Introduction
Inflammatory bowel disease (IBD) refers to a group
of idiopathic inflammatory disorders of the gastrointestinal (GI)
tract characterized by the manifestations of abdominal pain and
diarrhea, the course of which is a chronic-recurrent process.
Crohn's disease (CD) and ulcerative colitis (UC) are two types of
IBD and exhibit overlapping and different clinical and pathological
features (1). Its incidence in
developing countries has gradually increased over the past five
decades. The reported age-standardized incidence of IBD, CD and UC
in China was 1.77–3.14 per 100,000, 0.13–1.09 per 100,000 and
1.45–2.05 per 100,000, respectively (2–4). The
estimated prevalence of UC and CD in China was 11.6 and 1.4 per
100,000 persons, respectively (5).
IBD is diagnosed by the comprehensive analysis of
clinical findings, radiological imaging, invasive endoscopy and
histopathological examination. Due to a lack of a gold standard,
certain patients do not receive a definitive diagnosis using
current diagnostic criteria, and an additional 5–15% of chronic IBD
cases cannot be classified as UC or CD, and are defined as
indeterminate colitis (IC) or IBD unclassified (IBDU) (6). Furthermore, the pathogenesis of IBD
remains unclear, although it is proposed to be caused by
environmental effects and infection in genetically predisposed
individuals, and mediated by immune mechanisms. Recently, the
search for assistance with, or partial replacement of, diagnostic
means and the elucidation of the pathogenesis have become the focus
of IBD research, and serum antibody markers appear to be a good
combination of these two aspects. Since antineutrophil cytoplasmic
antibody (ANCA) and anti-Saccharomyces cerevisiae antibodies
(ASCA) were first discovered in the 1990s, increasing numbers of
serum antibodies have been identified, including Escherichia
coli outer membrane porin C antibody (anti-OmpC) and antibody
to CBir1 flagellin (anti-CBir1), amongst others.
The majority of IBD serological studies are
performed abroad, and the subjects are predominantly Caucasian.
However, it has been shown that the prevalence of antibodies
differs between locations or ethnic groups. Serological research in
the clinical diagnosis of IBD in China has only recently begun,
thus, there are few relevant reports and data. Furthermore, the
research conclusions have often been inconsistent and it has been
difficult to draw guidance that could be applied to all locations.
The aim of the present study was to evaluate the clinical value of
the abovementioned five serum antibodies (ANCA, ASCA-IgG and
ASCA-IgA, anti-OmpC and anti-CBir1) in Chinese IBD patients,
including their value for differentiating between IBD and non-IBD
(N-IBD) GI diseases, UC and CD. In addition, their association with
disease phenotypes (location, activity and complications) was
investigated.
Materials and methods
Sample collection
A case group was developed consisting of CD and UC
patients treated in the Department of Gastroenterology of Xiangya
Hospital, Central South University (Changsha, China) between March
2015 and November 2015. Gender- and age-matched patients with
non-IBD diseases (N-IBD) were defined as the disease control group,
and gender- and age-matched healthy individuals from the Physical
Examination Department of Xiangya Hospital served as the healthy
control group. Clinical data (gender, age, disease duration,
clinical manifestation, laboratory tests, endoscopic and
histological examinations) were recorded when the serum sample was
drawn. A total of 2 ml venous blood was obtained from each subject
(in the morning, fasted). All of the samples were spun at a speed
of 1,000 × g for 10 min within 2 h of collection, and the upper
serum was collected and frozen at −80°C until the assays were
performed.
IBD case inclusion criteria included typical
clinical manifestations, such as abdominal pain, diarrhea and
purulent stools. The diagnosis of CD or UC was established by
standard laboratory and radiological findings, and endoscopic
criteria according to the 2010 World Gastroenterology Organization
Practice Guidelines for IBD (1). CD
and UC were subgrouped according to the Montreal classification
(7).
The N-IBD group consisted of patients with non-IBD
GI disorders, including other gastrointestinal diseases (OGID) and
intestinal tuberculosis (TB). The patients with normal colonoscopy
and pathology, and normal imaging were considered as the healthy
control group. The exclusion criteria for all the subjects included
acute and chronic infection of the GI tract other than TB, and a
history of autoimmune diseases and cancer.
Written informed consent was obtained from all of
the participants and the present study was approved by the Ethics
Committees of Xiangya Hospital, Central South University.
Enzyme-linked immunosorbent assay
(ELISA)
All of the serum samples were analyzed using a
standardized ELISA to detect the five antibodies, and the ELISA
kits were obtained from Shen Yu Technology Co., Ltd. (Changsha,
China). All of the specimens and kit reagents were restored to room
temperature (20–25°C) before use. Sera were diluted 1:10 in
phosphate-buffered saline (PBS) and 100 µl of the diluted sera was
dispensed into the appropriate wells (2 wells per sample). For the
reagent blank, 100 µl diluent was dispensed in the 1A-well
position. The samples were incubated for 30 min at room
temperature, the liquid was removed from all of the wells, and the
wells were washed three times in a PBS-Tween solution (Shen Yu
Technology Co., Ltd.) followed by incubation, according to the
manufacturer's instructions, with 100 µl peroxidase-labeled goat
anti-human IgG or IgA (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany). Following the incubation for 30 min at room temperature,
the enzyme conjugate was removed from all of the wells. The wells
were then washed three times, and 100 µl 3,3′,
5,5′-tetramethylbenzidine substrate (Shen Yu Technology Co., Ltd.)
