Introduction
Among patients with lung cancer, 30–40% develop bone
metastases (1), while 40–50% develop
brain metastases (2,3). The median overall survival (OS) rate of
these patients with brain metastases is <1 year (3). Current therapeutic approaches for cancer
bone and brain metastasis include palliative radiotherapy and
systemic therapy with chemotherapy and targeted agents (3,4).
Platinum-based combination chemotherapy is the standard of care for
non-small cell lung cancer (NSCLC) (5); however, drug resistance limits the
therapeutic efficacy of combination chemotherapy in advanced NSCLC
(6). Among the key mechanisms by
which cancer cell acquire multi-drug resistance is the upregulation
of adenosine triphosphate (ATP)-binding cassette (ABC)
transporters, including P-glycoprotein (ABCB1), breast cancer
resistance protein (ABCG2) and multi-drug resistance associated
protein-1, in order to export a number of different
chemotherapeutics from the cytoplasm (7). The concept that cancer stem cells may
originate from a fusion between an ‘altered’ pre-malignant cell and
a bone marrow-derived stem cell has been proposed (8). Numerous studies have observed higher
expression of ABC transporters in cancer stem cells and significant
associations of cancer stem cells with multi-drug resistance and
tumor relapse (9–12). ABC transporter activity is dependent
on ATP (13) and cancer cells produce
ATP primarily through glycolysis, even under normal oxygen
concentrations, in a process of anaerobic glycolysis known as the
‘Warburg effect’ (14,15).
Anaerobic glycolysis produces only two ATPs per
glucose molecule and is less efficient than oxidative
phosphorylation, which produces ~38 ATPs per glucose molecule
(16,17). This suggests that tumor cells require
a larger supply of glucose compared with normal cells. Therefore,
it was hypothesized that brief and mild induction of hypoglycemia
during chemotherapy may increase the sensitivity of cancer cells to
chemotherapeutic agents, which may thus bring palliative benefit to
patients with advanced cancers by enabling a decrease in the
effective dose of chemotherapeutic drugs. The present report
describes an advanced pulmonary adenocarcinoma patient with
multiple bone and brain metastases who received treatments of
insulin-induced hypoglycemia followed by reduced doses of
chemotherapy. The study was approved by the Ethics Committee of
Changzhou Tumor Hospital (Changzhou, China) and the patient
provided written informed consent.
Case report
A 64-year-old male was referred to the Department of
Medical Oncology at Changzhou Tumor Hospital (Changzhou, China) in
May 2013 for advanced pulmonary adenocarcinoma with multiple bone
metastases. Two months prior, the patient presented with mild
coughing and pain in the lower back at a local hospital. During
this initial visit, a computed tomography (CT) scan revealed a mass
in the upper right lung and mediastinal and right hilar lymph node
enlargement. Adenocarcinoma cancer cells were detected by fiber
bronchoscope examination and cytological examination. A whole body
radionuclide bone scan identified increased radiation uptake in
multiple bones, suggesting widespread bone metastases. After 2
cycles of systemic chemotherapy (cisplatin 75 mg/m2 and
pemetrexed 500 mg/m2 on day 1 of a 3-week cycle) at the
local hospital, the patient experienced no marked relief of the
bone pain or irritable cough. The patient was prescribed tramadol
(100 mg; PO q12h) to ease the pain. The serum concentration of
carcinoembryonic antigen (CEA) increased from 456.7 ng/ml on first
admission to >1,000 ng/ml following chemotherapy.
On admission to Changzhou Tumor Hospital, the
patient complained of whole body bone pain, an irritating cough,
poor appetite, fatigue and substantial weight loss experienced for
~2 months. Prior to chemotherapy, the patient exhibited adequate
parameters of organ function, as follows: Adequate bone marrow
reserve [white blood cell count, >4,000/ml (reference range,
4,000–10,000/ml), platelet count, >100,000/ml (reference range,
100,000–300,000/ml)]; absence of heart disease and heart rate, 69
bpm [reference range, 60–100 bpm (18)]; serum creatinine, 49.3 µmol/l
(reference range, 45–84 µmol/l); serum bilirubin and alanine
aminotransferase, 7.8 µmol/l and 15 U/l, respectively (reference
ranges, 1.7–23.9 µmol/l and 13–35 U/l, respectively). References
ranges were according to manufacturer's guidelines unless otherwise
stated. The baseline levels of serum tumor markers prior to
chemotherapy are presented in Table
I. For measurement of the serum tumor markers CEA, carcinoma
antigen 15-3 (CA 15-3), CYFRA21-1 and neuron-specific enolase
(NSE), peripheral blood (2.0 ml) was collected in a vacuum tube (BD
Biosciences, Franklin Lakes, NJ, USA) without hematolysis or
lipemia from each participant following overnight fasting. Each
sample was centrifuged at 1,710 × g for 5 min at 4°C. The serum was
retained following centrifugation, and kept at 4°C until analysis.
