18126. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Estella C, Herrer I, Atkinson SP,
Quiñonero A, Martínez S, Pellicer A and Simón C: Inhibition of
histone deacetylase activity in human endometrial stromal cells
promotes extracellular matrix remodelling and limits embryo
invasion. PLoS One. 7:e305082012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jobling AI, Wan R, Gentle A, Bui BV and
McBrien NA: Retinal and choroidal TGF-β in the tree shrew model of
myopia: isoform expression, activation and effects on function. Exp
Eye Res. 88:458–466. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhuang H, Zhang R, Shu Q, Jiang R, Chang
Q, Huang X, Jiang C and Xu G: Changes of TGF-β2, MMP-2, and TIMP-2
levels in the vitreous of patients with high myopia. Graefes Arch
Clin Exp Ophthalmol. 252:1763–1767. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Guggenheim JA and McBrien NA:
Form-deprivation myopia induces activation of scleral matrix
metalloproteinase-2 in tree shrew. Invest Ophthalmol Vis Sci.
37:1380–1395. 1996.PubMed/NCBI
|
|
30
|
Siegwart JT Jr and Norton TT: Selective
regulation of MMP and TIMP mRNA levels in tree shrew sclera during
minus lens compensation and recovery. Invest Ophthalmol Vis Sci.
46:3484–3492. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chavey C, Mari B, Monthouel MN, Bonnafous
S, Anglard P, Van Obberghen E and Tartare-Deckert S: Matrix
metalloproteinases are differentially expressed in adipose tissue
during obesity and modulate adipocyte differentiation. J Biol Chem.
278:11888–11896. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Limaye AM, Desai KV, Chavalmane AK and
Kondaiah P: Regulation of mRNAs encoding MMP-9 and MMP-2, and their
inhibitors TIMP-1 and TIMP-2 by androgens in the rat ventral
prostate. Mol Cell Endocrinol. 294:10–18. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ma DH, Chen JK, Kim WS, Hao YX, Wu HC,
Tsai RJ, Hwang DG and Zhang F: Expression of matrix
metalloproteinases 2 and 9 and tissue inhibitors of
metalloproteinase 1 and 2 in inflammation-induced corneal
neovascularization. Ophthalmic Res. 33:353–362. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Webb KE, Henney AM, Anglin S, Humphries SE
and McEwan JR: Expression of matrix metalloproteinases and their
inhibitor TIMP-1 in the rat carotid artery after balloon injury.
Arterioscler Thromb Vasc Biol. 17:1837–1844. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lakatos G, Hritz I, Varga MZ, Juhász M,
Miheller P, Cierny G, Tulassay Z and Herszényi L: The impact of
matrix metalloproteinases and their tissue inhibitors in
inflammatory bowel diseases. Dig Dis. 30:289–295. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gomez DE, De Lorenzo MS, Alonso DF and
Andrade ZA: Expression of metalloproteinases (MMP-1, MMP-2, and
MMP-9) and their inhibitors (TIMP-1 and TIMP-2) in schistosomal
portal fibrosis. Am J Trop Med Hyg. 61:9–13. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Knittel T, Mehde M, Grundmann A, Saile B,
Scharf JG and Ramadori G: Expression of matrix metalloproteinases
and their inhibitors during hepatic tissue repair in the rat.
Histochem Cell Biol. 113:443–453. 2000.PubMed/NCBI
|
|
38
|
Figueira RC, Gomes LR, Neto JS, Silva FC,
Silva ID and Sogayar MC: Correlation between MMPs and their
inhibitors in breast cancer tumor tissue specimens and in cell
lines with different metastatic potential. BMC Cancer. 9:202009.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gress TM, Müller-Pillasch F, Lerch MM,
Friess H, Buchler M and Adler G: Expression and in-situ
localization of genes coding for extracellular matrix proteins and
extracellular matrix degrading proteases in pancreatic cancer. Int
J Cancer. 62:407–413. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Poruk KE, Firpo MA, Scaife CL, Adler DG,
Emerson LL, Boucher KM and Mulvihill SJ: Serum osteopontin and
tissue inhibitor of metalloproteinase 1 as diagnostic and
prognostic biomarkers for pancreatic adenocarcinoma. Pancreas.
42:193–197. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gardner J and Ghorpade A: Tissue inhibitor
of metalloproteinase (TIMP)-1: the TIMPed balance of matrix
metalloproteinases in the central nervous system. J Neurosci Res.