was dispensed and incubated for 15 min at room temperature. A total
of 100 µl of the stop solution was then added, and the optical
density (OD) of the reaction was read within 15 min at a wavelength
of 450 nm using an ELISA reader. As qualitative ELISA assays were
used, the cut-off values were determined according to the receiver
operating characteristic (ROC) curve, and an OD greater than the
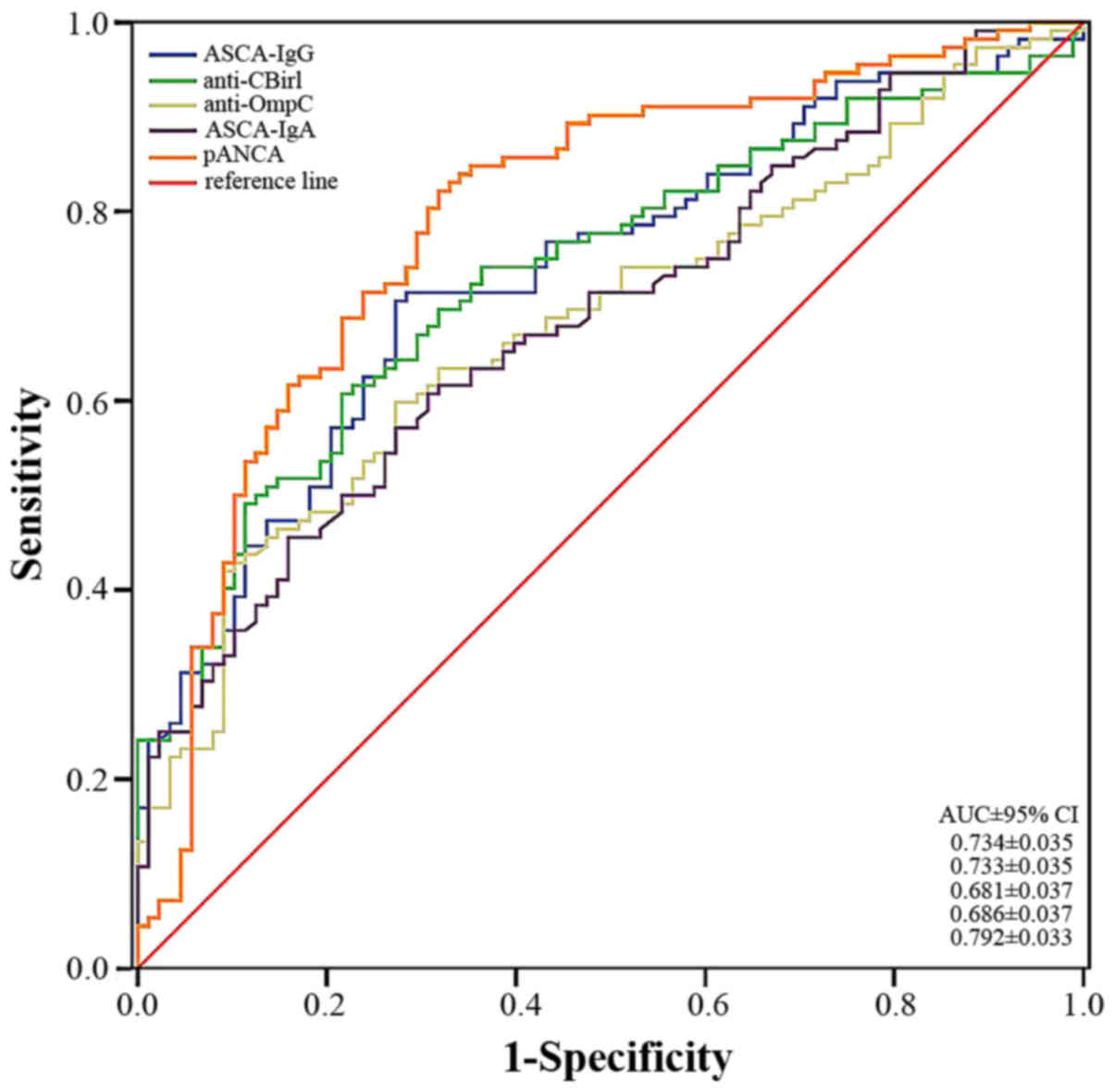
cut-off value was considered positive. The ROC curves for the five
antibodies are presented in Fig. 1.
The area under the curve (AUC) for the IBD versus N-IBD with the
five antibodies was ≥0.7, indicating their ability to diagnose IBD.
Perinuclear antineutrophil cytoplasmic antibody (pANCA) was clearly
the most accurate marker for differentiating patients with IBD from
patients with other diseases [AUC 0.792 and 95% confidence interval
(CI), ±0.033). In addition, ASCA-IgG and anti-CBir1 demonstrated
good diagnostic accuracy (AUC 0.734±0.035 and 0.733±0.035,
respectively).
Statistical analysis
Statistical analysis was performed using SPSS 19.0
(IBM SPSS, Armonk, NY, USA). The sensitivity, specificity, negative
predictive value (NPV) and positive predictive value (PPV) of
pANCA, ASCA (IgG and/or IgA), anti-OmpC, anti-CBir1 and their
different combinations were determined for distinguishing between
the UC, CD and control groups. For the data analyses, χ2
test or Fisher's exact test, as appropriate, were used to compare
the frequency of positive antibodies between the study groups. The
nonparametric Kruskal-Wallis test or Spearman's correlation assay
was utilized to compare the median levels of antibody titers (the
levels of the OD value) among different disease phenotypes.
P<0.05 was considered to indicate statistically significant
differences.
Results
Demographics
A total of 112 unrelated IBD patients (CD, n=71; UC,
n=41), 78 patients with N-IBDs and 31 normal healthy individuals
were enrolled in the present study. The N-IBD group consisted of
patients with chronic gastroenteritis (n=26), irritable bowel
syndrome (n=5), functional dyspepsia (n=5), GI polyps (n=10),
stromal tumor (n=4), diverticulosis (n=2), intestinal flora
imbalance (n=2), mixed hemorrhoids (n=2), gastroesophageal reflux
disease (n=1) and intestinal TB (n=21). The characteristics of the
four populations are provided in Table
I. No significant difference was identified in gender
composition among each group (P>0.05), whereas the CD group had
a significantly younger mean age (P<0.05). According to the
Montreal classification (7), UC
patients were classified as follows: Proctitis (E1), left-sided
colitis (E2), or pancolitis (E3), and the severity was classified
as S0 (remission; none occurred because all of the patients
enrolled in the present were inpatients), S1 (mild), S2 (moderate)
and S3 (severe). The CD patients were subgrouped by disease
behavior (B1, non-stricturing/nonpenetrating; B2, stricturing; and
B3, penetrating) and disease location (L1, terminal ileum; L2,
colon; L3, ileocolon; and L4, upper GI tract). According to the
Crohn's Disease Activity Index (CDAI) (8), the clinical activity of CD patients was
measured as mild, moderate or severe.
 | Table I.Clinical data of patients with CD and
UC, and N-IBD and healthy control subjects. |
Table I.
Clinical data of patients with CD and
UC, and N-IBD and healthy control subjects.
| Characteristic | CD n=71 | UC n=41 | N-IBDa n=78 | Healthy group
n=31 |
|---|
| Male/female | 49/22 | 24/17 | 44/34 | 20/11 |
| Mean age (years) | 33.9±13.4 | 46.1±13.7 | 45.9±15.7 | 42.1±13.9 |
| Range | 15–72 | 17–72 | 15–77 | 15–70 |
| Disease duration
(years) | 2.3±2.7 | 2.9±3.6 |
|
|
| Disease location: UC,
n (%) |
|
|
|
|
| E1,
Proctitis |
| 6
(14.6) |
|
|
| E2,
Left side |
| 21 (51.2) |
|
|
| E3,
Extensive |
| 14 (34.2) |
|
|
| Severity of UC, n
(%) |
|
|
|
|
| S1,
Mild |
|
| 13 (31.7) |
|
| S2,
Moderate |
|
| 19 (46.3) |
|
| S3,
Severe |
|
| 9
(22.0) |
|
| Disease location:
CD, n (%) |
|
|
|
|
| L1,
Terminal ileum | 31 (43.6) |
|
|
|
| L2,
Colon | 18 (25.4) |
|
|
|
| L3,
Ileocolon | 18 (25.4) |
|
|
|
| L4,
Upper GI | 4 (5.6) |
|
|
|
| Clinical disease
activity: CDAI, n (%) |
|
|
|
|
|
Mild | 15 (21.1) |
|
|
|
|
Moderate | 35 (49.3) |
|
|
|
|
Severe | 21 (29.6) |
|
|
|
| Disease behavior:
CD, n (%) |
|
|
|
|
| B1,
Non-stricturing, non-penetrating | 45 (63.4) |
|
|
|
| B2,
Stricturing | 22 (31.0) |
|
|
|
| B3,
Penetrating | 2 (2.8) |
|
|
|
| B2
+ B3, Stricturing and penetrating | 2 (2.8) |
|
|
|
Diagnostic precision of a single
antibody
The unique antibody patterns for the five antibodies
are provided in Table II. ASCA-IgG
was positive in 52.1% of CD patients compared with 31.7% of UC
patients (P<0.05), 17.5% of OGID patients and only 6.5% of the
healthy group. The presence of ASCA-IgA in CD patients was 33.8%,
which was not significantly higher than that observed in the UC
patients (17.1%), although was markedly higher than those observed
in the OGID (8.8%) and healthy (0%) groups. Of the CD patients with
positive ASCA-IgG, 37.8% exhibited positive ASCA-IgA. The
prevalence of pANCA was significantly higher in the UC group
(53.7%) than in the CD, OGID and healthy control groups (21.1, 7.0
and 3.2%, respectively). In addition, the prevalence of pANCA was
significantly higher in the CD group than in the OGID and healthy
control groups. Of the CD patients, 57.7 and 50.7% were positive
for anit-CBir1 and anti-OmpC, respectively, which were
significantly higher than in the UC group (17.1 and 31.7%), the
OGID group (12.3 and 17.5%) and the healthy control group (6.5 and
3.2%). No significant difference was identified between the OGID
group and the healthy group in all five antibodies. The prevalence
of anti-OmpC was greater in the CD group than in the TB group,
while the prevalence of the other four antibodies showed no
significant difference between the CD, UC and the TB group.
 | Table II.Prevalence of five individual
antibodies in each group. |
Table II.