The assays were performed within 5 days after samples acquisition.
Electrochemiluminescence immunization with an immunology analyzer
(Roche Cobas e601; Roche Diagnostics, Basel, Switzerland) was
performed to evaluate the tumor markers (19). All tumor marker detection kits were
purchased from Roche Diagnostics. According to the manufacturer's
recommendations, the reference values of the markers were as
follows: CEA, <5.0 ng/ml; CA 15-3, <39 U/ml; CYFRA21-1,
<3.3 ng/ml; and NSE, <17.0 ng/ml.
 | Table I.Changes in the serum levels of tumor
markers during chemotherapy. |
Table I.
Changes in the serum levels of tumor
markers during chemotherapy.
|
| Tumor markers |
|---|
|
|
|
|---|
| Chemotherapy
cycle | CEA (ng/ml) | CA 15-3 (U/ml) | CYRA21-1 (ng/ml) | NSE (ng/ml) |
|---|
| Baseline | >1,000.0 | 159.7 | 165.0 | 35.6 |
| Second | >1,000.0 | 121.8 | 6.5 | 24.0 |
| Third | >1,000.0 | 97.9 | 5.5 | 15.0 |
| Fourth | >1,000.0 | 74.7 | 3.6 | 12.1 |
| Fifth | 941.6 | 50.0 | 2.8 | 9.5 |
| Sixth | 747.5 | 48.0 | 2.5 | 10.1 |
| Seventh | 721.8 | 43.4 | 1.6 | 10.2 |
| Eighth | 395.3 | 41.8 | 1.2 | 10.0 |
To prepare for chemotherapy combined with glucose
inhibition, the patient fasted after 10 pm on the day before
chemotherapy to achieve a consistent blood glucose level, though
was allowed to drink water. On each day of chemotherapy, the
baseline blood glucose levels were tested with a glucometer (SD
Codefree; SD BioSensor, Inc., Suwon, South Korea) using standard
procedures described by the manufacturer, and the range was
determined to be 4.5–5.4 mmol/l [reference range, ≤6.1 mmol/l
(20)]. Subsequently, insulin
(Nanjing Xinbai Pharmaceutical Co., Ltd., Nanjing, China) at 0.2
U/kg body weight was administered to the patient intravenously
(i.v.). After ~20 min, when the blood glucose levels had dropped to
2.5–3.0 mmol/l, chemotherapy was initiated, namely through
sequential i.v. injections of navelbine (10 mg; Jiangsu Hengrui
Medicine Co., Ltd., Lianyungang, China), cisplatin (10 mg) and
fluorouracil (250 mg; both from Qilu Pharmaceutical Co., Ltd.,
Jinan, China) administered over ~10 min. Following chemotherapy on
all treatment days, blood glucose level dropped to 1.6–2.5 mmol/l,
and was subsequently elevated to 7–9 mmol/l by i.v. injection with
20 ml 50% glucose. Following each round of treatment, the patient
was able to eat a full meal. The chemotherapy treatments were
administered 7 times/month for 8 months (56 rounds in total).
During each treatment, the patient experienced only mild
discomforts of mild heart palpitations and increased sweating.
Following the treatments, the patient generally experienced an
increase in appetite but no nausea or vomiting. Additionally, the
patient's heart rate (69–96 bpm) and blood pressure [90/60-130/88
mmHg; reference range, 90/60-140/90 mmHg (21)] changed little throughout the induced
changes in blood glucose levels.
After 3 rounds of chemotherapy, the patient noted a
reduction in pain levels. After 2 months, the patient noted an
obvious reduction in coughing. After ~4 months, the patient's
weight increased from 58 kg (measured on admission) to 63 kg. The
serum levels of tumor markers also generally declined gradually
(Table I). During the 56 rounds of
chemotherapy, the patient presented with grade 1 anemia once
following the fifth cycle of chemotherapy, with a serum hemoglobin
(Hb) concentration of 110 g/l [reference range of serum Hb
concentration in males, 131–172 g/l (22)].
