Introduction
Urokinase (UK), also termed UK-type plasminogen
activator (uPA), is a type of serine protease present in humans and
other animals, used clinically as a thrombolytic agent in the
treatment of severe or massive deep venous thrombosis (DVT),
pulmonary embolism, myocardial infarction, and occluded intravenous
(IV) or dialysis cannulas. However, UK is not particularly
selective for clot-bound plasminogen (it binds almost equally to
freely circulating plasminogen and clot-bound plasminogen), and
causes significant fibrinogenolysis and clot fibrinolysis. To the
best of our knowledge, prourokinase (Pro-UK; also termed
single-chain UK-type PA, single-chain pro-UK, scu-PA, pro-UK, pro
u-PA and PUK) has only been evaluated in stroke by a single study
(1). Pro-UK is a zymogen with little
fibrin affinity, but has an equivalent fibrin specificity to tissue
PA (tPA) (2). Intra-arterial local
rpro-UK infusion has previously been associated with superior
recanalization in acute thrombotic/thromboembolic stroke when
compared with a placebo (3). The
safety and efficacy of the thrombolytic agent, pro-UK, in the
treatment of DVT of the lower limbs have been investigated in an
open, uncontrolled, pilot study (4).
The results of this pilot study indicated that pro-UK was
thrombolytic in DVT and that it may be administered simultaneously
with a conventional heparin treatment. Recombinant human
(rh)Pro-UK, is a novel type of thrombolytic, which preferentially
activates plasminogen on the fibrin surface and induces
fibrin-selective clot lysis. It has the advantages of more potent
efficacy and less adverse reactions in comparison with other
thrombolytics (5). Advantages of
thrombolytic therapy using rhPro-UK for patients with acute
myocardial infarction include its reliable curative effect and high
safety (6). The aim of the present
study was to investigate the effects of rhPro-UK in rabbit models
of thromboembolic stroke at 3, 4.5 and 6 h therapeutic time
windows, particularly regarding its effects on thrombolysis rate,
patency rate (recanalization) and intracerebral hemorrhage.
Materials and methods
Animals
Adult male and female rabbits, weighing 2.0–3.0 kg
[SCXK(JING)2014-0003] were obtained from Longan Experimental Animal
Breeding Center(Beijing, China). The present study was performed in
strict accordance with the recommendations in the Guide for the
Care and Use of Laboratory Animals of the Tianjin Institute of
Pharmaceutical Research (Tianjin, China).
Drugs, reagents and devices
rhPro-UK (batch no. 201400401) was obtained from
Shanghai Tasly Pharmaceutical Co., Ltd. (Shanghai, China).
Recombinant human tissue plasminogen activator (rt-PA; batch no.
403437) was obtained from Boehringer Ingelheim (Ingelheim am Rhein,
Germany). UK (batch no. 041603023) was obtained from Biochemical
Pharmaceutical Co., Ltd. (Tianjin, China).
The rabbit α2-AP ELISA kit (batch no.
201606) was provided by Bio-Swamp Co., Ltd. (Wuhan, China), and
assay kits for activated partial thromboplastin time (APTT; batch
no. 021603A), prothrombin time (PT; batch no. 011504A), thrombin
time (TT; batch no. 031601D) and fibrinogen (FIB; batch no.
041603A) were obtained from MD Pacific (Tianjin, China)
Biotechnology Co., Ltd. (Tianjin, China), technetium
(99Tcm) sodium was obtained from Atomic High Tech
Isotope Pharmaceutical Co., Ltd. (Tianjin, China).
A JC1000-PC Medical Gamma Counter was obtained from
Kaipu Electromechanical Co., Ltd. (Xi'an, China), RT-6100
microplate reader was purchased from Rayto Life Science Co., Ltd.,
(Guangzhou, China) and a PARBER blood coagulation factor analyzer
was obtained from Beijing SHIDI Scientific Instrument Company
(Beijing, China).
Establishing the experimental embolism
model
Embolus preparation
Embolism was established as previously described
(7,8)
with some modification. Briefly, labeled mixture was obtained from
0.5 ml eluted radioactive sodium (radioactive intensity, 92.5
MBq/ml) with 30 µl stannous chloride (5 mg/ml). Of this, 20 µl was
added into 1 ml rabbit anticoagulant blood and incubated for 30 min
at 37°C. Mixture (50 µl) with an equal volume of CaCl2
(0.5 M) and bovine thrombin (50 IU/ml) were added into the rabbit
autologous blood and a PE90 pipe (inner diameter, 0.86 mm; outer
diameter, 1.27 mm) was used to collect a clot sample; the clot was
solidified at 37°C for 2 h and sliced to 10 mm. The radioactivity
of the thrombus was evaluated using a JC1000-PC Medical Gamma
Counter following three washes with normal saline (5 min/wash).
