Introduction
Heart failure (HF) and atrial fibrillation (AF)
occurring concomitantly is one of the most common cardiac
conditions encountered in the clinic, and the incidence of HF
complicated by AF has been increasing (1,2). During the
pathophysiological process, HF and AF influence and promote each
other, forming a vicious circle (3).
Due to the side-effects and low efficiency of anti-arrhythmic
drugs, an increasing number of clinicians are developing a keen
interest in non-drug therapy for HF and AF (4). In recent years, catheter ablation (CA)
has provided a novel direction for the treatment of HF complicated
with AF, based on its advantages in the restoration of sinus
rhythm, improvement of cardiac function and long-term prognosis
(5). However, one previous
meta-analysis study demonstrated that the ablation success rate of
HF patients with AF is only ~60% (6).
Therefore, how to reduce the recurrence rate of AF with HF has
become a hot research topic. It has been demonstrated that statins
may prevent the occurrence of AF by virtue of their being
antioxidants and anti-inflammatory agents, and through the
stabilization of cardiomyocyte membranes (7,8). These
characteristics of statins are closely linked with the pathological
processes of HF and AF. The GISSI-HF trial, which explored the
effects of rosuvastatin in patients with chronic heart failure,
demonstrated that rosuvastatin is able to reduce the occurrence of
AF in patients with HF (9).
Furthermore, Xia et al (10)
demonstrated that rosuvastatin decreases the early recurrence of AF
following electrical cardioversion with reduced asymmetric
dimethylarginine levels. However, the effects of rosuvastatin on
the recurrence rate of AF in patients with HF have yet to be fully
elucidated. Therefore, the present study aimed to ascertain whether
rosuvastatin was able to reduce the recurrence rate following CA
for AF in patients with HF.
Materials and methods
Patient population
All patients diagnosed with HF and AF who underwent
CA of AF between June 2012 and May 2014 were recruited in the
present study. Both the definition and classification of AF and HF
were based on the guidelines of 2014's American College of
Cardiology (ACC)/American Heart Association Task Force on Practice
Guidelines (AHA)/Heart Rhythm Society (HRS) (5), 2013's American College of Cardiology
Foundation (ACCF)/AHA/HF guidelines (11) and the functional classification of the
New York Heart Association (NYHA) (12). Patients who were suffering from
moderate-to-severe valvular disease, inflammatory diseases,
coronary artery disease, a previous incidence of AF/CA, surgery,
trauma, hyperthyroidism or hypothyroidism, anomalous pulmonary
venous connection, revealed left atrium thrombus or anticoagulation
contraindications were excluded. Eligible patients were randomly
assigned in proportions of 1:1:1 to receive an additional 10 mg
rosuvastatin daily (group 1), an additional 20 mg rosuvastatin
daily (group 2), or only a conventional treatment of HF following
ablation [group 3, the control group: Specifically, patients with
heart failure were treated with an appropriate pharmacological
therapy, such as angiotensin-converting enzyme
inhibitors/angiotensin receptor blockers (ACEI/ARBs), β-blocker,
digoxin or diuretics, except in the case of treatment with
rosuvastatin, when necessary, according to the 2016 ESC Guidelines
for the Diagnosis and Treatment of Acute and Chronic Heart Failure
(13)]. Rosuvastatin
(Crestor®; 10 mg, 7 pills per packet) was obtained from
AstraZeneca (Cambridge, UK).
The present study was approved by the Ethics
Committee of the First Affiliated Hospital of Zhengzhou University
Institute (Zhengzhou, China), and informed consent was obtained
from the patients prior to their participation in this study.
Procedure of radiofrequency CA
All the subjects received warfarin therapy for ≥1
month prior to the surgery, maintaining the international
normalized ratio between 2.0–3.0. Warfarin was discontinued 3 days
prior to the surgery, and bridging with low-molecular-weight
heparin (Chia Tai Haier Pharmaceutical Co., Ltd., Qingdao, China)
was performed. Within 24 h prior to the surgery, transesophageal
echocardiography was performed, or a pulmonary vein computed
tomography angiography examination was conducted, to exclude left
atrial thrombus. In addition, anti-arrhythmic drug therapy was
stopped ≥1 week prior to the ablation, with the exception of
amiodarone, which was orally administered (0.2 mg) three times a
day 7 days prior to the ablation. Following local anesthesia, the
right and left femoral vein were punctured using Seldinger's
technique (two 8F sheets were placed on the right femoral vein, and
one 6F sheet was placed on the left femoral vein). Subsequently, a
10-pole mapping electrode (Lasso®; Biosense Webster,
Inc., Diamond Bar, CA, USA) was inserted into the coronary sinus
through the 6F sheath. Next, a Swartz™ sheath (St. Jude Medical,
Inc., St. Paul, MN, USA) was used for the transseptal puncture.