A metabolic response assessment with F-18
fluorodeoxy glucose (18F-FDG) positron emission
tomography (PET; Infinia vc Hawkeye 4; GE Healthcare, Chicago, IL,
USA) (23) and a brain metastases
response assessment with magnetic resonance imaging (MRI; Magnetom
Trio; Siemens Healthineers, Erlangen, Germany) (24) were performed on the patient. A
baseline 18F-FDG PET/CT scan prior to the start of
chemotherapy (Fig. 1) identified
increased 18F-FDG uptake in the right hilum, right upper
lung, shoulder blades, thoracic vertebrae, lumbar, sacrum,
bilateral iliac crest and pelvis, and the largest tumor-to-normal
(T/N) ratio was 9.1 [normal T/N range, ≤1.0 (23)] in the lumbar, which indicated
increased glucose metabolism in the tumor regions. After ~4 months
of chemotherapy, 18F-FDG PET/CT exhibited decreased
18F-FDG uptake in the same lesions (Fig. 1). 18F-FDG uptake was
further decreased after ~8 months, and the greatest T/N ratio (9.1)
had decreased to 1.0, which was paralleled by a reduction in the
size of the right upper lung lesion (Fig.
1). Prior to the start of chemotherapy, the head MRI exhibited
multiple metastases in the brain, while after 4 months of
chemotherapy, MRI identified reductions in the volumes of the brain
metastases (data not shown).
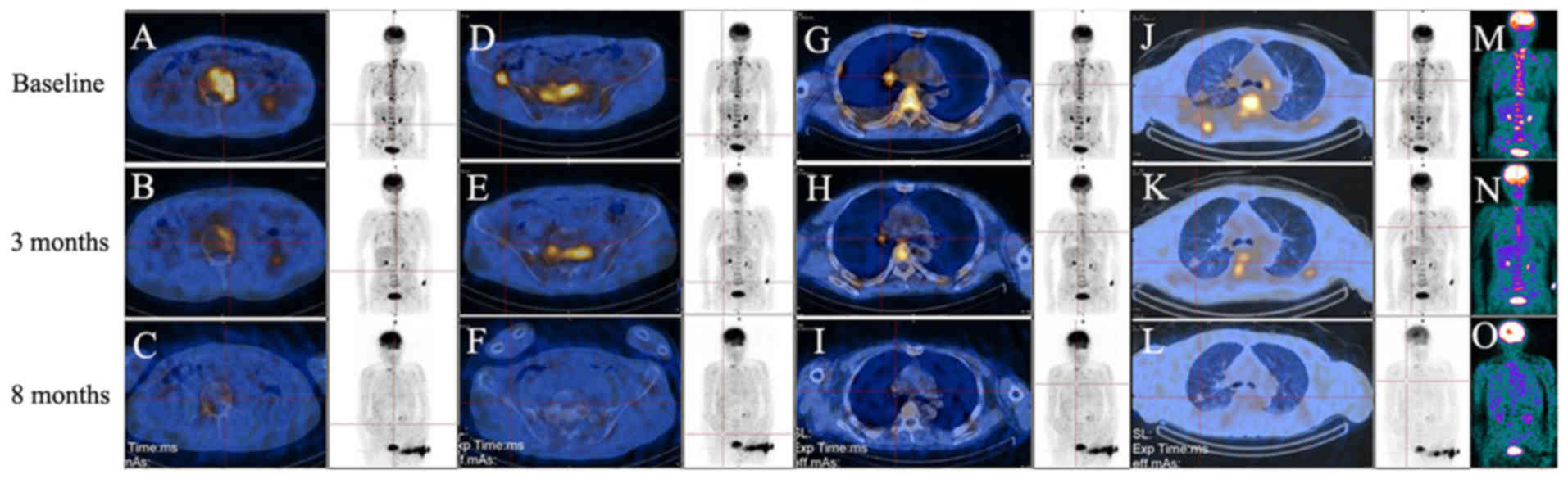 | Figure 1.(A-O) 18F-FDG PET/CT scans.