Establishing the embolism model
The rabbits were anesthetized using 20% urethane (1
g/kg) and fixed on the operating table. The cervical midline skin
was incised and the right carotid artery, internal carotid artery
(ICA) and external carotid artery (ECA) were separated. Following
ligation and transection of the ECA, the modified PE90 (reducing
the optical density to 0.4 mm at one end) with blood clot samples
was injected with a 2 ml syringe via the ECA into the ICA. The
radioactive intensity of the forelimb cortex (1.0 mm posterior and
5.5 mm lateral to the bregma) was detected using a JC1000-PC
Medical Gamma Counter prior to and following embolus injection
(9–11). When the radiation intensity was >2
times greater than the background signal, the model preparation for
thromboembolism was considered successful (8). All rabbits were resuscitated following
completion of the thrombolysis assay.
Grouping
The animals with thromboembolic stroke were randomly
divided into 6 groups as follows: Model group (saline solution),
the rhPro-UK (2.5×, 5× and 10×104 U/kg) groups and the
positive groups (5×104 U/kg UK and 4.5 mg/kg rt-PA). In
addition, the rabbits in the sham group without occlusion by
autologous blood clots were administered saline solution. A total
of 10 rabbits in each group were treated at 3, 4.5 and 6 h after
occlusion via IV infusion.
Thrombolysis assay
The radioactive intensity was detected using a
medical gamma counter before and after drug administration for 15,
30, 45, 60, 75, 90, 105 and 120 min. A thrombolysis rate >50%
was considered as the patency rate (12) and calculated as follows: Thrombolysis
rate (%) = [(n0x k-nt)/(n0 × k)] ×100% where
k=e−0.6931× (t/6.02), n0is the radioactive
intensity before administration, ntis the radioactive
intensity after different drug administration times and t is the
time after administration.
Intracerebral hemorrhage assay
The animals were sacrificed 24 h after treatment
under anesthesia with 20% urethane (1 g/kg). Brains were removed
subsequent to perfusion and coronally sliced into 5 mm sections.
The hemorrhage was estimated used a semi-quantitative method by
counting the number of sections where hemorrhage was present
(13–16). Each brain slice has two ‘faces’ and
the score counting criteria were 1 for a hemorrhage on 1 ‘face’ and
2 for a hemorrhage on 2 ‘faces’, then the total bleeding score was
calculated. Three types of hemorrhage were identified as follows:
i) Hemorrhagic infarction or red speckling of an area, usually
surrounded by soft infarcted tissue; ii) punctate hemorrhages or
isolated small red marks within the tissue; and iii) parenchymatous
intracerebral hemorrhages, a large homogeneous mass of blood within
the tissue.
Blood coagulation factor determination
Blood was collected by heart puncture and
anticoagulated with 3.8% sodium citrate and the plasma was obtained
by centrifugation (4°C, 1,000 × g for 10 min). PT, APTT, TT and FIB
were evaluated using a solidification method, according to
manufacturer's instructions of the PT, APTT, TT and FIB assay kits,
with the PARBER Blood Coagulation Factor Analyzer. Levels of
α2-AP were also measured via ELISA with the RT-6100
microplate reader.
Statistical analysis
Values are presented as the mean ± standard error of
the mean, normal distribution data were analyzed using one-way
analysis of variance and non-normal data were evaluated using a
nonparametric test, the Kruskal Wallis test. The counting data are
expressed as ratios (%) and using the χ2 test P<0.05
was considered to indicate a statistically significant
difference.
Results
Effect of rhPro-UK on
thrombolysis
At the 3 h therapeutic time window, the patency rate
of rhPro-UK(2.5×, 5× and 10×104) was 10% (P>0.05),
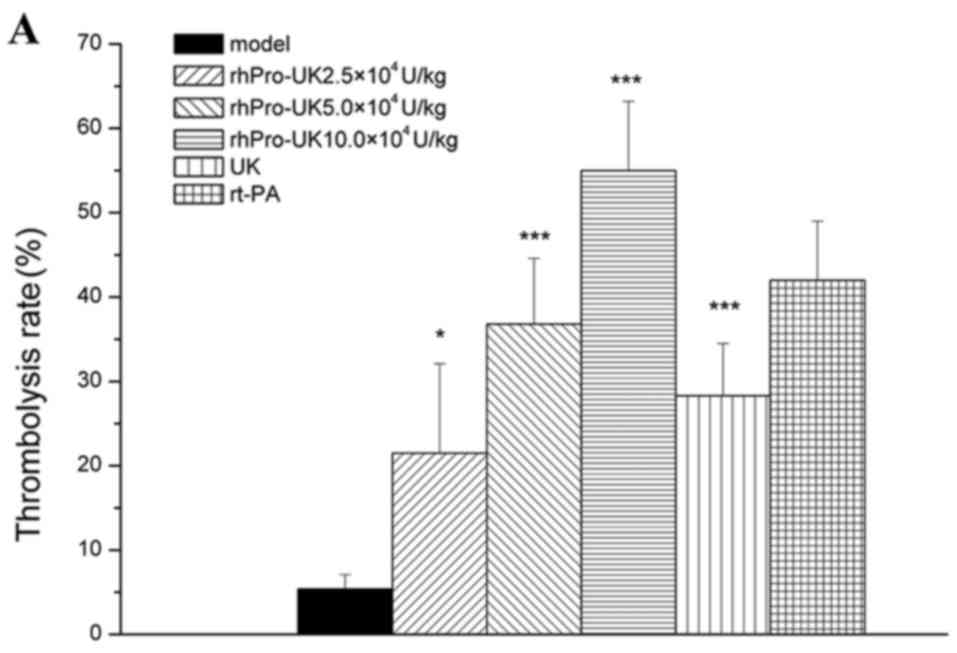
40% (P<0.05) and 70% (P<0.001), and the thrombolysis rate
reached 21.5% (P<0.05), 36.8% (P<0.001) and 55.0%
(P<0.001), respectively. At 4.5 h post-embolism, the patency
rate was increased to 10% (P>0.05), 30% (P<0.05) and 50%
(P<0.01), and the thrombolysis rate reached 18.8% (P<0.05),
29.9% (P<0.01) and 49.0% (P<0.001), respectively. At 6 h
post-embolism, the patency rate was increased to 20% (P>0.05),
30% (P<0.05) and 40% (P<0.01), and the thrombolysis rate
reached 14.7% (P<0.05), 24.1% (P<0.01) and 35.7%
(P<0.001).