Prevalence of five individual
antibodies in each group.
| Antibody | CD (n=71 | UC (n=41) | TB (n=21) | OGID (n=57) | Healthy (n=31) |
|---|
| ASCA-IgG (%) | n37
(52.1)a,b | 13 (31.7) | 8 (38.1) | 10 (17.5) | 2 (6.5) |
| ASCA-IgA (%) | n24
(33.8)b | 7 (17.1) | 3 (14.3) | 5 (8.8) | 0 (0.0) |
| pANCA (%) | n15
(21.1)b | 22
(53.7)c,d | 7 (33.3) | 4 (7.0) | 1 (3.2) |
| Anti-CBir1 (%) | n41
(57.7)a,b | 7 (17.1) | 9 (42.9) | 7 (12.3) | 2 (6.5) |
| Anti-OmpC (%) | n36
(50.7)a,b | 13 (31.7) | 4
(19.0)e | 10 (17.5) | 1 (3.2) |
As there were no significant differences between the
OGID group and healthy control group for all five antibodies, the
OGID group and the healthy group were combined and served as the
control group (n=88), which was compared to the CD or UC groups.
Sensitivity, specificity, PPV and NPV data for the five antibodies
individually are provided in Table
III. The sensitivity of ASCA-IgG and ASCA-IgA for CD was 52.1
and 33.8%, respectively, and the specificity was 86.4 and 94.3%,
whereas PPV was 75.5 and 82.8%, respectively. The sensitivity and
specificity of pANCA for UC were 53.7 and 94.3%, PPV was 81.5%, and
NPV was 81.4%. When tested alone, the sensitivities of anti-CBir1
and anti-OmpC for CD were 57.7 and 50.7%, respectively and the
specificities were 89.8 and 87.5%, respectively. Due to the
difference of anti-OmpC between CD and TB, the corresponding
sensitivity, specificity, PPV, and NPV were calculated.
 | Table III.Sensitivity, specificity, PPV and NPV
of diagnosis for CD or UC. |
Table III.
Sensitivity, specificity, PPV and NPV
of diagnosis for CD or UC.
| Antibody | Comparison | Sensitivity
(%) | Specificity
(%) | PPV (%) | NPV (%) |
|---|
| ASCA-IgG | CD vs. control |
52.1 |
86.4 | 75.5 | 69.1 |
| ASCA-IgA | CD vs. control |
33.8 |
94.3 | 82.8 | 63.8 |
| pANCA | UC vs. control |
53.7 |
94.3 | 81.5 | 81.4 |
| Anti-CBir1 | CD vs. control |
57.7 |
89.8 | 82.0 | 72.5 |
| Anti-OmpC | CD vs. control |
50.7 |
87.5 | 76.6 | 68.8 |
| Anti-OmpC | CD vs. TB |
50.7 |
81.0 | 90.0 | 32.7 |
Diagnostic accuracy of combined
antibodies
The diagnostic significance of the combined
detection of four CD-associated antibodies is presented in Table IV. The sensitivity of ASCA-IgA for CD
was low, but it increased to 66.2% when combined with ASCA-IgG. The
specificity of ASCA-IgG/IgA was 83%. When combined with anti-CBir1,
the sensitivity of ASCA (ASCA-IgA and/or IgG) for CD increased to
80.3%, and specificity decreased to 76.1%. The combination of
anti-OmpC and ASCA had a sensitivity of 85.9% for CD, and the
specificity was 73.9%. If a positive screening test was defined as
the presence of at least one positive antibody, then the
three-antibody panel (ASCA, anti-CBir1 and anti-OmpC) had an
overall sensitivity of 91.5% for CD, with a specificity of 69.3%.
Therefore, it was concluded that the diagnostic efficiency of
combining the detection of anti-OmpC and ASCA was the highest.
 | Table IV.Combination of ASCA-IgG, ASCA-IgA,
anti-CBir1 and anti-OmpC in the diagnosis of CD. |
Table IV.
Combination of ASCA-IgG, ASCA-IgA,
anti-CBir1 and anti-OmpC in the diagnosis of CD.
| Antibody | Comparison % | Sensitivity % | Specificity % | PPV % | NPV % |
|---|
| ASCA-IgA/G | CD vs. control | 66.2 | 83 | 75.8 | 75.3 |
| Anti-CBir1 and
ASCA | CD vs. control | 80.3 |
76.1 | 73.1 | 82.7 |
| Anti-OmpC and
ASCA | CD vs. control | 85.9 |
73.9 | 72.6 | 86.7 |
| Anti-OmpC,
anti-CBir1 and ASCA | CD vs. control | 91.5 |
69.3 | 70.7 | 91.0 |
The diagnostic precision of the combined testing of
ASCA and pANCA, with or without anti-CBir1 and/or anti-OmpC, in IBD
(CD and UC, n=112) is presented in Table
V. When compared with the control group (OGID and healthy group
combined), pANCA+/ASCA+ had a sensitivity of
72.3% and a specificity of 78.4% for IBD. However, the addition of
anti-CBir1 improved the sensitivity to 82.1%, while it slightly
decreased the specificity to 71.6%. However, after adding
anti-OmpC, the sensitivity of pANCA+/ ASCA+
for IBD was 85.7%, with a specificity of 69.3%. Finally, 89.3% of
IBD patients were positive for ≥1 of the five antibodies, although
the specificity and PPV decreased.
 | Table V.Diagnostic precision of different
combinations of antibodies for inflammatory bowel disease. |
Table V.