Equivalent full body images on each row are shown. To indicate the
changes in 18F-FDG uptake, areas of lesions (red
intersections) with increased 18F-FDG uptake are
displayed. A baseline 18F-FDG PET/CT scan prior to the
start of chemotherapy identified increased 18F-FDG
uptake in multiple bones (A, lumbar; D, right iliac crest; M,
colored full body image), in (G) the right hilum and in (J) the
right upper lung. Following chemotherapy treatments,
18F-FDG PET/CT scans identified gradually reduced
18F-FDG uptake in all the above lesions after 3 months
(B, E, H, K and N) and 8 months (C, F, I, L and O) of chemotherapy
treatments. 18F-FDG PET/CT, F-18 fluorodeoxy glucose
positron electron tomography/computed tomography. |
At ~2 months after completion of the chemotherapy
course, the patient presented with changes in his emotional state,
and although the volumes of the brain metastases had been obviously
reduced, MRI identified widespread soft meningeal metastases.
Testing of bronchoscopy biopsy tissue for epidermal growth factor
(EGFR) mutation by direct sequencing (Applied Biosystems; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) indicated that the
patient harbored EGFR exon 21 heterozygous mutations (data not
shown), and thus was eligible to receive tyrosine kinase inhibitor
(TKI) treatment. The patient received whole brain radiotherapy
(doses and schedule unknown) followed by TKI treatment (gefitinib,
250 mg/day; AstraZeneca, Cambridge, UK) for ~8 months at the
initial local hospital. However, the patient finally succumbed due
to meningeal metastasis. The progression-free survival following
glucose inhibition combined with chemotherapy was about 11 months,
and the OS rate following all treatments was 25 months.
Discussion
Increased activity of drug efflux pumps, including
of the ABC superfamily, is considered to be critical in removing
chemotherapeutic agents from cancer cells and causing chemotherapy
failure (7). The activity of drug
efflux pumps is dependent on ATP hydrolysis, and cancer cells
produce ATP primarily through glycolysis, even under normal oxygen
concentrations (the Warburg effect) (14,15). Thus,
inhibition of tumor glycolysis may impact tumor growth by depleting
cellular energy, and may be particularly effective when combined
with cancer cell sensitization to therapeutic drugs (25–27). A
number of glycolytic inhibitors that target the glycolytic enzymes
have been tested, and may be feasible approaches for targeting the
limited metabolic behaviors of cancer cells (28–30).
Although targeting glucose metabolism seems a viable strategy for
cancer therapy, the potential toxic effects of inhibiting metabolic
enzymes on normal cells need to be determined in future studies.
Distant solid tumor metastases are generally characterized by
markedly increased glycolytic activity compared with primary solid
tumors, and cancer cells in metastases are typically more resistant
to chemotherapy than primary cancer cells (31). In the present lung cancer patient
presenting with widespread bone and brain metastases, the
18F-FDG PET/CT scan identified increased
18F-FDG uptake in multiple bones and in the primary lung
tumor, indicating increased glucose uptake and thus the presence of
cancer cells. Meanwhile, the head MRI detected multiple metastases
in the brain. It was hypothesized that a brief reduction in blood
sugar levels may cause a reduction in ATP production in cancer
cells, which in turn may lead to increased sensitivity of the
cancer cells to lower dose chemotherapy agents. The present case
report first confirmed that a 10–15 min periods of controlled
hypoglycemia (1.6–3.0 mmol/l) was safe. During hypoglycemia, the
patient experienced only mild heart palpitations and increases in
sweating, and when blood glucose levels were returned to normal
range with 20 ml 50% glucose solution (i.v.), the patient recovered
from the hypoglycemic symptoms. Secondly, administering reduced
doses of chemotherapeutics during mild hypoglycemia was an
effective treatment method for the patient with advanced lung
cancer. The 18F-FDG PET/CT scans taken every 3–5 months
demonstrated that glucose uptake in the tumor lesions was gradually
reduced during the chemotherapy course. Notably, PET/CT scan
following completion of the chemotherapy course indicated that
glucose uptake values in all cancer lesions were almost equivalent
to those in adjacent normal tissues. After 4 months of
chemotherapy, the MRI identified decreased tumor volumes of the
brain metastases. Furthermore, the patient noted pain relief and
his cough was alleviated. His appetite also increased, his weight
increased from 58 to 63 kg, and nausea and vomiting were
absent.