At the 3, 4.5 and 6 h therapeutic time windows, the
patency rate of 5×104 U/kg UK was 20% (P>0.05), 20%
(P>0.05) and 0% (P>0.05), respectively and the patency rate
of 4.5 mg/kg rt-PA was 40% (P<0.05), 30% (P<0.05) and 20%
(P>0.05). rhProUK (5×104 U/kg) marginally increased
the thrombolysis rate compared with 5×104 U/kg UK (36.8
vs. 28.3%, 29.9 vs. 22.6% and 24.1 vs. 13.2%; Table I and Fig.
1A-C).
 | Table I.Effect of rhPro-UK on patency rate in
thromboembolic rabbit models (means ± standard error; n=10). |
Table I.
Effect of rhPro-UK on patency rate in
thromboembolic rabbit models (means ± standard error; n=10).
| Therapeutic time
window (h) | Group | Dose (×104
U/kg) | Patency rate (%) |
|---|
|
| Sham | – | – |
|
| Model | – | 0 |
| 3 | rhPro-UK | 2.5 | 10 |
|
|
rhPro-UK | 5 | 40a,d |
|
|
rhPro-UK | 10 | 70c |
|
| UK | 5 | 20 |
|
| rt-PA | 4.5 mg/kg | 40a |
|
| Sham | – | – |
|
| Model | – | 0 |
| 4.5 | rhPro-UK | 2.5 | 10 |
|
| rhPro-UK | 5 | 30a,d |
|
| rhPro-UK | 10 | 50b |
|
| UK | 5 | 20 |
|
| rt-PA | 4.5 mg/kg | 30a |
|
| Sham | – | – |
|
| Model | – | 0 |
| 6 | rhPro-UK | 2.5 | 20 |
|
| rhPro-UK | 5 | 30a,d |
|
| rhPro-UK | 10 | 40b |
|
| UK | 5 | 0 |
|
| rt-PA | 4.5 mg/kg | 20 |
Effect of rhPro-UK on thrombolysis
time
At the 3 h therapeutic time window, the thrombolysis
rate of 2.5×104 U/kg rhPro-UK significantly increased at
75, 105 and 120 min, at 60–120 min for 5×104 U/kg
rhPro-UK and at 15–120 min for 10×104 U/kg rhPro-UK,
respectively. In addition, UK and rt-PA increased the thrombolysis
rate. The thrombolysis times of rhPro-UK(2.5×, 5× and
10×104) were 117.0, 105.0 and 77.5 min, respectively and
the thrombolysis times for UK and rt-PA were 109.0 and 97.5 min,
respectively.
At the 4.5 h therapeutic time window, the
thrombolysis rate of 2.5×104 U/kg rhPro-UK significantly
increased at 75 and 120 min, at 30–120 min for 5×104
U/kg rhPro-UK and at 45–120 min for 10×104 U/kg.
Furthermore, UK and rt-PA increased the thrombolysis rate. The
thrombolysis times of rhPro-UK(2.5×, 5× and 10×104) were
119.5, 111.0 and 105.5 min, and were 111.5 and 101.5 min for UK and
rt-PA, respectively.
At the 6 h therapeutic time window, the thrombolysis
rate of 2.5×104 U/kg rhPro-UK significantly increased at
75–120 min, at 30 and 75–120 min for 5×104 U/kg
rhPro-UK, and at 30–120 min for 10×104 U/kg rhPro-UK. In
addition, UK and rt-PA increased the thrombolysis rate. The
thrombolysis time of rhPro-UK(2.5×, 5× and 10×104) were
113.0, 111.0 and 103.5, respectively, and the thrombolysis rates of
UK and rt-PA were 113.0 and 107.0 min (Tables II–IV).
 | Table II.Effect of rhPro-UK on thrombolysis
rate (%) and thrombolysis time in thromboembolic rabbit models at
the 3 h therapeutic window (means ± standard error; n=10). |
Table II.