Diagnostic precision of different
combinations of antibodies for inflammatory bowel disease.
| Combination | Sensitivity
(%) | Specificity
(%) | Positive predictive
value (%) | Negative predictive
value (%) |
|---|
|
pANCA+/ASCA+ | 72.3 | 78.4 | 81.0 | 69.0 |
|
pANCA+/ASCA+/anti-CBir1+ | 82.1 | 71.6 | 78.6 | 75.9 |
|
pANCA+/ASCA+/anti-OmpC+ | 85.7 | 69.3 | 78.0 | 79.2 |
|
pANCA+/ASCA+/anti-CBir1+/anti-OmpC+ | 89.3 | 64.8 | 76.3 | 82.6 |
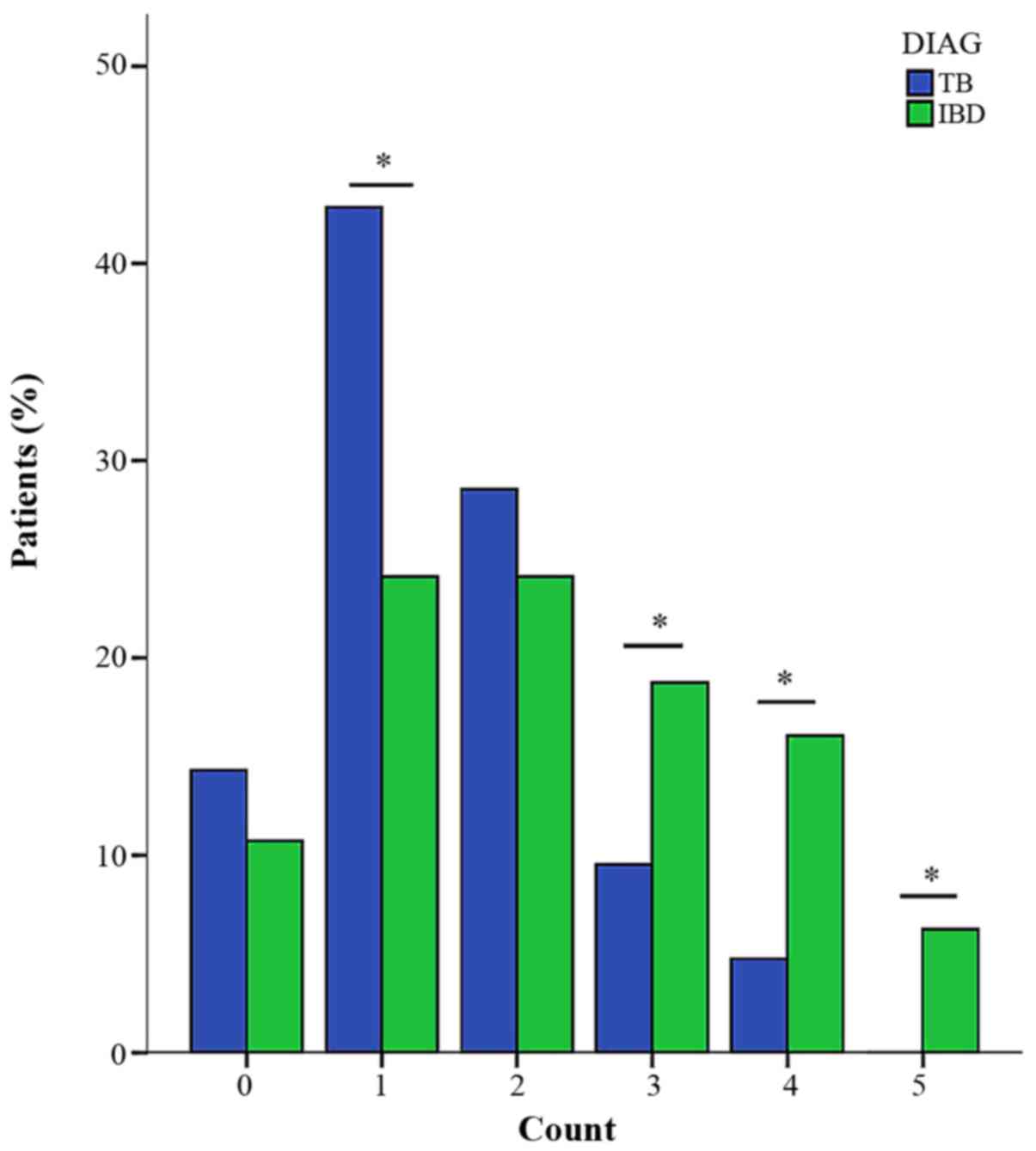
The number of positive antibodies in the
five-antibody panel between IBD and the control group (Fig. 2), and between the IBD and the TB groups
(Fig. 3) was compared. This finding
indicated that the control group was more likely to have no
positive antibody, and there was no significant difference between
IBD and the control group when there was just one positive
antibody. However, the percentage of patients that had ≥2 positive
antibodies in the IBD group was significantly higher. As
demonstrated in Fig. 3, when the
number was 0 or 2, no difference between IBD and TB was identified,
whereas the TB group was more likely to have 1 positive antibody,
and the IBD group was more likely to have ≥3 antibodies.
Serologic antibodies in the
differential diagnosis of CD and UC
From the abovementioned results (Table II), it was identified that ASCA and
pANCA were unique for CD and UC, respectively; however, ASCA was
positive in 41.5% of UC patients and pANCA was positive in 21.1% of
CD patients, which decreased their ability to differentiate between
patients with UC and CD. Therefore, the diagnostic accuracy of
ASCA+/pANCA− and
pANCA+/ASCA− in the differential diagnosis of
CD and UC was evaluated, and the results are presented in Table VI. The sensitivity of
ASCA+/pANCA− for distinguishing CD and UC was
50.7%, and the specificity was 80.5%. While the combination of
pANCA+/ASCA− had a specificity of 94.4% for
differentiating between UC and CD.
 | Table VI.ASCA and pANCA for single or combined
differential diagnosis of CD and UC. |
Table VI.
ASCA and pANCA for single or combined
differential diagnosis of CD and UC.
| Comparison | Antibody | Sensitivity
(%) | Specificity
(%) | PPV (%) | NPV (%) |
|---|
| CD vs. UC |
ASCA+ | 66.2 | 58.5 | 73.4 | 50 |
|
|
ASCA+/pANCA− | 50.7 | 80.5 | 81.8 | 51.5 |
| UC vs. CD |
pANCA+ | 53.7 | 78.9 | 59.5 | 74.7 |
|
|
pANCA+/ASCA− | 31.7 | 94.4 | 76.5 | 70.5 |
The importance of newly identified antibodies was
noted by the observation that ASCA-negative CD patients may be
positive for anti-CBir1 and anti-OmpC. That result indicated that
the two differentiated between the ASCA-negative CD patients and
the control subjects. In addition, anti-OmpC had a differential
value between CD and UC, whereas anti-CBir1 had no such value
(P=0.112). However, neither anti-CBir1 nor anti-OmpC was able to
distinguish pANCA-positive CD from UC.