74:801–806. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Singh RD, Haridas N, Patel JB, Shah FD,
Shukla SN, Shah PM and Patel PS: Matrix metalloproteinases and
their inhibitors: correlation with invasion and metastasis in oral
cancer. Indian J Clin Biochem. 25:250–259. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wallard MJ, Pennington CJ,
Veerakumarasivam A, Burtt G, Mills IG, Warren A, Leung HY, Murphy
G, Edwards DR, Neal DE and Kelly JD: Comprehensive profiling and
localisation of the matrix metalloproteinases in urothelial
carcinoma. Br J Cancer. 94:569–577. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kossakowska AE, Huchcroft SA, Urbanski SJ
and Edwards DR: Comparative analysis of the expression patterns of
metalloproteinases and their inhibitors in breast neoplasia,
sporadic colorectal neoplasia, pulmonary carcinomas and malignant
non-Hodgkins lymphomas in humans. Br J Cancer. 73:1401–1408. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ma J, Wang J, Fan W, Pu X, Zhang D, Fan C,
Xiong L, Zhu H, Xu N, Chen R and Liu S: Upregulated TIMP-1
correlates with poor prognosis of laryngeal squamous cell
carcinoma. Int J Clin Exp Pathol. 7:246–254. 2013.PubMed/NCBI
|
|
46
|
Ylisirniö S, Höyhtyä M and
Turpeenniemi-Hujanen T: Serum matrix metalloproteinases −2, −9 and
tissue inhibitors of metalloproteinases −1, −2 in lung cancer -
TIMP-1 as a prognostic marker. Anticancer Res. 20:1311–1316.
2000.PubMed/NCBI
|
|
47
|
Moore CS and Crocker SJ: An alternate
perspective on the roles of TIMPs and MMPs in pathology. Am J
Pathol. 180:12–16. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
McBrien NA, Cornell LM and Gentle A:
Structural and ultrastructural changes to the sclera in a mammalian
model of high myopia. Invest Ophthalmol Vis Sci. 42:2179–2187.
2001.PubMed/NCBI
|
|
49
|
Rada JA and Brenza HL: Increased latent
gelatinase activity in the sclera of visually deprived chicks.
Invest Ophthalmol Vis Sci. 36:1555–1565. 1995.PubMed/NCBI
|
|
50
|
Rada JA, Perry CA, Slover ML and Achen VR:
Gelatinase A and TIMP-2 expression in the fibrous sclera of myopic
and recovering chick eyes. Invest Ophthalmol Vis Sci. 40:3091–3099.
1999.PubMed/NCBI
|
|
51
|
Schippert R, Brand C, Schaeffel F and
Feldkaemper MP: Changes in scleral MMP-2, TIMP-2 and
TGFβ-2 mRNA expression after imposed myopic and hyperopic
defocus in chickens. Exp Eye Res. 82:710–719. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Eichler W, Friedrichs U, Thies A, Tratz C
and Wiedemann P: Modulation of matrix metalloproteinase and TIMP-1
expression by cytokines in human RPE cells. Invest Ophthalmol Vis
Sci. 43:2767–2773. 2002.PubMed/NCBI
|
|
53
|
Wang X, Zhu Y, Tao H, Jin C, Liu Y, Lu X,
Hu X and Fan C: Interaction of ERK1/2 and Smad2/3 signaling
pathways in TGF-β1-induced TIMP-3 expression in rat chondrocytes.
Arch Biochem Biophys. 564:229–236. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Leivonen SK, Lazaridis K, Decock J,
Chantry A, Edwards DR and Kähäri VM: TGF-β-elicited induction of
tissue inhibitor of metalloproteinases (TIMP)-3 expression in
fibroblasts involves complex interplay between Smad3, p38α, and
ERK1/2. PLoS One. 8:e574742013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Narcisi R, Quarto R, Ulivi V, Muraglia A,
Molfetta L and Giannoni P: TGF β-1 administration during ex vivo
expansion of human articular chondrocytes in a serum-free medium
redirects the cell phenotype toward hypertrophy. J Cell Physiol.
227:3282–3290. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Luna J, Masamunt MC, Llach J, Delgado S
and Sans M: Palm oil tocotrienol rich fraction reduces
extracellular matrix production by inhibiting transforming growth
factor-β1 in human intestinal fibroblasts. Clin Nutr. 30:858–864.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Todorova L, Gürcan E, Westergren-Thorsson
G and Miller-Larsson A: Budesonide/formoterol effects on
metalloproteolytic balance in TGFβ-activated human lung
fibroblasts. Respir Med. 103:1755–1763. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Su S, Grover J, Roughley PJ, DiBattista
JA, Martel-Pelletier J, Pelletier JP and Zafarullah M: Expression
of the tissue inhibitor of metalloproteinases (TIMP) gene family in
normal and osteoarthritic joints. Rheumatol Int. 18:183–191. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Morris KJ, Cs-Szabo G and Cole AA:
Characterization of TIMP-3 in human articular talar cartilage.
Connect Tissue Res. 51:478–490. 2010. View Article : Google Scholar : PubMed/NCBI
|