Effect of rhPro-UK on thrombolysis
rate (%) and thrombolysis time in thromboembolic rabbit models at
the 3 h therapeutic window (means ± standard error; n=10).
|
|
|
| Time after
administration (min) |
|---|
|
|
|
|
|
|---|
| Group | Dose
(×104 U/kg) |
Pre-administration | 15 | 30 | 45 | 60 | 75 | 90 | 105 | 120 |
|---|
| Model | – | 0 |
3.0±1.7 |
3.4±2.9 |
3.1±2.9 |
5.5±4.5 |
2.0±2.5 |
4.3±3.7 |
5.5±4.0 |
5.4±1.7 |
| hPro-UK | 2.5 | 0 |
0.2±4.2 |
5.2±4.3 |
6.2±3.8 |
8.0±5.1 |
15.2±4.8a |
17.6±7.4 |
23.6±6a |
21.5±10.6a |
| rhPro-UK | 5 | 0 |
10.3±2.6d |
7.6±2.4d |
9.2±3.1d |
23.5±4.5a,d |
25.9±4.3c,d |
33.4±5.8b,d |
32.4±6.1b,d |
36.8±7.8c,d |
| rhPro-UK | 10 | 0 |
17.4±2.7b |
28.2±5.1c |
38.5±6.5c |
39.3±8.5c |
42.7±7.8c |
49.2±9.7c |
53.3±8.8c |
55.0±8.2c |
| UK | 5 | 0 |
8.8±4.8 |
9.7±4.6 |
13.6±2.8a |
16.8±3.8 |
16.8±3.4a |
22.1±7.1a |
22.2±7.2 |
28.3±6.2c |
| rt-PA | 4.5 mg/kg | 0 |
9.5±3.4 |
16.1±4.4a |
19.1±6.9a |
23.8±7.6a |
30.8±9.2c |
33.4±8.5b |
35.6±8.1b |
42.0±7.0c |
 | Table IV.Effect of rhPro-UK on thrombolysis
rate (%) and thrombolysistime in thromboembolic rabbit models at
the 6 h therapeutic window (means ± standard error; n=10). |
Table IV.
Effect of rhPro-UK on thrombolysis
rate (%) and thrombolysistime in thromboembolic rabbit models at
the 6 h therapeutic window (means ± standard error; n=10).
|
|
|
| Time after
administration (min) |
|---|
|
|
|
|
|
|---|
| Group | Dose
(×104 U/kg) |
Pre-administration | 15 | 30 | 45 | 60 | 75 | 90 | 105 | 120 |
|---|
| Model | – | 0 |
−4.6±3.3 |
0.1±1.5 |
0.4±3.2 |
2.0±4.5 |
−2.8±4.4 |
−3.8±4.2 |
−10.9±5.5 |
−6.2±5.4 |
| rhPro-UK | 2.5 | 0 |
1.7±2.7 |
3.4±3.7 |
10.9±5.2 |
16.8±4.0 |
15.9±7.2a |
17.7±5.5b |
14.7±4.2b |
14.7±8.7a |
| rhPro-UK | 5 | 0 |
1.9±3.2d |
9.0±2.7a,d |
10.1±3.4d |
11.5±5.8d |
18.7±6.0b,d |
18.0±5.8b,d |
12.7±7.9b,d |
24.1±6.6b,d |
| rhPro-UK | 10 | 0 |
0.2±4.3 |
8.9±3.7a |
15.2±3.5b |
20.9±5.7a |
24.9±6.0c |
27.3±7.0c |
30.1±7.2c |
35.7±6.8c |
| UK | 5 | 0 |
4.5±3.8 |
3.1±2.7 |
1.7±3.2 |
2.7±3.2 |
8.0±3.5 |
6.6±2.4 |
5.5±1.9 |
13.2±3.1a |
| rt-PA | 4.5 mg/kg | 0 |
3.7±3.2 |
7.6±3.0 |
9.0±3.6 |
16.2±7.7 |
28.3±7.8c |
30.3±8.9c |
28.2±7.4c |
30.7±7.5c |
Effect of rhPro-UK on bleeding
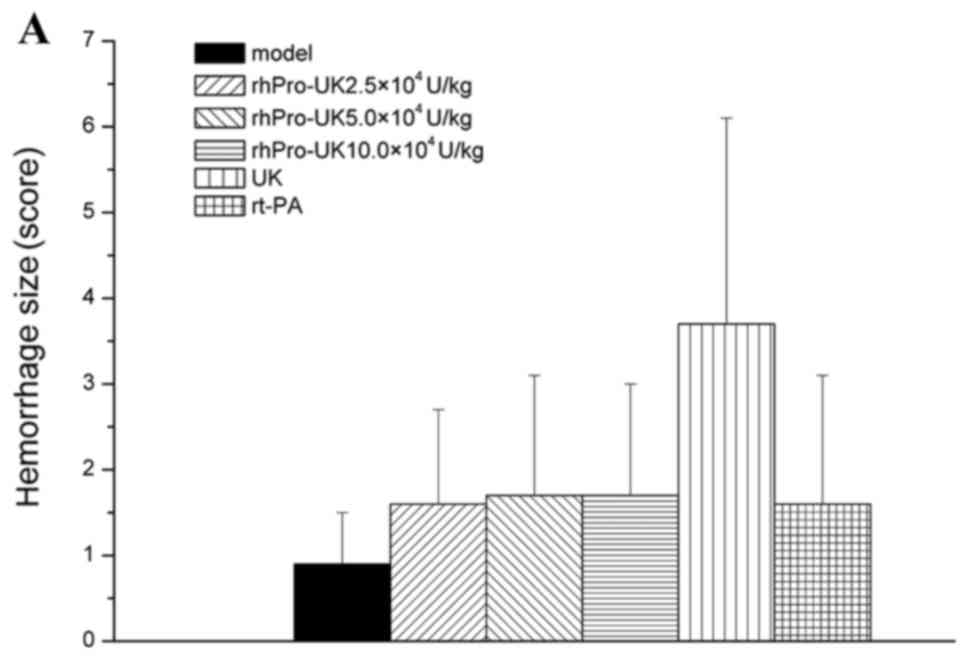
In the different therapeutic time windows, no