Bone and brain metastases are highly common
secondary localizations of disease in patients with lung cancer,
and are associated with substantial negative effects on patient
quality of life and survival (1,3). The
median survival time of patients with these secondary lesions has
been reported as 3–7 months (32–34), and
the patients frequently require therapeutic intervention.
Zoledronic acid is the first and seemingly only bisphosphonate that
has exhibited efficacy in the treatment of bone metastases in a
randomized phase III trials (35,36).
Previous treatments for brain metastases have focused on symptom
palliation with whole brain radiotherapy and steroids (3). The TKIs, gefitinib and erlotinib, are
also effective options for the treatment of bone and brain
metastases, particularly in patients with EGFR mutation (37). Despite advances in the targeted
treatment of NSCLC over past years, chemotherapy remains of key
importance in the treatment of advanced NSCLC. Platinum-based
combination chemotherapy has been a standard of treatment for
metastatic NSCLC for more than 30 years, though reaches a
therapeutic plateau (3). Therefore,
there has been focus on the combination of novel agents with
chemotherapy to optimize efficacy, patient survival and overcome
acquired resistance (38). It has
been proposed that insulin as a pharmacological agent induces a
switch from a non-cycling to cycling status in tumor cells
(39), thus increasing the uptake of
chemotherapeutic agents (40) and
their cytotoxic effect in cancer cells (41). In the current patient, insulin-induced
hypoglycemia followed by reduced-dose chemotherapy drugs was
observed to reduce the glucose uptake and sizes of metastatic tumor
lesions, and to improve the patient's quality of life, notably by
reducing bone pain and analgesic use, improving appetite and
increasing body weight. It may be speculated that hypoglycemia
inactivates ABC transporter pump activity, particularly in cancer
cells due to the Warburg effect. This may cause increased
accumulation of chemotherapeutic agents in cancer cells. However,
further experiments are required to verify these mechanisms. From
the current treatment study, it may be proposed that
insulin-induced hypoglycemia followed by low dose chemotherapy is a
viable choice for the palliative care of patients with advanced
solid tumors, particularly of those patients who can not tolerate
or have failed traditional chemotherapy regimens. Future studies of
similar cases are now required to validate the feasibility of this
treatment method.
Acknowledgements
The authors would like to thank personnel at the
Changzhou Tumor Hospital (Changzhou, China) who were involved in
the present study, and Dr Tom C. Tsang (Arizona Cancer Center,
University of Arizona, Tucson, USA) for his advice on treatment.
The present study was supported by the Science and Technology
Planning Project of Changzhou, Jiangsu Province (grant no.
CE20165052), the Science and Technology Planning Project of
Changzhou Health Bureau (grant no. ZD201616), the Research Project
of the Health Department of Jiangsu Province (grant no. Z201616),
the 333 Talents Training Project of Jiangsu Province (grant no.
2016 III-0727; BRA2017114) and the Key Medical Innovation Talents
Training Project of Changzhou (grant no. 2016CZLJ021). The abstract
was presented at the 24th Biennial Congress of the European
Association for Cancer Research July 9–12, 2016 in Manchester, UK
and published as abstract no. 899 in European Journal of Cancer 61
(Suppl 1), 2016.
References
|
1
|
Tsuya A, Kurata T, Tamura K and Fukuoka M:
Skeletal metastases in non-small cell lung cancer: A retrospective
study. Lung Cancer. 57:229–232. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mamon HJ, Yeap BY, Jänne PA, Reblando J,
Shrager S, Jaklitsch MT, Mentzer S, Lukanich JM, Sugarbaker DJ,
Baldini EH, et al: High risk of brain metastases in surgically
staged IIIA non-small-cell lung cancer patients treated with
surgery, chemotherapy, and radiation. J Clin Oncol. 23:1530–1537.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lemjabbar-Alaoui H, Hassan OU, Yang YW and
Buchanan P: Lung cancer: Biology and treatment options. Biochim
Biophys Acta. 1856:189–210. 2015.PubMed/NCBI
|
|
4
|
Owonikoko TK, Arbiser J, Zelnak A, Shu HK,
Shim H, Robin AM, Kalkanis SN, Whitsett TG, Salhia B, Tran NL, et
al: Current approaches to the treatment of metastatic brain
tumours. Nat Rev Clin Oncol. 11:203–222. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rossi A, Chiodini P, Sun JM, O'Brien ME,
von Plessen C, Barata F, Park K, Popat S, Bergman B, Parente B, et
al: Six versus fewer planned cycles of first-line platinum-based
chemotherapy for non-small-cell lung cancer: A systematic review
and meta-analysis of individual patient data. Lancet Oncol.