Correlation between antibodies and
disease phenotype
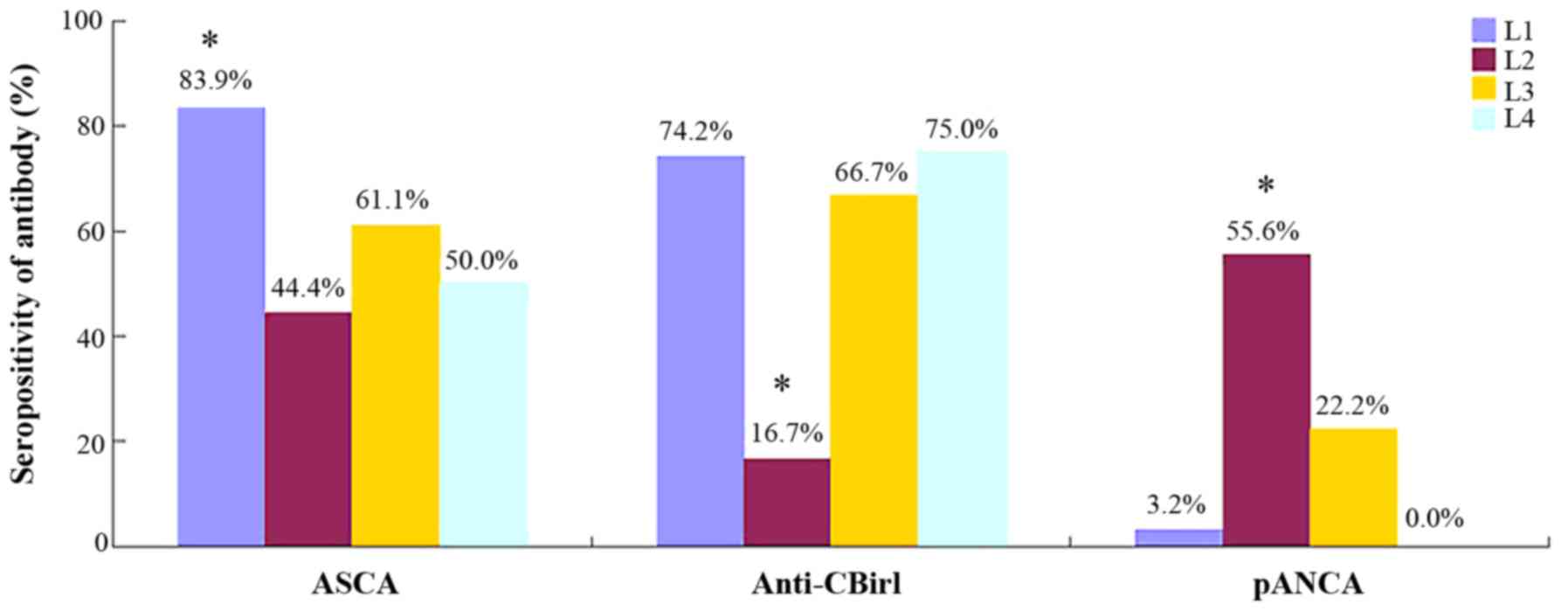
Higher ASCA seropositivity was identified in CD
patients with ileal lesions (L1) compared to patients with colonic
(L2) disease, ileo-colonic (L3) disease or upper GI tract
involvement (L4) (83.9% vs. 44.4, 61.1 or 50%, respectively;
P<0.05). The ASCA seropositivity was not significantly different
in the L2, L3, and L4 groups (P>0.05). Significantly higher
ASCA-IgG prevalence was observed in patients with complicated
(stricturing, penetrating or lesion of the anus) disease, compared
with patients with non-complicated (B1) phenotype (75 vs. 33.3%;
P=0.001). However, no significant difference was identified in the
prevalence of ASCA-IgA between complicated diseases and simple
diseases. According to ASCA titers in ASCA-IgG-positive CD, no
significant difference among location subgroups or disease
behaviors was identified. Furthermore, no correlation was observed
between the disease severity and ASCA seropositivity in the
patients in the current study.
pANCA is present with a significantly higher
frequency in CD patients with only colonic disease (L2, 55.6%)
compared with patients with ileal lesions (L1, 3.2%), ileocolonic
lesions (L3, 22.2%) or upper GI tract involvement (L4, 0%); no
significant difference was found among the latter three
subtypes.
The prevalence of anti-CBir1 was different among
distinct locations of CD and multiple comparisons showed that those
patients with colonic disease had lower seropositivity compared
with patients with the other three phenotypes; although there was
no significant difference among the other three subtypes. In CD
with positive anti-CBir1, the anti-CBir1 titers were not
significantly different among the different location phenotypes.
There was no correlation between the presence of anti-CBir1 and
disease behavior or activity. Qualitative anti-OmpC was not
significantly different among the CD locations, but it was more
common in the patients with complications than in those with pure
inflammation. In CD with positive anti-OmpC, there was no positive
correlation between anti-OmpC titers and complications. Similar to
anti-CBir1, anti-OmpC was not associated with CD activity. The
correlation between antibodies and CD phenotype are shown in
Figs. 4 and 5.
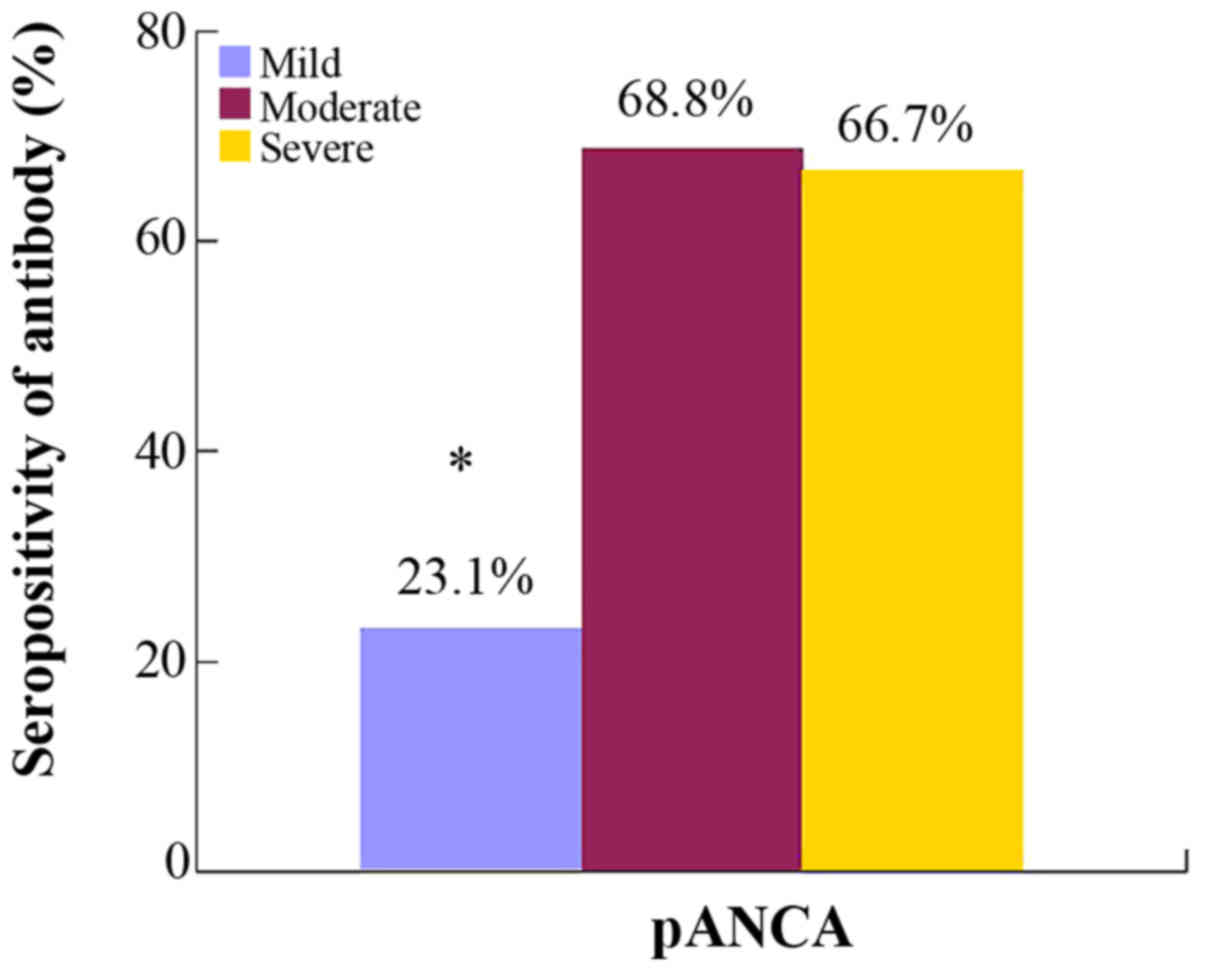
In UC patients, the presence of pANCA was not
significantly different among the disease locations. The result of
multiple comparison indicated that the patients with moderate and
severe disease (S2 and S3) had significantly higher pANCA
seropositivity than those with mild (S1) disease, whereas the two
former were compared, and no difference was observed (Fig. 6). However, in UC with positive pANCA
expression, the antibody titers of S2 and S3 were not higher than
in S1.