After the transseptal puncture, heparin was administered at a
concentration of 100 U/kg to the patients, and the Swartz™ sheath
was continuously rinsed with heparin saline. After directing the
Swartz™ sheath to the pulmonary vein ostium along the guide-wire,
both sides of the pulmonary vein angiography were subsequently
performed to determine the anatomical location of the pulmonary
vein openings. Ten pole-circular mapping electrodes
(Lasso®; Biosense Webster, Inc.) were subsequently
directed to each pulmonary vein ostium through the Swartz™ sheath,
recording the pulmonary vein potential in order to guide pulmonary
vein ablation. A 3.5 mm saline irrigated ablation catheter
(Thermo-cool Navistar; Biosense Webster, Inc.) was subsequently
delivered to the left atrium through the Swartz™ sheath. The left
atrium and pulmonary veins were reconstructed according to their
three-dimensional structure following the guidance system of
CARTO® (Biosense Webster, Inc.). Guided by the
CARTO® system, the 3.5 mm saline infusion thermostat
catheter was used to perform the annular ablation along the
orifices of the right and left pulmonary veins. The following
discharge parameters were set: 34–40 W; flow rate of 17–25 ml/min
(2 ml/min when ablation was not being performed); maximum
temperature, 43°C; discharge time for each point of 20–30 sec. The
end-point was determined by the pulmonary vein and the atrium
achieving two-way electrical isolation. The majority of patients
with paroxysmal and early persistent AF required pulmonary vein
isolation alone, although a large number of the patients with
prolonged and permanent AF were required to have an additional
complex fractionated electrogram ablation, or a cavotricuspid
isthmus or linear ablation. After the ablation, if atrial
fibrillation was not restored to normal sinus rhythm, or a switch
was made to atrial tachycardia or atrial flutter, then electrical
cardioversion was performed.
Follow-up
The detailed medical history of the patient was
obtained, and an echocardiography examination, 24 h Holter
monitoring and a series of basic laboratory analyses (see below)
were performed prior to the procedure. The follow-up period of the
study was 18 months. Patients were followed at 1, 3, 6, 12 and 18
months through the outpatient clinic following the surgery. At the
time-point of each follow-up visit, patients received 24 h Holter
monitoring, and were also asked about any symptoms associated with
the presence of arrhythmia. If the patients were suspected of
having had an emerging arrhythmia, but no evidence of this was
available at the time of examination, additional 24 h Holter
monitoring and short-duration follow-up were performed. If
palpitations arose or the patients experienced any symptoms of
arrhythmia during the follow-up, 24 h Holter monitoring was
instantly administered. At the time of 6 and 12 months following
CA, an echocardiography was performed, and the laboratory analyses,
including assessing the levels of hypersensitive C-reactive protein
(hs-CRP) and N-terminal pronatriuretic peptide (NT-proBNP) and
estimating the glomerular filtration rate (eGFR), were performed
for all the participants. All patients received warfarin for 3
months postoperatively, if there was no recurrence of AF and the
CHADS2 score (5) was ≥2. The
electrocardiogram was assessed to confirm whether there had been
recurrence of any AF or atrial tachycardia for ≥30 sec. AF
occurring within 3 months was not considered to be a recurrence
(since this fell within the ‘blanking period’, during which time
recurrences were often managed medically). The primary end-point of
this trial was the recurrence of any AF or atrial tachycardia of
>30 sec duration. In addition, the major adverse events were
defined as mortality, muscle-associated symptoms, HF
hospitalization, or stroke.
Laboratory assessment and
echocardiography
The blood samples were collected from the elbow
while the patients were supine following a rest period. Plasma
hs-CRP levels were analyzed using an automated hs-CRP method
(IMMULITE®; Diagnostic Products Corp., Los Angeles, CA,
USA). The plasma NT-proBNP level was analyzed by
electrochemiluminescence in an E170 immunoassay analyzer
(Hoffmann-LaRoche, Basel, Switzerland). The eGFR values were
calculated using the Modification of Diet in Renal Disease (MDRD)
equation modified by the Japanese coefficient (0.881), as described
previously (14): [GFR (ml/min per
1.73 m2) = 0.881 × 186 × age−0.203 ×
Scr−1.154 (if female, × 0.742). The equation provides an
accurate eGFR value within the range of GFR <60 ml/min/1.73
m2]. For the echocardiography parameters, the left
ventricular ejection fraction (LVEF) was calculated using Simpson's
method, whereas the left atrial diameter (LAD) and left ventricular
end-diastolic diameter (LVEDD) diameter were assessed using M-mode
methods.