significant difference (P>0.05) was identified between rhPro-UK
(2.5×, 5× and 10×104 U/kg) on hemorrhage type and number
compared with the model group. At 3, 4.5 and 6 h, respectively,
rhPro-UK (5×104 U/kg) treatment exhibited similar
hemorrhage numbers when compared with UK treatment (20 vs. 30%, 20
vs. 30% and 30 vs. 40%), and the hemorrhage size also slightly
decreased (1.7 vs. 3.7, 2.2 vs. 4.4 and 2.5 vs. 4.1; Table V and Fig.
2A-C).
 | Table V.Effect of rhPro-UK on hemorrhage in
thromboembolic rabbit models (n=10). |
Table V.
Effect of rhPro-UK on hemorrhage in
thromboembolic rabbit models (n=10).
|
|
|
| Hemorrhage
type |
|
|---|
|
|
|
|
|
|
|---|
| Therapeutic time
window (h) | Group | Dose
(×104 U/kg) | NH | PT | HI | ICH | Total subjects with
hemorrhage (%) |
|---|
|
| Sham | – | 10 | 0 | 0 | 0 | 0 (0) |
|
| Model | – | 8 | 1 | 1 | 0 | 2 (20)a |
| 3 | rhPro-UK | 2.5 | 8 | 0 | 2 | 0 | 2 (20)b |
|
| rhPro-UK | 5 | 8 | 1 | 1 | 0 | 2 (20)b,c |
|
| rhPro-UK | 10 | 8 | 1 | 1 | 0 | 2 (20)b |
|
| UK | 5 | 8 | 1 | 0 | 2 | 3 (30)b |
|
| rt-PA | 4.5 mg/kg | 8 | 1 | 0 | 1 | 2 (20)b |
|
| Sham | – | 10 | 0 | 0 | 0 | 0 (0) |
|
| Model | – | 8 | 0 | 1 | 1 | 10
(10)a |
| 4.5 | rhPro-UK | 2.5 | 8 | 0 | 2 | 0 | 2 (20)b |
|
| rhPro-UK | 5 | 8 | 0 | 1 | 1 | 2 (20)b,c |
|
| rhPro-UK | 10 | 7 | 1 | 2 | 0 | 3 (30)b |
|
| UK | 5 | 7 | 0 | 2 | 1 | 3 (30)b |
|
| rt-PA | 4.5 mg/kg | 7 | 0 | 2 | 1 | 3 (30)b |
|
| Sham | – | 10 | 0 | 0 | 0 | 0 (0) |
|
| Model | – | 8 | 0 | 2 | 0 | 2 (20)a |
| 6 | rhPro-UK | 2.5 | 8 | 1 | 1 | 0 | 2 (20)b |
|
| rhPro-UK | 5 | 7 | 0 | 2 | 1 | 3 (30)b,c |
|
| rhPro-UK | 10 | 7 | 0 | 2 | 1 | 3 (30)b |
|
| UK | 5 | 6 | 1 | 2 | 1 | 4 (40)b |
|
| rt-PA | 4.5 mg/kg | 7 | 0 | 3 | 0 | 3 (30)b |
Effect of rhPro-UK on blood
coagulation factor
Compared with the model group, no significant
difference (P>0.05) was identified between rhPro-UK (2.5×, 5×
and 10×104 U/kg) on PT, TT, APTT and FIB for the
different time windows. UK treatment extended TT and APTT, and
reduced FIB, furthermore rt-PA prolonged APTT and reduced FIB
slightly. rhPro-UK (5×104 U/kg)exerted lighter effects
on TT, APTT and FIB when compared with UK treatment (Table VI).
 | Table VI.Effect of rhPro-UK on blood
coagulation factors in thromboembolic rabbit models (means ±
standard error; n=10). |
Table VI.