The results of the correlation analysis demonstrated
no correlation between IBD disease location, activity and
complications. The Kruskal-Wallis test indicated the titers of
ASCA, anti-CBir1 and anti-OmpC were not correlated with the
duration of CD and the titer of pANCA was not correlated with the
duration of UC.
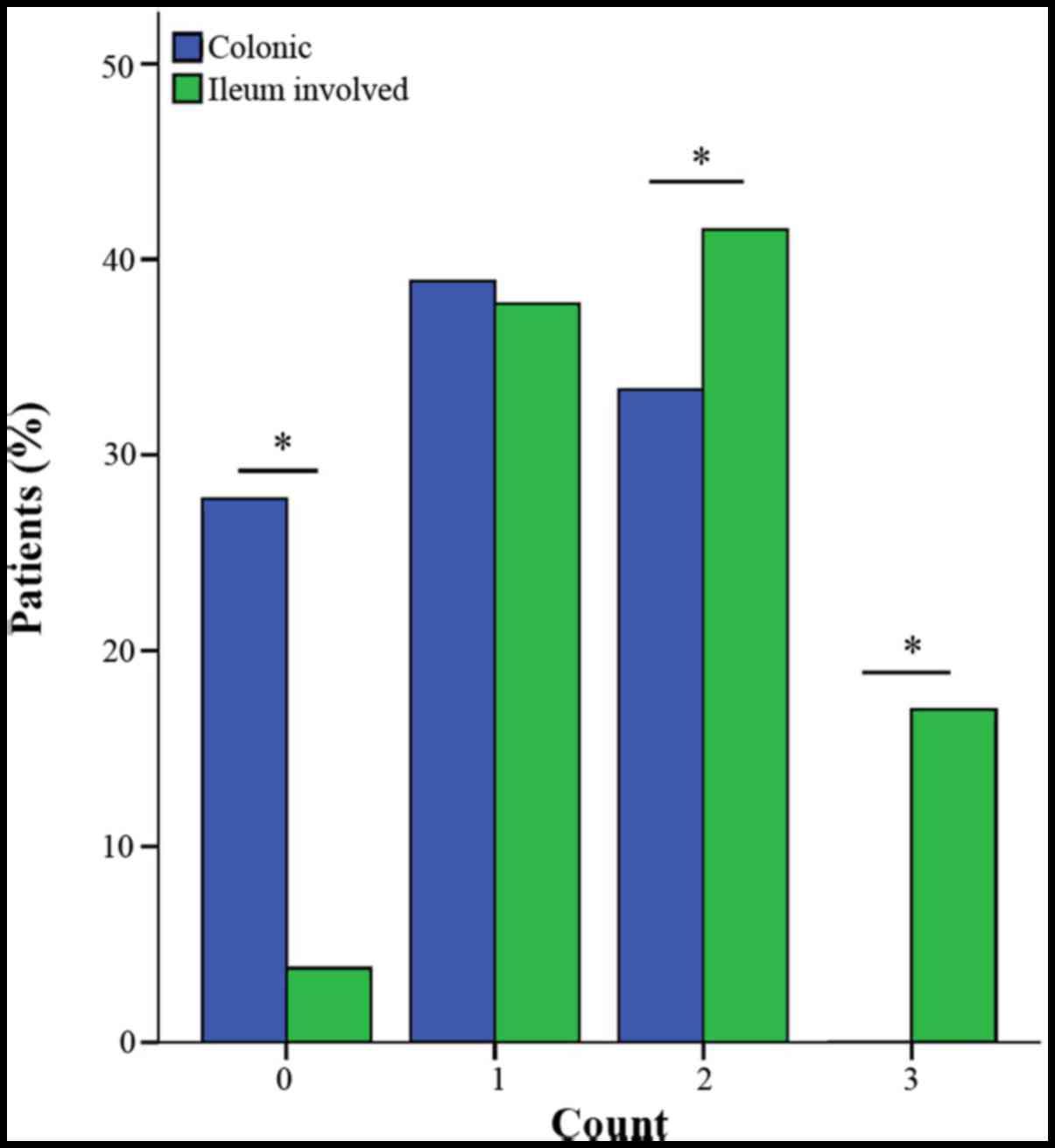
The determination of the disease phenotype by the
combined test of antibodies was evaluated (Fig. 7). When anti-OmpC, anti-CBir1 and ASCA
were combined and all three antibodies were negative, the ratio of
colonic CD was significantly higher than CD with ileum involvement.
When only one antibody was positive, there was no difference
between the two. When there were 2 or more antibodies positive, the
ratio of CD with ileum involvement was significantly higher than
colonic CD. However, no correlation between disease behavior and
the number of positive antibodies was observed in the patients.
Discussion
ASCA and pANCA were the earliest identified
serological antibodies correlated with IBD, and the studies date
back to the 1990s. ASCA are targeted at the phosphopeptidomannan of
the cell wall of Saccharomyces cerevisiae (9), including ASCA-IgG and ASCA-IgA. Previous
studies have shownthat ASCA was detected in more CD patients
(39–70%) and their healthy relatives (25–20%) than in healthy
individuals without family history and in UC patients (0–5% and
10–15%, respectively) (10). ANCAs are
autoanibodies directed against the cytosolic components of
neutrophil granules and are represented by three main staining
patterns as follows: Cytoplasmic granular, perinuclear and
atypical. ANCAs were initially described in primary vasculitis,
such as Wegener granulomatous, and the correlation between pANCA
and UC was first established in 1990 (11). Studies have found that the prevalence
of pANCA was 50–70% in UC patients, 6–20% in CD patients and only
0–2.5% in healthy individuals, although the prevalence of pANCA in
the healthy relatives of UC was not identified to be higher than
that in ordinary people (10).
In the present study, the seroprevalence of ASCA-IgG
in CD was 52.1%, which was significantly higher than that in UC,
OGIDs and the healthy group, and the seroprevalence of ASCA-IgA in
CD was also significantly higher than in the two control groups.
Similarly, 53.7% of UC patients are seropositive for pANCA,
significantly higher than in CD and the control groups. These
results were consistent with studies that have been performed
abroad (10,12) and in domestic research (13,14).
Anti-CBir1 and anti-OmpC are newly identified
bacterial antigen antibodies that target flagellin CBir1 and E.
coli outer membrane poreC, respectively. According to
previous studies, the seropositivity of anti-CBir1 in CD, UC and
healthy control subjects was 50–57, 6–16 and 8–15%, respectively
(15). The antigen proteins of
anti-OmpC were originally proposed to cross-react with pANCA, as
the expression level of the IgG antibody in UC patients increased
more than in healthy individuals (16). However, later experiments demonstrated
that the IgA of anti-OmpC was more common in CD patients (17), and therefore IgA is generally detected.