Statistical analysis
The measured data were expressed as the mean ±
standard deviation. Baseline characteristics of different groups
were compared using the Student's t-test. Enumeration data were
represented as n, and as a percentage. Comparisons of categorical
variables were made using the Chi-square test or Fisher's exact
test. Kaplan-Meier survival curves were used to estimate AF-free
survival, and the differences between the curves were compared
using the log-rank test. Univariate and multivariate Cox
proportional hazard regression analyses were performed to identify
the independent predictors of AF recurrence. Data were analyzed
using SPSS statistical software, version 17.0 (SPSS, Inc., Chicago,
IL, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Study population
A total of 107 patients with the complications of HF
and AF were recruited in the present study. Baseline
characteristics of the patients are listed in Table I. No statistically significant
differences were identified with respect to age, gender, duration
of AF, LVEDD, LVEF, type of AF, NYHA functional class,
complications, or other aspects among the different groups
(Table II). The major adverse events
occurred in 4 patients (all were HF hospitalizations) among the
participants, and there were no significant differences regarding
major adverse events among the 3 groups (P=0.767) (Table III). No patients were lost during the
follow-up.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristic | Value |
|---|
| Age, years | 63.3±13.6 |
| Female, n (%) | 58 (54.2) |
| Smoking, n (%) | 17 (15.9) |
| Hypertension, n
(%) | 21 (19.6) |
| Diabetes mellitus, n
(%) | 17 (15.9) |
| Type of AF, n
(%) |
|
Paroxysmal | 24 (22.4) |
|
Persistent | 45 (42.1) |
|
Permament | 38 (35.5) |
| Duration of AF,
months | 37.8 (4.0–70.3) |
| Comorbidities |
|
| Laboratory data |
|
| hs-CRP,
mg/l | 2.00±1.21 |
| eGFR,
ml/min/1.73 m2 | 63.3±18.2 |
| NT-proBNP
level, pg/dl | 399.9
(65.3–887.5) |
| Echocardiographic
parameters |
|
| LAD,
mm | 47.5±7.9 |
| LVEDD,
mm | 55.6±10.1 |
| LVEF,
% | 45.2±14.1 |
| NYHA
functional class | 2.3±0.6 |
| Medical therapy, n
(%) |
|
| ACEI or
ARB | 53 (49.5) |
|
Diuretic | 60 (56.1) |
|
β-blockers | 61 (57.0) |
|
Digoxin | 22 (20.6) |
|
Calcium-channel blockers | 13 (12.1) |
| Ablation procedure, n
(%) |
|
| PVI | 107 (100.0) |
| PVI with
additional procedure | 88 (82.2) |
| Antiarrhythmic drug
use, n (%) |
|
| Class
1 | 16 (14.9) |
| Class
3 | 34 (31.8) |
 | Table II.Comparison of baseline characteristics
of different groups. |
Table II.
Comparison of baseline characteristics
of different groups.
| Parameter | Group 1 (n=36) | Group 2 (n=36) | Group 3 (n=35) | P-value |
|---|
| Age, years | 62.2±11.7 | 63.1±14.2 | 62.7±13.0 | 0.31 |
| Female, n (%) | 22 (61.1) | 20 (55.6) | 16 (45.7) | 0.73 |
| Smoking, n (%) | 6
(16.7) | 7
(19.4) | 4
(11.4) | 0.19 |
| Hypertension, n
(%) | 7
(19.4) | 6
(16.7) | 4
(11.4) | 0.91 |
| Diabetes mellitus,
n (%) | 8
(22.2) | 9
(30.0) | 12 (18.2) | 0.49 |
| Type of AF |
|
|
|
|
|
Paroxysmal, n (%) | 6
(16.7) | 7
(19.4) | 11 (31.4) | 0.11 |
|
Persistent, n (%) | 17 (47.2) | 15 (41.7) | 13 (37.1) | 0.94 |
|
Permanent, n (%) | 13 (36.1) | 14 (38.9) | 11 (31.4) | 0.76 |
| Duration of AF,
months | 37.3
(3.3–79.9) | 34.2
(2.3–66.5) | 49.0
(4.2–83.5) | 0.45 |
| Laboratory
data |
|
|
|
|
| hs-CRP,
mg/l | 2.17±0.82 | 1.84±1.06 | 2.03±1.34 | 0.69 |
| eGFR,
ml/min/1.73 m2 | 62.1±17.0 | 67.5±20.3 | 61.2±18.7 | 0.21 |
|
NT-proBNP level, pg/dl | 365.9±136.8 | 421.7±182.1 | 359.5±129.3 | 0.41 |
| Echocardiographic
parameters |
|
|
|
|
| LAD,
mm | 46.7±9.1 | 47.3±7.2 | 47.9±9.2 | 0.72 |
| LVEDD,
mm | 56.9±9.6 | 53.7±11.0 | 54.1±9.3 | 0.66 |
| LVEF,
% | 46.3±14.6 | 45.2±13.5 | 49.5±15.7 | 0.76 |
| NYHA
functional class | 2.5±0.8 | 2.3±0.5 | 2.2±0.7 | 0.89 |
| Medical
therapy |
|
|
|
|
| ACEI or
ARB, (n, %) | 18 (50.0) | 21 (58.3) | 15 (42.9) | 0.29 |
|
Diuretic | 26 (72.2) | 16 (44.4) | 18 (51.4) | 0.93 |
|
β-blockers | 17 (47.2) | 24 (66.7) | 20 (57.1) | 0.62 |
|
Digoxin | 6
(16.7) | 8
(22.2) | 8
(22.9) | 0.11 |
|
Calcium-channel blockers | 3 (8.3) | 6
(16.7) | 4
(11.4) | 0.59 |
| Ablation procedure
(n, %) |
|
|
|
|
|
PVI | 36
(100.0) | 36
(100.0) | 35
(100.0) | NA |
| PVI
with additional procedure | 28 (77.8) | 31 (86.1) | 29 (82.9) | 0.88 |
| Antiarrhythmic drug
use, n (%) |
|
|
|
|
| Class
1 | 3 (8.3) | 4
(11.1) | 7
(20.0) | 0.57 |
| Class
3 | 12 (33.3) | 15 (41.7) | 7
(20.0) | 0.09 |
 | Table III.Major adverse events during the
follow-up. |
Table III.