15:1254–1262. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Leon G, MacDonagh L, Finn SP, Cuffe S and
Barr MP: Cancer stem cells in drug resistant lung cancer: Targeting
cell surface markers and signaling pathways. Pharmacol Ther.
158:71–90. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gottesman MM, Fojo T and Bates SE:
Multidrug resistance in cancer: Role of ATP-dependent transporters.
Nat Rev Cancer. 2:48–58. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
He X, Tsang TC, Pipes BL, Ablin RJ and
Harris DT: A stem cell fusion model of carcinogenesis. J Exp Ther
Oncol. 5:101–109. 2005.PubMed/NCBI
|
|
9
|
Castillo V, Valenzuela R, Huidobro C,
Contreras HR and Castellon EA: Functional characteristics of cancer
stem cells and their role in drug resistance of prostate cancer.
Int J Oncol. 45:985–994. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu C and Alman BA: Side population cells
in human cancers. Cancer Lett. 268:1–9. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Haraguchi N, Utsunomiya T, Inoue H, Tanaka
F, Mimori K, Barnard GF and Mori M: Characterization of a side
population of cancer cells from human gastrointestinal system. Stem
Cells. 24:506–513. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hirschmann-Jax C, Foster AE, Wulf GG,
Nuchtern JG, Jax TW, Gobel U, Goodell MA and Brenner MK: A distinct
‘side population’ of cells with high drug efflux capacity in human
tumor cells. Proc Natl Acad Sci USA. 101:pp. 14228–14233. 2004;
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fletcher JI, Haber M, Henderson MJ and
Norris MD: ABC transporters in cancer: More than just drug efflux
pumps. Nat Rev Cancer. 10:147–156. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Epstein T, Xu L, Gillies RJ and Gatenby
RA: Separation of metabolic supply and demand: Aerobic glycolysis
as a normal physiological response to fluctuating energetic demands
in the membrane. Cancer Metab. 2:72014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Thorne JL and Campbell MJ: Nuclear
receptors and the Warburg effect in cancer. Int J Cancer.
137:1519–1527. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gatenby RA and Gillies RJ: A
microenvironmental model of carcinogenesis. Nat Rev Cancer.
8:56–61. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rich PR: The molecular machinery of
Keilin's respiratory chain. Biochem Soc Trans. 31:1095–1105. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Magder SA: The ups and downs of heart
rate. Crit Care Med. 40:239–245. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao W, Yu H, Han Z, Gao N, Xue J and Wang
Y: Clinical significance of joint detection of serum CEA, SCCA, and
bFGF in the diagnosis of lung cancer. Int J Clin Exp Pathol.
8:9506–9511. 2015.PubMed/NCBI
|
|
20
|
Shaye K, Amir T, Shlomo S and Yechezkel S:
Fasting glucose levels within the high normal range predict
cardiovascular outcome. Am Heart J. 164:111–116. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Frese EM, Fick A and Sadowsky HS: Blood
pressure measurement guidelines for physical therapists. Cardiopulm
Phys Ther J. 22:5–12. 2011.PubMed/NCBI
|
|
22
|
Wu X, Zhao M, Pan B, Zhang J, Peng M, Wang
L, Hao X, Huang X, Mu R, Guo W, et al: Complete blood count
reference intervals for healthy Han Chinese adults. PLoS One.
10:e01196692015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang P, Meng Z, Tan J, Jia Q and Zhang F:
An improved method for measurement of target-to-background ratio in
assessing mediastinal lesions on 18F-FDG coincidence SPECT/CT
imaging. Nucl Med Commun. 31:398–404. 2010.PubMed/NCBI
|
|
24
|
Noebauer-Huhmann IM, Szomolanyi P,
Kronnerwetter C, Widhalm G, Weber M, Nemec S, Juras V, Ladd ME,
Prayer D and Trattnig S: Brain tumours at 7T MRI compared to
3T-contrast effect after half and full standard contrast agent
dose: Initial results. Eur Radiol. 25:106–112. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hsu PP and Sabatini DM: Cancer cell
metabolism: Warburg and beyond. Cell. 134:703–707. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dang CV, Hamaker M, Sun P, Le A and Gao P:
Therapeutic targeting of cancer cell metabolism. J Mol Med (Berl).