Effect of rhPro-UK on blood
coagulation factors in thromboembolic rabbit models (means ±
standard error; n=10).
| Therapeutic time
window (h) | Group | Dose
(×104 U/kg) | PT (s) | TT (s) | APTT (s) | FIB (g/l) |
|---|
|
| Sham | – |
7.1±1.4 |
17.3±3.2 |
26.5±5.5 |
3.4±0.8 |
|
| Model | – |
6.3±1.3g |
19.5±2.8g |
27.8±3.7g |
3.5±1.2g |
| 3 | rhPro-UK | 2.5 |
6.7±0.8 |
22.1±3.0 |
28.2±4.0 |
3.6±0.6 |
|
| rhPro-UK | 5 |
6.2±0.5 |
20.6±2.0a |
29.5±5.4a |
3.4±0.9b |
|
| rhPro-UK | 10 |
6.6±0.6 |
21.5±4.9 |
31.2±4.1c |
3.2±0.8 |
|
| UK | 5 |
7.2±1.2 |
25.3±5.9d |
34.0±4.3e |
2.4±0.6c |
|
| rt-PA | 4.5 mg/kg |
7.1±1.1 |
19.4±1.8 |
32.4±3.4d |
2.6±0.7c |
|
| Sham | – |
6.2±0.5 |
19.7±3.7 |
29.6±4.6 |
3.3±0.8 |
|
| Model | – |
6.6±1.4g |
20.7±3.6g |
30.0±3.1g |
3.3±0.6g |
| 4.5 | rhPro-UK | 2.5 |
6.2±0.6 |
19.7±1.7 |
28.5±4.5 |
3.5±0.5 |
|
| rhPro-UK | 5 |
7.6±3.2 |
19.4±3.2b |
29.5±5.7a |
3.6±0.9f |
|
| rhPro-UK | 10 |
6.9±0.9 |
22.4±2.8 |
31.6±3.0 |
3.4±1.0 |
|
| UK | 5 |
6.7±1.2 |
24.2±4.0c |
34.6±5.5c |
2.2±0.8c |
|
| rt-PA | 4.5 mg/kg |
6.6±0.5 |
20.0±2.3 |
34.0±3.8c |
2.5±0.9 |
|
| Sham | – |
6.3±0.4 |
18.7±2.4 |
26.1±3.5 |
3.5±0.7 |
|
| Model | – |
6.4±0.5g |
19.2±2.1g |
29.0±3.4g |
3.5±0.6g |
| 6 | rhPro-UK | 2.5 |
6.7±0.6 |
19.4±3.6 |
29.1±5.4 |
3.7±0.8 |
|
| rhPro-UK | 5 |
6.4±0.7 |
18.5±2.1f |
31.6±3.5 |
3.4±1.1 |
|
| rhPro-UK | 10 |
6.6±0.8 |
20.9±4.0 |
30.7±4.3 |
3.3±0.9 |
|
| UK | 5 |
6.4±0.7 |
23.6±3.4d |
34.1±5.1d |
2.3±0.8d |
|
| rt-PA | 4.5 mg/kg |
6.7±0.8 |
20.6±1.8 |
32.9±4.1c |
2.5±0.8c |
Effect of rhPro-UK on
α2-AP
At the 3 h therapeutic time window, rhPro-UK(2.5×,
5× and 10×104 U/kg) reduced α2-AP by 5.3%
(P>0.05), 5.3% (P>0.05) and 18.1% (P<0.05), respectively.
In addition, UK and rt-PA reduced α2-AP by 29.2%
(P<0.01) and 22.7% (P<0.05), respectively. Compared with UK,
5×104 U/kg rhPro-UK exerts a smaller influence on
α2-AP (9.7 vs. 29.2%).
At the 4.5 h therapeutic time window, rhPro-UK
(2.5×, 5× and 10×104 U/kg)reduced α2-AP by
2.4%(P>0.05), 6.5%(P>0.05) and 17.8% (P<0.05).
Furthermore, UK and rt-PA reduced α2-AP by 25.3%
(P<0.01) and 19.8% (P<0.05), respectively. rhPro-UK
(5×104 U/kg) exerts a smaller influence on
α2-AP when compared with UK(6.5 vs. 25.3%).
At the 6 h therapeutic time window, rhPro-UK (2.5×,
5× and 10×104 U/kg) reduced α2-AP by 5.7%
(P>0.05), 12.7% (P>0.05) and 22.2% (P<0.01). In addition,
UK and rt-PA reduced α2-AP by 30.2% (P<0.001) and
25.6% (P<0.01). Compared with UK, 5×104 U/kg rhPro-UK
exerts a smaller influence on α2-AP when compared with
UK (12.7 vs. 30.2%; Fig. 3A-C).