Major adverse events during the
follow-up.
| Major adverse
event | Group 1 | Group 2 | Group 3 | P-value |
|---|
| Stroke | 0 | 0 | 0 | – |
| All cause
death | 0 | 0 | 0 | – |
| Muscle-related
symptoms | 0 | 0 | 0 | – |
| HF
hospitalization | 2 | 1 | 1 | 0.767 |
Ablation outcomes
Successful ablation was accomplished in all
patients. Pulmonary vein isolation was successfully accomplished in
all patients (100%). Additional complex fractionated electrogram
ablation, cavotricuspid isthmus or linear ablation were performed
on a total of 88 patients. No severe complications occurred in any
patients. All the patients were restored to a sinus rhythm prior to
leaving hospital. Two patients had pericardial effusion, one
patient had left groin hematoma due to an inadvertent puncture, but
following treatment, all the patients were effectively healed and
discharged.
Follow-up results
Up to the end of the follow-up, 68 patients (63.6%)
were free from AF. In group 1, 14 persons (38.9%) suffered
recurrence of AF; in group 2, the recurrence rate was 22.2%, while
there were 17 individuals (48.6%) who suffered from recurrence in
group 3. The results demonstrated that treatment with 10 mg
rosuvastatin daily following ablation did not reduce the recurrence
rate of AF in patients with HF in comparison with the control group
(38.9 vs. 48.6%; P=0.879), whereas treatment with 20 mg
rosuvastatin daily following CA was able to significantly decrease
the recurrence rate of AF compared with group 1 (22.2 vs. 38.9%, P
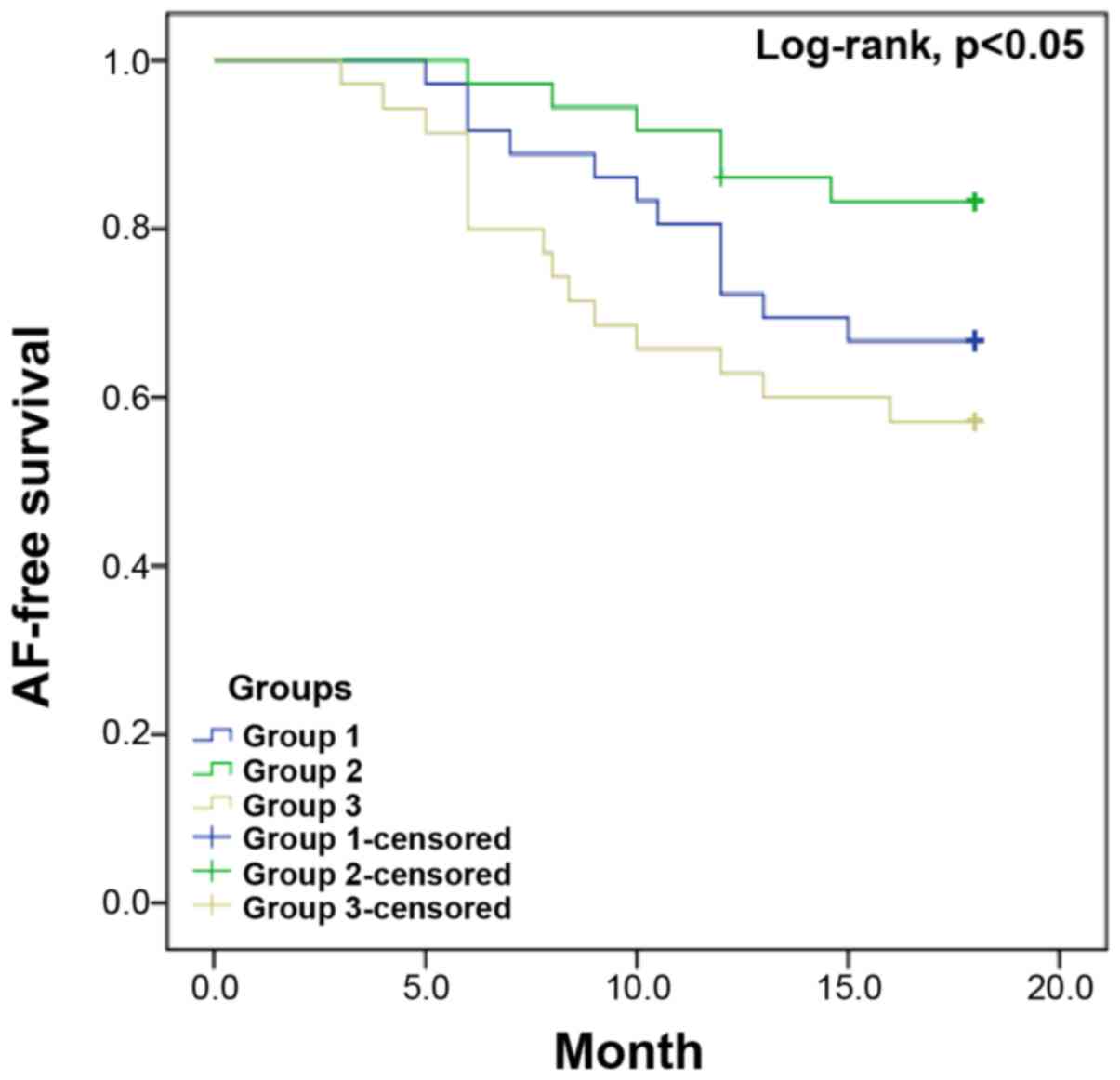
=0.013) and group 3 (22.2% vs. 48.6%, P=0.021). The Kaplan-Μeier
curves (Fig. 1) demonstrated that
treatment with 20 mg rosuvastatin daily following CA (group 2)
could significantly decrease the recurrence rate of AF compared
with group 1and group 3 (P<0.05, by log-rank test).