89:205–212. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Birsoy K, Sabatini DM and Possemato R:
Untuning the tumor metabolic machine: Targeting cancer metabolism:
A bedside lesson. Nat Med. 18:1022–1023. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rosano C: Molecular model of hexokinase
binding to the outer mitochondrial membrane porin (VDAC1):
Implication for the design of new cancer therapies. Mitochondrion.
11:513–519. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jae HJ, Chung JW, Park HS, Lee MJ, Lee KC,
Kim HC, Yoon JH, Chung H and Park JH: The antitumor effect and
hepatotoxicity of a hexokinase II inhibitor 3-bromopyruvate: In
vivo investigation of intraarterial administration in a rabbit VX2
hepatoma model. Korean J Radiol. 10:596–603. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wallace DC: Mitochondria and cancer:
Warburg addressed. Cold Spring Harb Symp Quant Biol. 70:363–374.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Winquist RJ, Boucher DM, Wood M and Furey
BF: Targeting cancer stem cells for more effective therapies:
Taking out cancer's locomotive engine. Biochem Pharmacol.
78:326–334. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Patchell RA, Tibbs PA, Regine WF, Dempsey
RJ, Mohiuddin M, Kryscio RJ, Markesbery WR, Foon KA and Young B:
Postoperative radiotherapy in the treatment of single metastases to
the brain: A randomized trial. JAMA. 280:1485–1489. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Patchell RA, Tibbs PA, Walsh JW, Dempsey
RJ, Maruyama Y, Kryscio RJ, Markesbery WR, Macdonald JS and Young
B: A randomized trial of surgery in the treatment of single
metastases to the brain. N Engl J Med. 322:494–500. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Coleman RE: Metastatic bone disease:
Clinical features, pathophysiology and treatment strategies. Cancer
Treat Rev. 27:165–176. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rosen LS, Gordon D, Tchekmedyian S,
Yanagihara R, Hirsh V, Krzakowski M, Pawlicki M, de Souza P, Zheng
M, Urbanowitz G, et al: Zoledronic acid versus placebo in the
treatment of skeletal metastases in patients with lung cancer and
other solid tumors: A phase III, double-blind, randomized trial -
the Zoledronic Acid Lung Cancer and Other Solid Tumors Study Group.
J Clin Oncol. 21:3150–3157. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rosen LS, Gordon D, Tchekmedyian NS,
Yanagihara R, Hirsh V, Krzakowski M, Pawlicki M, De Souza P, Zheng
M, Urbanowitz G, et al: Long-term efficacy and safety of zoledronic
acid in the treatment of skeletal metastases in patients with
nonsmall cell lung carcinoma and other solid tumors: A randomized,
phase III, double-blind, placebo-controlled trial. Cancer.
100:2613–2621. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Masters GA, Temin S, Azzoli CG, Giaccone
G, Baker S Jr, Brahmer JR, Ellis PM, Gajra A, Rackear N, Schiller
JH, et al: American Society of Clinical Oncology Clinical Practice:
Systemic therapy for stage IV non-small-cell lung cancer: American
Society of Clinical Oncology Clinical Practice Guideline Update. J
Clin Oncol. 33:3488–3515. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Belani CP: Optimizing chemotherapy for
advanced non-small cell lung cancer: Focus on docetaxel. Lung
Cancer. 50 Suppl 2:3–8. 2005. View Article : Google Scholar
|
|
39
|
Gross GE, Boldt DH and Osborne CK:
Perturbation by insulin of human breast cancer cell cycle kinetics.
Cancer Res. 44:3570–3575. 1984.PubMed/NCBI
|
|
40
|
Oster JB and Creasey WA: Enhancement of
cellular uptake of ellipticine by insulin preincubation. Eur J
Cancer Clin Oncol. 17:1097–1103. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Alabaster O, Vonderhaar BK and Shafie SM:
Metabolic modification by insulin enhances methotrexate
cytotoxicity in MCF-7 human breast cancer cells. Eur J Cancer Clin
Oncol. 17:1223–1228. 1981. View Article : Google Scholar : PubMed/NCBI
|