In a previous study, the seroprevalence of anti-OmpC ranged from
5–11% in UC patients, 20–55% of CD patients were seropositive for
anti-OmpC and the prevalence in healthy control subjects was only
5% (17). Similar to the
above-mentioned studies, the present study showed that the
seroprevalence of anti-CBir1 and anti-OmpC in CD were significantly
higher than that in UC, OGIDs or healthy individuals. When tested
independently, anti-CBir1 and anti-OmpC had limited sensitivity,
although their specificity was good. In addition, the sensitivity,
specificity, and positive and negative predictive value of
anti-CBir1 were all higher than anti-OmpC. Therefore, it seemed
that the diagnostic value of anti-CBir1 was better than anti-OmpC;
however, to the best of our knowledge, there is no study comparing
the two.
Regarding the differential diagnosis of intestinal
TB and IBD in the present study, four antibodies had no
differential ability except for anti-OmpC. Similarly, Makharia
et al (18) found that ASCA or
pANCA could not distinguish between TB and IBD; however, studies
comparing the expression of anti-CBir1 or anti-OmpC between IBD and
TB are limited. In the present study, the PPV of anti-OmpC
differentiating between CD and TB was as high as 90%, and it may be
used as a marker for the differential diagnosis of TB and CD;
however, this requires further confirmation.
A combined test for ASCA-IgG and IgA may increase
the diagnostic accuracy for CD, which is consistent with a previous
report (19). The addition of
anti-CBir1 increased the sensitivity of ASCA (ASCA-IgA and/or IgG)
for CD from 66.2 to 80.3% with the specificity slightly decreasing
(from 83 to 76.1%); the sensitivity increased to 85.9% when joined
with anti-OmpC in ASCA and the specificity remained at 73.9%. Our
results were comparable with the conclusion of a study by Zholudev
et al (20). When the four
above-mentioned antibodies were detected, the sensitivity for CD
was 91.5%, but the specificity significantly decreased. The
combined test of pANCA and ASCA had a higher sensitivity for IBD
than that of any of the antibodies along, and the addition of
anti-CBir1 improved the sensitivity of this combination to 82.1%,
with a specificity of 71.6%. The addition of anti-OmpC increased
the sensitivity of the pANCA and ASCA combination to 85.7%, with a
specificity of 69.3%. It may be concluded that the detection of an
individual antibody has a limited sensitivity and a high
specificity, which is not suitable for screening IBD in patients
with GI symptoms, but rather is suitable as an adjunctive tool for
patients in whom an endoscopic examination does not provide a
certain diagnosis. The combined detection of antibodies improves
diagnostic sensitivity and slightly decreases specificity, which
may serve as a non-invasive screening tool. In particular, for the
novel serological antibodies, the diagnostic value will be greater
in the combined test than when tested independently.
Previous data have indicated that the higher the
number of positive antibodies, the more possible the diagnosis for
IBD (17). The present study also
confirmed that when there were ≥2 positive antibodies, IBD patients
could be distinguished from OGIDs and healthy individuals.
Furthermore, an increased percentage of IBD patients with an
increasing diversity of the immune response was demonstrated, with
the highest odds in patients that were positive for all five
antibodies. Additionally, patients positive for ≥3 antibodies were
more likely to be diagnosed as IBD rather than TB, although the
seropositivity of a single antibody between IBD and TB was
similar.
Combined testing rather than evaluating individual
antibodies is more useful in identifying IBD subtypes. The
ASCA+/pANCA− profile had the best combined
sensitivity and specificity for distinguishing CD from UC at 50.7
and 80.5%, respectively. The reverse profile of
pANCA+/ASCA- was most specific for differentiating UC
from CD, with a specificity of 94.4%. These results are also
consistent with a previous report (21).
The novel antibodies, anti-CBir1 and anti-OmpC, may
help to diagnose ASCA-negative CD, and independent from ASCA,
anti-OmpC may allow differentiation between ASCA-negative CD
patients and ASCA-negative UC patients. This finding was consistent
with the study by Joossens et al (17), although it was different from the
conclusion that anti-CBir1 or anti-OmpC could differentiate
pANCA-positive UC from pANCA-positive CD (which was not established
in the current study). However, other studies also confirmed that
anti-CBir1 was able to differentiate between pANCA-positive UC and
CD (15). This discrepancy may result
from differences regarding the pathogenesis between Chinese and
Western individuals.
ASCA-IgG and IgA are associated with the disease
location of CD, their seropositivity were highest in ileal CD. The
proportion of ASCA-IgG-positive CD patients was significantly
higher in complicated diseases (stenosis, perforation and anal
diseases) compared with patients with uncomplicated diseases. These
results have been proven by previous studies (19,22).
However, in ASCA-positive CD patients, the correlation between ASCA
titers and disease locations or disease behavior was not identified
in the present study. It was known that the prevalence of ASCA was
not associated with CD severity, and the present study drew the
same conclusion. Data showed that pANCA was associated with UC-like
CD (13,23), and the current study found that the
proportion of pANCA-positives was higher in colonic CD patients
than in the other subgroups.
Anti-CBir1 was the first bacterial antigen antibody
that could induce colitis in rat models, which increased the
detection of small-bowel disease, UC-like disease, and complicated
disease, such as fibrostenosis or internal-penetrating disease
(24). In the present study,
qualitative anti-CBir1 was negatively associated with the colonic
CD population; however, the quantitative level of anti-CBir1 was
found to be unassociated with disease locations in
anti-CBir1-positive CD. Unlike certain studies that were conducted
on a western population, anti-CBir1 expression was not identified
to be associated with CD complications in the current patients.
Previous studies demonstrated that anti-OmpC was not associated
with CD locations (23), and the
present result was consistent with this finding. Furthermore, it
was found that anti-OmpC expression was associated with CD behavior
and had a higher prevalence in complicated CD. In addition, there
was an association between anti-OmpC titer level and disease
behaviors. Previous data showed that the presence of ASCA in CD
patients was independent of disease activity and duration (19). In the present study, ASCA, anti-CBir1
and anti-OmpC were independent of CD activity and the disease
course.
Many foreign studies demonstrated that pANCA was not
associated with the UC phenotype (23,25).
However, it has been shown in Chinese UC patients that pANCA was
more frequent with extensive disease (26) and with active disease (27). In the present study, the prevalence of
pANCA was significantly higher in moderate to severe UC than in
mild UC, but it was unrelated with UC extension. In pANCA-positive
UC, the titer of pANCA did not increase as the disease activity
increased.
The results of the combined test of three antibodies
(anti-OmpC, anti-CBir1 and ASCA) indicated that when the number of
positive antibodies was ≥2, the CD patients were more likely to
have ileum involvement. The above result was consistent with
previous review articles (23).
Furthermore, no association between the quantity of antibody and
the disease behavior was identified in the present study.
In the present study, up to five antibodies were
detected, alone and in combination, for the diagnosis of IBD. Their
correlation with disease phenotype was analyzed in depth. To the
best of our knowledge, these results are the first to indicate that
anti-OmpC may potentially be the antibody that differentiates
between CD and TB. The subjects of the current study were primarily
Chinese individuals in a limited area, which was somewhat
representative. However, there were also certain limitations. The
duration of the study was short and the number of cases was
correspondingly small rather than a large cohort of IBD patients. A
larger sample size and a more diverse population are required in
order that the conclusions are more applicable. Previous studies
have shown that serum antibody markers predicted the occurrence and
progress of IBD (28,29). The present study was a retrospective,
non-prospective study, the association between the antibodies and
the progress of diseases were not tracked, and the prediction of
antibodies on the treatment response was not examined. Therefore,
follow-up experimental studies are required in future.