There were no differences in the baseline parameters
of LVEF, NYHA functional class, LAD, NT-proBNP, LVEDD and hs-CRP
levels in groups 1, 2 and 3. However, in comparison with baseline
parameters, LVEF, LAD, NT-proBNP and hs-CRP levels all exhibited
improvements in the 3 groups. Regrettably, an inspection of the
parameters mentioned above did not reveal any statistically
significant differences among groups 1, 2 and 3, with the exception
of the levels of hs-CRP and LAD during the follow-up (Table IV).
 | Table IV.Baseline and follow-up changes in
LAD, LVEF, hs-CRP and NT-proBNP. |
Table IV.
Baseline and follow-up changes in
LAD, LVEF, hs-CRP and NT-proBNP.
| Group | Parameter | Baseline | 6 months after
ablation | 12 months after
ablation |
|---|
| 1 | LAD, mm | 46.7±9.1 | 45.1±6.2 |
43.3±4.3a,c |
|
| LVEF, % | 46.3±14.6 | 49.5±14.3 |
51.4±12.3c |
|
| hs-CRP, mg/l | 2.17±0.82 |
0.96±0.61a |
0.47±0.26a–d |
|
| NT-proBNP,
pg/dl | 365.9±136.8 |
282±97.3c |
193±65.9c,d |
| 2 | LAD, mm | 47.3±7.2 | 43.2±6.2 |
39.5±5.8c,d |
|
| LVEF, % | 45.2±13.5 |
48.5±10.4c |
50.1±9.8c |
|
| hs-CRP, mg/l | 1.84±1.06 |
0.35±0.47b,c |
0.23±0.19c,b |
|
| NT-proBNP,
pg/dl | 421.7±182.1 |
312±107.1c |
213±87.4c,d |
| 3 | LAD, mm | 47.9±9.2 | 44.9±5.7 |
44.3±5.6a,c |
|
| LVEF, % | 49.5±15.7 | 51.2±13.5 |
52.7±14.2c |
|
| hs-CRP, mg/l | 2.03±1.34 |
1.11±0.57a |
0.79±0.54a,c |
|
| NT-proBNP,
pg/dl | 359.5±129.3 |
248±79.4c |
181±64.3c |
Univariate analysis (Table
V) revealed that the female gender, hs-CRP, non-paroxysmal AF,
duration of AF, LVEF and LAD were associated with higher recurrence
rates. When multivariate analysis was used to adjust for relevant
confounders, only hs-CRP, duration of AF and LAD were associated
with higher rates of recurrence (Table
VI).
 | Table V.Univariate analysis comparing
patients with and without AF recurrence. |
Table V.
Univariate analysis comparing
patients with and without AF recurrence.
| Variable | HR (95% CI) | P-value |
|---|
| Age, years | 1.02
(0.99–1.04) | 0.723 |
| Female | 2.84
(1.81–4.04) | 0.005 |
| Smoking | 0.85
(0.20–3.03) | 0.973 |
| Non-PAF | 0.74
(0.34–0.98) | 0.025 |
| Duration of AF,
months |
1.03(1.01–1.05) | 0.001 |
| Hypertension | 1.07
(0.46–2.43) | 0.998 |
| Diabetes
mellitus | 2.01
(0.57–8.34) | 0.254 |
| hs-CRP, mg/l | 1.42
(1.03–1.89) | 0.003 |
| eGFR,
ml/min/1.73m2 | 0.48
(0.16–1.35) | 0.101 |
| NT-proBNP level,
pg/dl | 1.03
(0.72–3.01) | 0.104 |
| LAD, mm | 1.08
(1.01–1.32) | 0.011 |
| LVEDD, mm | 1.00
(0.97–1.31) | 0.713 |
| LVEF, % | 1.13
(1.06–1.40) | 0.035 |
| ACEI or ARB
use | 1.56
(0.87–2.88) | 0.728 |
| Diuretic use | 1.29
(0.43–1.97) | 0.281 |
| β-blocker use | 1.26
(0.84–1.90) | 0.728 |
| Digoxin use | 0.87
(0.56–1.43) | 0.590 |
| Calcium-channel
blocker use | 1.70
(1.30–2.62) | 0.727 |
| Antiarrhythmic drug
use |
|
|
| Class
1 |
1.03(0.69–1.52) | 0.444 |
| Class
3 |
1.37(1.02–2.29) | 0.997 |
 | Table VI.Multivariate analysis comparing
patients with and without AF recurrence. |
Table VI.