Discussion
Stroke is the second leading cause of mortality
worldwide and the number one cause of disability in the USA
(17). IV tPA remains the only drug
that has been approved by the United States Food and Drug
Administration for its treatment (18). However, the perception of marginal
utility, high risk of intracerebral bleeding, and/or high liability
associated with its administration discourage its administration
(19), although the American Heart
Association has deemed it an acceptable alternative therapy and
many stroke centers offer it to patients within 6 h of a major
acute stroke (20). These limitations
reflect the requirement for more effective thrombolic drugs.
rhPro-UK has more potent efficacy and fewer adverse reactions in
comparison with other thrombolytics due to the fibrin-selective
clot lysis. The rhPro-UK in the present study was from Tasly
Pharmaceutical Co., Ltd., generated from Chinese hamster ovary cell
expression using a genetic engineering method, and is typically
used to treat acute myocardial infarction (21). The present study evaluated IV
thrombolysis with rhPro-UK in rabbit acute cerebral infarction at
3, 4.5 and 6 h therapeutic time windows.
The results confirmed that the thrombolysis rate and
patency rate (recanalization rate) increased as the time window
shortened. At 3, 4.5 and 6 h therapeutic time windows, the
thrombolysis rate of 5×104 U/kg rhProUK was 36.8, 29.9
and 24.1%, respectively and the patency rate was 40, 30 and 30%.
The thrombolysis rate of 10×104 U/kg rhPro-UK was 55.0,
49.0 and 35.7% and the patency rate was 70, 50, 40% at 3, 4.5 and 6
h therapeutic time windows, respectively. rhPro-UK treatment
increased the thrombolysis rate slightly when compared with UK
(36.8 vs. 28.3%, 29.9 vs. 22.6% and 24.1 vs. 13.2%). Consistent
with the present study, del Zoppo et al (3) reported a phase II randomized trial of
rhPro-UK by direct arterial delivery in acute middle cerebral
artery stroke, local IV rhPro-UK infusion at 5.5 h from symptom
onset was associated with superior recanalization in acute
thrombotic/thromboembolic stroke when compared with a placebo. In
addition, Tirschwell et al (22) reported a PROACT II trial including 180
patients with acute ischemic stroke, despite an increased frequency
of early symptomatic intracranial hemorrhage, treatment with Pro-UK
within 6 h of the onset of acute ischemic stroke caused by middle
cerebral artery occlusion significantly improved the clinical
outcome at 90 days.
In addition, it was found that rhPro-UK (2.5×, 5×
and 10×104 U/kg) did not increase bleeding compared with
the model group (P>0.05), and the hemorrhage size of the
5×104 U/kg rhPro-UK group was slightly decreased
compared with the UK treatment group at different time points.
rhPro-UK (5×104 U/kg) had less of an influence on PT,
TT, APTT, FIB and α2-APwhen compared with UK. This
finding is comparable to a study by Zhang et al (23), where rhPro-UK did not effect FIB, PA
or α2-AP, and the effect on bleeding time, clotting time
and bleeding quantity per unit time was less than those of UK.
Plasmin is an enzyme that participates in
fibrinolysis. α2-AP is a serine protease inhibitor
responsible for inactivating plasmin. Its rapid reaction with
plasmin results in the formation of an inactive complex
(plasmin-α2-AP complex; PAP), which is composed of one
molecule of each component. Therefore, the method that was used for
measuring α2-AP in the present study only indirectly
reflects the actual fibrinolytic activity, and thus presents a
limitation of this study. Determination of PAP may be more
appropriate in future studies.
In conclusion, IV rhPro-UK exerted therapeutic
effects on thromboembolic stroke rabbit models within a 6 h time
frame, influencing thrombolysis and recanalization (patency rate)
with reduced risk of cerebral hemorrhage.
References
|
1
|
Furlan A, Higashida R, Wechsler L, Gent M,
Rowley H, Kase C, Pessin M, Ahuja A, Callahan F, Clark WM, et al:
Intra-arterial prourokinase for acute ischemic stroke. The PROACT
II study: A randomized controlled trial. Prolyse in acute cerebral
thromboembolism. JAMA. 282:2003–2011. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gurewich V, Pannell R, Louie S, Kelley P,
Suddith RL and Greenlee R: Effective and fibrin-specific clot lysis
by a zymogen precursor form of urokinase (pro-urokinase). A study
in vitro and in two animal species. J Clin Invest. 73:1731–1739.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
del Zoppo GJ, Higashida RT, Furlan AJ,
Pessin MS, Rowley HA and Gent M: PROACT: A phase II randomized
trial of recombinant pro-urokinase by direct arterial delivery in
acute middle cerebral artery stroke. PROACT investigators. Prolyse
in acute cerebral thromboembolism. Stroke. 29:4–11. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Moia M, Mannucci PM, Pini M, Prandoni P
and Gurewich V: A pilot study of pro-urokinase in the treatment of
deep vein thrombosis. Thromb Haemost. 72:430–433. 1994.PubMed/NCBI
|
|
5
|
Ning RX, Wang R and Cui XY: A new
thrombolytic drug: Recombinant human prourokinase. Zhongguo Xin Yao
Zazhi. 17:430–432. 2008.(In Chinese).