References
|
1
|
Bernstein CN, Fried M, Krabshuis JH, Cohen
H, Eliakim R, Fedail S, Gearry R, Goh KL, Hamid S, Khan AG, et al:
World gastroenterology organization practice guidelines for the
diagnosis and management of IBD in 2010. Inflamm Bowel Dis.
16:112–124. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zeng Z, Zhu Z, Yang Y, Ruan W, Peng X, Su
Y, Peng L, Chen J, Yin Q, Zhao C, et al: Incidence and clinical
characteristics of inflammatory bowel disease in a developed region
of Guangdong Province, China: A prospective population-based study.
J Gastroenterol Hepatol. 28:1148–1153. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhao J, Ng SC, Lei Y, Yi F, Li J, Yu L,
Zou K, Dan Z, Dai M, Ding Y, et al: First prospective,
population-based inflammatory bowel disease incidence study in
mainland of China: The emergence of ‘western’ disease. Inflamm
Bowel Dis. 19:1839–1845. 2013.PubMed/NCBI
|
|
4
|
Yang H, Li Y, Wu W, Sun Q, Zhang Y, Zhao
W, Lv H, Xia Q, Hu P, Li H and Qian J: The incidence of
inflammatory bowel disease in Northern China: A prospective
population-based study. PLoS One. 9:e1012962014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ye L, Cao Q and Cheng J: Review of
inflammatory bowel disease in China. Sci World J. 2013:2964702013.
View Article : Google Scholar
|
|
6
|
Arai R: Serologic markers: Impact on early
diagnosis and disease stratification in inflammatory bowel disease.
Postgrad Med. 122:177–185. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Satsangi J, Silverberg MS, Vermeire S and
Colombel JF: The Montreal classification of inflammatory bowel
disease: Controversies, consensus, and implications. Gut.
55:749–753. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Best WR, Becktel JM, Singleton JW and Kern
F Jr: Development of a Crohn's disease activity index. National
cooperative Crohn's disease study. Gastroenterology. 70:439–444.
1976.PubMed/NCBI
|
|
9
|
Main J, McKenzie H, Yeaman GR, Kerr MA,
Robson D, Pennington CR and Parratt D: Antibody to Saccharomyces
cerevisiae (bakers' yeast) in Crohn's disease. BMJ. 297:1105–1106.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Peyrin-Biroulet L, Standaert-Vitse A,
Branche J and Chamaillard M: IBD serological panels: Facts and
perspectives. Inflamm Bowel Dis. 13:1561–1566. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rump JA, Schölmerich J, Gross V, Roth M,
Helfesrieder R, Rautmann A, Lüdemann J, Gross WL and Peter HH: A
new type of perinuclear anti-neutrophil cytoplasmic antibody
(p-ANCA) in active ulcerative colitis but not in Crohn's disease.
Immunobiology. 181:406–413. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim BG, Kim YS, Kim JS, Jung HC and Song
IS: Diagnostic role of anti-Saccharomyces cerevisiae mannan
antibodies combined with antineutrophil cytoplasmic antibodies in
patients with inflammatory bowel disease. Dis Colon Rectum.
45:1062–1069. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou F, Xia B, Wang F, Shrestha UK, Chen
M, Wang H, Shi X, Chen Z and Li J: The prevalence and diagnostic
value of perinuclear antineutrophil cytoplasmic antibodies and
anti-Saccharomyces cerevisiae antibodies in patients with
inflammatory bowel disease in mainland China. Clin Chim Acta.
411:1461–1465. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lawrance IC, Murray K, Hall A, Sung JJ and
Leong R: A prospective comparative study of ASCA and pANCA in
Chinese and caucasian IBD patients. Am J Gastroenterol.
99:2186–2194. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Papp M and Lakatos PL: Serological studies
in inflammatory bowel disease: How important are they? Curr Opin
Gastroenterol. 30:359–364. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Landers CJ, Cohavy O, Misra R, Yang H, Lin
YC, Braun J and Targan SR: Selected loss of tolerance evidenced by
Crohn's disease-associated immune responses to auto- and microbial
antigens. Gastroenterology. 123:689–699. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Joossens S, Reinisch W, Vermeire S, Sendid
B, Poulain D, Peeters M, Geboes K, Bossuyt X, Vandewalle P,
Oberhuber G, et al: The value of serologic markers in indeterminate
colitis: A prospective follow-up study. Gastroenterology.
122:1242–1247. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Makharia GK, Sachdev V, Gupta R, Lal S and
Pandey RM: Anti-Saccharomyces cerevisiae antibody does not
differentiate between Crohn's disease and intestinal tuberculosis.
Dig Dis Sci. 52:33–39. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gologan S, Iacob R, Preda C, Vadan R,
Cotruta B, Catuneanu M, Iacob S, Constantinescu I, Gheorghe L,
Iobagiu S, et al: Higher titers of anti-Saccharomyces cerevisiae
antibodies IgA and IgG are associated with more aggressive
phenotypes in Romanian patients with Crohn's disease. J
Gastrointestin Liver Dis. 21:39–44. 2012.PubMed/NCBI
|
|
20
|
Zholudev A, Zurakowski D, Young W,
Leichtner A and Bousvaros A: Serologic testing with ANCA, ASCA, and
anti-OmpC in children and young adults with Crohn's disease and
ulcerative colitis: Diagnostic value and correlation with disease
phenotype. Am J Gastroenterol. 99:2235–2241. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Reese GE, Constantinides VA, Simillis C,
Darzi AW, Orchard TR, Fazio VW and Tekkis PP: Diagnostic precision
of anti-Saccharomyces cerevisiae antibodies and perinuclear
antineutrophil cytoplasmic antibodies in inflammatory bowel
disease. Am J Gastroenterol. 101:2410–2422. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang Z, Li C, Zhao X, Lv C, He Q, Lei S,
Guo Y and Zhi F: Anti-Saccharomyces cerevisiae antibodies associate
with phenotypes and higher risk for surgery in Crohn's disease: A
meta-analysis. Dig Dis Sci. 57:2944–2954. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Prideaux L, De Cruz P, Ng SC and Kamm MA:
Serological antibodies in inflammatory bowel disease: A systematic
review. Inflamm Bowel Dis. 18:1340–1355. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Targan SR, Landers CJ, Yang H, Lodes MJ,
Cong Y, Papadakis KA, Vasiliauskas E, Elson CO and Hershberg RM:
Antibodies to CBir1 flagellin define a unique response that is
associated independently with complicated Crohn's disease.
Gastroenterology. 128:2020–2028. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kuna AT: Serological markers of
inflammatory bowel disease. Biochem Med (Zagreb). 23:28–42. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cioffi M, Rosa AD, Serao R, Picone I and
Vietri MT: Laboratory markers in ulcerative colitis: Current
insights and future advances. World J Gastrointest Pathophysiol.
6:13–22. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang X and Dong E: Anti-neutrophil
cytoplasmic antibodies in patients with inflammatory bowel
diseases. Chin J Integr Med. 3:15–18. 2000.(In Chinese).
|
|
28
|
Israeli E, Grotto I, Gilburd B, Balicer
RD, Goldin E, Wiik A and Shoenfeld Y: Anti-Saccharomyces cerevisiae
and antineutrophil cytoplasmic antibodies as predictors of
inflammatory bowel disease. Gut. 54:1232–1236. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Devlin SM and Dubinsky MC: Determination
of serologic and genetic markers aid in the determination of the
clinical course and severity of patients with IBD. Inflamm. Bowel
Dis. 14:125–128, 132–133. 2008. View Article : Google Scholar
|