Multivariate analysis comparing
patients with and without AF recurrence.
| Variable | HR (95% CI) | P-value |
|---|
| Female | 2.56
(0.97–3.58) | 0.084 |
| Non-PAF | 0.87
(0.41–1.92) | 0.155 |
| Duration of AF,
months | 1.14
(1.09–1.18) | 0.011 |
| LVEF, % | 1.17
(0.83–1.90) | 0.314 |
| LAD, mm | 1.12
(1.06–1.67) | 0.049 |
| hs-CRP, mg/l | 1.37
(1.11–1.92) | 0.002 |
Discussion
The present study evaluates the effects of a high
dose of rosuvastatin in preventing AF recurrence following CA in
patients with HF. At the same time, the data illustrate that CA may
significantly improve cardiac function in patients with HF and AF,
which corroborates the conclusions of previously published studies
(15–17). Furthermore, the present study has
revealed that hs-CRP, duration of AF and LAD are independent
predictors of higher recurrence rates of AF in patients with
HF.
It is well known that statins act as
anti-inflammatory and anti-oxidative stress agents, except in the
case of regulating lipid metabolism (18,19). In
recent times, the role of statins in the treatment of AF has been
attracting more attention. Atrium NADPH oxidase 2 (NOX2) serves an
important role in the pathophysiological process of AF: The
stronger the atrium NOX2 activity, the higher the incidence of AF
(20,21). Recalde et al (22) demonstrated that increased AF
susceptibility in mice with myocardial-specific NOX2 overexpression
was prevented by short-term statin treatment. It may be inferred
that the cause could possibly be due to high doses of rosuvastatin
inhibiting the activity of NOX2. The role of inflammation in AF is
well established. The randomized, placebo-controlled JUPITER trial
confirmed that increased levels of hs-CRP were associated with an
increased incidence of AF (23).
Similarly to the present study, the GISSI-HF trial also
demonstrated that administering 10 mg rosuvastatin daily could
significantly improve the incidence of new-onset AF in HF patients,
although chronic AF patients exhibited no benefits resulting from
rosuvastatin therapy (9). Another
possible reason for rosuvastatin decreasing the recurrence rate of
AF could be via its powerful functions as an anti-inflammatory and
anti-oxidative stress agent. Explanations for why small doses of
rosuvastatin were not able to attenuate the recurrence rate may be
due to the strength of the anti-inflammatory, anti-oxidative
stress, and also that the ability to inhibit the activity of NOX2
is weak. It was observed in the present study that 20 mg
rosuvastatin administered daily may prevent the recurrence of AF in
patients with HF, although further research is required to
elucidate the precise mechanism.
With the rapid development of intervention therapy,
the standing of CA in the treatment of AF has been steadily
improving. The latest RAAFT-2 (24)
and MANTRA-PAF trials (25) have
demonstrated that, as the first-line treatment, CA for paroxysmal
AF had improved long-term effects compared with anti-arrhythmic
drug therapy. In addition, Rillig et al (26) demonstrated that, for patients with a
reduced ejection fraction, CA improved the patients' ejection
fraction of 35% (the baseline value) by increasing it to 56.5%
(P<0.01), and LAD was reduced (from 50 to 46 mm; P<0.01). The
present study also demonstrated that cardiac function and the
parameters of NT-proBNP and LAD improved in groups 1, 2 and 3,
although rosuvastatin therapy was not demonstrated to be associated
with an improved cardiac function following CA compared with the
control group. This may be associated with the type of statin,
since a recent meta-analysis of randomized trials illustrated how
atorvastatin, and not rosuvastatin, improved cardiac function in
HF, and the potential explanation could have been that the uptake
of hydrophilic rosuvastatin by the heart is extremely low (27). Another explanation may be that CA
itself improved cardiac function significantly, and therefore
statins were not responsible for the improvements observed.
Loricchio et al (28) demonstrated that CRP was an independent
factor for recurrence of AF in patients with persistent AF.
Similarly to that study, the present study also revealed that
hs-CRP is an independent risk factor for recurrence of AF in
patients with HF, indicating that inflammation exerted an important
role in the germination and maintenance of AF in patients with HF.
The basic mechanism to account for this phenomenon would be that
infiltration of inflammatory mediators promoted the atrial
fibrosis, increasing the risk of AF (29). Increases in the size of the left atrium
are conducive to the formation of the matrix, which makes it easier
for the occurrence and maintenance of AF (30). With the extension of time of AF, the
left atrium gradually expanded. Enlargement of the left atrium
causes myocardial cell deformation, leading to changes in the
mechanisms of ion-channel electrophysiology, and therefore the
excitability and self-regulation of the myocardium may increase
(31). These factors would explain why
LAD and the duration of AF are of value in predicting the
recurrence of AF in patients with HF.