|
|
6
|
Liu Y and Wang L: Efficacy and safety of
thrombolytic therapy with recombinant human prourokinase for acute
myocardial infarction in 50 cases. Chin Pharm J. 15:76–78. 2015.(In
Chinese).
|
|
7
|
Thomas GR, Thibodeaux H, Bennett WF,
Refino CJ, Badillo JM, Errett CJ and Zivin JA: Optimized
thrombolysis of cerebral clots with tissue-type plasminogen
activator in a rabbit model of embolic stroke. J Pharmacol Exp
Ther. 264:67–73. 1993.PubMed/NCBI
|
|
8
|
Hao CH, Xu XW, Ma YZ, Zhang R, Sun SY,
Wang WT, et al: Application of 99Tcm tracer technique in rabbit
cerebral thromboembolic stroke. Yaowu Pingjia Yanjiu. 40:648–651.
2017.(In Chinese).
|
|
9
|
Zhang L, Zhang RL, Jiang Q, Ding G, Chopp
M and Zhang ZG: Focal embolic cerebral ischemia in the rat. Nat
Protoc. 10:539–547. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Feng L, Liu J, Chen J, Pan L and Feng G:
Establishing a model of middle cerebral artery occlusion in rabbits
using endovascular interventional techniques. Exp Ther Med.
6:947–952. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang Z, Zhang RL, Jiang Q, Raman SB,
Cantwell L and Chopp M: A new rat model of thrombotic focal
cerebral ischemia. J Cereb Blood Flow Metab. 17:123–135. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yu B, Tong J and Li HF: Thrombolytic
efficacy of rt-PA in embolic stroke of rabbits by 99Tcm trace. J
Isot. 18:160–163. 2005.
|
|
13
|
Thomas GR, Thibodeaux H, Errett CJ,
Badillo JM, Keyt BA, Refino CJ, Zivin JA, Bennett WF, et al: A
long-half-life and fibrin-specific form of tissue plasminogen
activator in rabbit models of embolic stroke and peripheral
bleeding. Stroke. 25:2072–2078. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bovill EG, Terrin ML, Stump DC, Berke AD,
Frederick M, Collen D, Feit F, Gore JM, Hillis LD, Lambrew CT, et
al: Hemorrhagic events during therapy with recombinant tissue-type
plasminogen activator, heparin, and aspirin for acute myocardial
infarction. Results of the thrombolysis in myocardial infarction
(TIMI), Phase II trial. Ann Intern Med. 115:256–265. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Clark WM, Madden KP, Lyden PD and Zivin
JA: Cerebral hemorrhagic risk of aspirin or heparin therapy with
thrombolytic treatment in rabbits. Stroke. 22:872–8761991.
View Article : Google Scholar
|
|
16
|
Chapman DF, Lyden P, Lapchak PA, Nunez S,
Thibodeaux H and Zivin J: Comparison of TNK with wild-type tissue
plasminogen activator in a rabbit embolic stroke model. Stroke.
32:748–752. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mozaffarian D, Benjamin EJ, Go AS, Arnett
DK, Blaha MJ, Cushman M, de Ferranti S, Després JP, Fullerton HJ,
Howard VJ, et al: Heart disease and stroke statistics-2015 update:
A report from the American heart association. Circulation.
131:29–322. 2015. View Article : Google Scholar
|
|
18
|
Amar AP, Griffin JH and Zlokovic BV:
Combined neurothrombectomy or thrombolysis with adjunctive delivery
of 3K3A-activated protein C in acute ischemic stroke. Front Cell
Neurosci. 9:3442015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
So Relle and Ruth MP: Breaking news: The
‘biggest, baddest’ controversy in EM. Emerg Med News. 35:26–27.
2013.
|
|
20
|
Furlan AJ and Abou-Chebl A: The role of
recombinant pro-urokinase (r-pro-UK) and intra-arterial
thrombolysis in acute ischaemic stroke: The PROACT trials. Prolyse
in acute cerebral thromboembolism. Curr Med Res Opin. 18:44–47.
2002. View Article : Google Scholar
|
|
21
|
Prourokinase Clinical Trial Group, ; Li T,
Xiao Ch, Liu R and Liu L: Multicenter phase III study of
recombinant prourokinase for acute myocardial infarction with
ST-segment evaluation. J Med Res. 42:26–31. 2013.
|
|
22
|
Tirschwell DL, Coplin WM, Becker KJ,
Vogelzang P, Eskridge J, Haynor D, Cohen W, Newell D, Winn HR and
Longstreth WT Jr: Intra-arterial urokinase for acute ischemic
stroke: Factors associated with complications. Neurology.
57:1100–1103. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang ZG, Xiao CZ, Hu XW, Xu ZP, Liu JX,
Yang SJ, Ren JP, Liao MY, Shi XC and Wu BA: Investigation of
pharmacodynamics, pharmacology, and toxicology of domestic human
prourokinase. Sci Sin Vitae. 41:1024–1029. 2011. View Article : Google Scholar
|