In conclusion, the findings of the present study
have demonstrated that treatment with 20 mg rosuvastatin daily
following CA may significantly decrease the recurrence rate of AF
in patients with HF. The results also demonstrate that LAD, hs-CRP
and duration of AF are all independent predictors of AF recurrence
in patients with HF. Furthermore, the present study also highlights
how CA may improve cardiac function in patients with HF and AF.
Acknowledgements
We are thankful for the assistance of the
participants of this study at the Department of Cardiology of The
First Affiliated Hospital of Zhengzhou University.
References
|
1
|
Lloyd-Jones DM, Larson MG, Leip EP, Beiser
A, D'Agostino RB, Kannel WB, Murabito JM, Vasan RS, Benjamin EJ and
Levy D: Framingham Heart Study: Lifetime risk for developing
congestive heart failure-The Framingham Heart Study. Circulation.
106:3068–3072. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lloyd-Jones DM, Wang TJ, Leip EP, Larson
MG, Levy D, Vasan RS, D'Agostino RB, Massaro JM, Beiser A, Wolf PA
and Benjamin EJ: Lifetime risk for development of atrial
fibrillation - The Framingham Heart Study. Circulation.
110:1042–1046. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Anselmino M, Matta M and Gaita F: Catheter
ablation of atrial fibrillation in patients with heart failure: Can
we break the vicious circle? Eur J Heart Fail. 17:1003–1005. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cooper HA, Bloomfield DA, Bush DE, Katcher
MS, Rawlins M, Sacco JD and Chandler M: AFFIRM Investigators:
Relation between achieved heart rate and outcomes in patients with
atrial fibrillation (from the Atrial Fibrillation Follow-up
Investigation of Rhythm Management [AFFIRM] Study). Am J Cardiol.
93:1247–1253. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
January CT, Wann LS, Alpert JS, Calkins H,
Cigarroa JE, Cleveland JC Jr, Conti JB, Ellinor PT, Ezekowitz MD,
Field ME, et al: ACC/AHA Task Force Members: 2014 AHA/ACC/HRS
guideline for the management of patients with atrial fibrillation:
Executive summary: A report of the American College of
Cardiology/American Heart Association Task Force on practice
guidelines and the Heart Rhythm Society. Circulation.
130:2071–2104. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Anselmino M, Matta M, D'Ascenzo F, Bunch
TJ, Schilling RJ, Hunter RJ, Pappone C, Neumann T, Noelker G, Fiala
M, et al: Catheter ablation of atrial fibrillation in patients with
left ventricular systolic dysfunction: A systematic review and
meta-analysis. Circ Arrhythm Electrophysiol. 7:1011–1018. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Young-Xu Y, Jabbour S, Goldberg R, Blatt
CM, Graboys T, Bilchik B and Ravid S: Usefulness of statin drugs in
protecting against atrial fibrillation in patients with coronary
artery disease. Am J Cardiol. 92:1379–1383. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Horwich TB, MacLellan WR and Fonarow GC:
Statin therapy is associated with improved survival in ischemic and
non-ischemic heart failure. J Am Coll Cardiol. 43:642–648. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Maggioni AP, Fabbri G, Lucci D, Marchioli
R, Franzosi MG, Latini R, Nicolosi GL, Porcu M, Cosmi F, Stefanelli
S, et al: GISSI-HF Investigators: Effects of rosuvastatin on atrial
fibrillation occurrence: Ancillary results of the GISSI-HF trial.
Eur Heart J. 30:2327–2336. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xia W, Yin Z, Li J, Song Y and Qu X:
Effects of rosuvastatin on asymmetric dimethylarginine levels and
early atrial fibrillation recurrence after electrical
cardioversion. Pacing Clin Electrophysiol. 32:1562–1566. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yancy CW, Jessup M, Bozkurt B, Butler J,
Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi
JL, et al: American College of Cardiology Foundation; American
Heart Association Task Force on Practice Guidelines: 2013 ACCF/AHA
guideline for the management of heart failure: A report of the
American College of Cardiology Foundation/American Heart
Association Task Force on Practice Guidelines. J Am Coll Cardiol.
62:e147–e239. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hunt SA, Abraham WT, Chin MH, Feldman AM,
Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K,
et al: American College of Cardiology, American Heart Association
Task Force on Practice Guidelines, American College of Chest
Physicians, International Society for Heart and Lung
Transplantation, Heart Rhythm Society: ACC/AHA 2005 Guideline
Update for the Diagnosis and Management of Chronic Heart Failure in
the Adult: A report of the American College of Cardiology/American
Heart Association Task Force on Practice Guidelines (Writing
Committee to Update the 2001 Guidelines for the Evaluation and
Management of Heart Failure): Developed in collaboration with the
American College of Chest Physicians and the International Society
for Heart and Lung Transplantation: Endorsed by the Heart Rhythm
Society. Circulation. 112:e154–e235. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ponikowski P, Voors AA, Anker SD, Bueno H,
Cleland JG, Coats AJ, et al: ESC Guidelines for the Diagnosis and
Treatment of Acute and Chronic Heart Failure. Rev Esp Cardiol.
69:11672016.PubMed/NCBI
|
|
14
|
Imai E, Horio M, Nitta K, Yamagata K,
Iseki K, Tsukamoto Y, Ito S, Makino H, Hishida A and Matsuo S:
Modification of the modification of diet in Renal Disease (MDRD)
study equation for Japan. Am J Kidney Dis. 50:927–937. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hunter RJ, Berriman TJ, Diab I, Kamdar R,
Richmond L, Baker V, Goromonzi F, Sawhney V, Duncan E, Page SP, et
al: A randomized controlled trial of catheter ablation versus
medical treatment of atrial fibrillation in heart failure (the
CAMTAF trial). Circ Arrhythm Electrophysiol. 7:31–38. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Thihalolipavan S and Morin DP: Atrial
fibrillation and heart failure: Update 2015. Prog Cardiovasc Dis.
58:126–135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Machino-Ohtsuka T, Seo Y, Ishizu T, Sugano
A, Atsumi A, Yamamoto M, Kawamura R, Machino T, Kuroki K, Yamasaki
H, et al: Efficacy, safety, and outcomes of catheter ablation of
atrial fibrillation in patients with heart failure with preserved
ejection fraction. J Am Coll Cardiol. 62:1857–1865. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Maron DJ, Fazio S and Linton MF: Current
perspectives on statins. Circulation. 101:207–213. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liao JK and Laufs U: Pleiotropic effects
of statins. Annu Rev Pharmacol Toxicol. 45:89–118. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim YM, Guzik TJ, Zhang YH, Zhang MH,
Kattach H, Ratnatunga C, Pillai R, Channon KM and Casadei B: A
myocardial Nox2 containing NAD(P)H oxidase contributes to oxidative
stress in human atrial fibrillation. Circ Res. 97:629–636. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Reilly SN, Jayaram R, Nahar K, Antoniades
C, Verheule S, Channon KM, Alp NJ, Schotten U and Casadei B: Atrial
sources of reactive oxygen species vary with the duration and
substrate of atrial fibrillation: Implications for the
antiarrhythmic effect of statins. Circulation. 124:1107–1117. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Recalde A, Carnicer R, Simon J, Reilly S,
Verheule S, Shah AM, et al: Increased atrial fibrillation
susceptibility in mice with myocardial specific NOX2 overexpression
is prevented by short term statin treatment. Eur Heart J.
35:11182014.
|
|
23
|
Peña JM, MacFadyen J, Glynn RJ and Ridker
PM: High-sensitivity C-reactive protein, statin therapy, and risks
of atrial fibrillation: An exploratory analysis of the JUPITER
trial. Eur Heart J. 33:531–537. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Walfridsson H, Walfridsson U, Nielsen JC,
Johannessen A, Raatikainen P, Janzon M, Levin LA, Aronsson M,
Hindricks G, Kongstad O, et al: Radiofrequency ablation as initial
therapy in paroxysmal atrial fibrillation: Results on
health-related quality of life and symptom burden. The MANTRA-PAF
trial. Europace. 17:215–221. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Morillo CA, Verma A, Connolly SJ, Kuck KH,
Nair GM, Champagne J, Sterns LD, Beresh H, Healey JS and Natale A:
RAAFT-2 investigators: Radiofrequency ablation vs antiarrhythmic
drugs as first-line treatment of paroxysmal atrial fibrillation
(RAAFT-2): A randomized trial. JAMA. 311:692–700. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rillig A, Makimoto H, Wegner J, Lin T,
Heeger C, Lemes C, Fink T, Metzner A, Wissner E, Mathew S, et al:
Six-year clinical outcomes after catheter ablation of atrial
fibrillation in patients with impaired left ventricular function. J
Cardiovasc Electrophysiol. 26:1169–1179. 2015. View Article : Google Scholar
|
|
27
|
Takagi H and Umemoto T: Atorvastatin, not
rosuvastatin, improves cardiac function in heart failure: A
meta-analysis of randomized trials. Int J Cardiol. 155:296–299.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Loricchio ML, Cianfrocca C, Pasceri V,
Bianconi L, Auriti A, Calo L, Lamberti F, Castro A, Pandozi C,
Palamara A, et al: Relation of C-reactive protein to long-term risk
of recurrence of atrial fibrillation after electrical
cardioversion. Am J Cardiol. 99:1421–1424. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schiffrin EL: The immune system: Role in
hypertension. Can J Cardiol. 29:543–548. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xu J, Cui G, Esmailian F, Plunkett M,
Marelli D, Ardehali A, Odim J, Laks H and Sen L: Atrial
extracellular matrix remodeling and the maintenance of atrial
fibrillation. Circulation. 109:363–368. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jalife J and Kaur K: Atrial remodeling,
fibrosis, and atrial fibrillation. Trends Cardiovasc Med.
25:475–484. 2015. View Article : Google Scholar : PubMed/NCBI
|