Introduction
Evidence has been accumulating over several decades
that industrial chemicals cause neurodevelopmental damage (1). Trimethyltin (TMT) is a particularly
potent organotin chemical, formerly used for a variety of
industrial and agricultural purposes, which has been extensively
investigated and used as a model neurotoxin for investigating
selective brain dysfunction and delayed neuronal cell death
(2–4).
Various regions known to be affected by TMT include the
hippocampus, preform cortex, amygdaloid nucleus, neocortex, basal
ganglia, cerebellum, brain stem, spinal cord, dorsal root ganglia,
olfactory cortex, retina and inner ear (5). The implications of the hippocampus in
memory processes and neurogenesis are well known and it is likely
that the hippocampus would be the first region of the brain to be
affected by TMT-induced memory and behavioral changes. The
molecular pathogenesis of TMT intoxication is hypothesized to be a
complex event. Different theories have been proposed to explain the
underlying mechanism of the neurotoxic action of TMT, including
glutamate excitotoxicity, intracellular calcium overload and
impairment of neurotransmission (6,7), along with
oxidative stress (8,9). Although the underlying cellular
mechanisms remain unknown, TMT undoubtedly causes profound
behavioral changes, and affects learning and memory ability in
exposed mammals, as indicated by poor performances in the water
maze test, Y-maze test and passive avoidance test.
Ginseng is a deciduous perennial plant that belongs
to the Araliaceae family. Panax ginseng, cultivated in China, Korea
and the USA (Panax quinquefolium L) represents the most
extensively investigated species. The pharmacological and
therapeutic effects of ginseng are attributed to ginsenosides,
which have been demonstrated to affect the central nervous system
(CNS), cardiovascular system, endocrine secretion, immune function
and metabolism, as well as possessing anti-stress and -aging
properties (10–13). Ginsenosides are classified into three
major categories, namely protopanaxatriols (PPTs; ginsenoside Rg1,
Re, Rg2, Rh1 and Rf), protopanaxadiol (PPD; ginsenoside Rb1, Rb2,
Rd, Rg3 and Rh2) and oleanolic acid derivates (ginsenoside Ro).
Ginsenoside has become the focus of numerous research groups due to
its involvement and protective action in various types of
manipulated neuronal damage or dysfunction models in vivo
and in vitro (14–19). Certain classical models, such as
cerebral ischemia (14,15), chemical-induced excitotoxicity
(16,17)
and Alzheimer's disease (18,19) have received particular attention.
However, these lines of evidence support the interaction between
ginsenosides and the CNS, indicating that ginsenosides have more
potential for other underlying neuronal diseases. Based upon the
above-mentioned evidence of ginsenosides in the CNS, the present
study examined the impact of ginsenoside Rd on TMT-induced
neurotoxicity to elucidate the potential neuroprotective
potential.
Materials and methods
Chemicals
TMT chloride was purchased from Sigma-Aldrich (Merck
KGaA, Darmstadt, Germany). Ginsenoside Rd, with a purity of
>98%, was generously provided by the Central Research Institute
of KT&G (Daejeon, South Korea). Ginsenoside was dissolved in
dimethyl sulfoxide as a 10-mg/ml solution. All other chemicals in
the present study were of analytical grade.
Animals
Male ICR mice (age, 8 weeks; n=35–40) were obtained
from Dahan Biolink (Eumseong, South Korea) in present study. The
mice were housed in groups in a room maintained at 24±2°C under
artificial lighting from 7:00 a.m. to 7:00 p.m., with free access
to food and water. All experimental procedures complied with the
Guide for the Care and Use of Laboratory Animals issued by the
National Institutes of the Committee of Animal Experiments
(Chungnam National University, Daejeon, South Korea).
Primary culture of hippocampal
neurons
Hippocampi were aseptically dissected from
Sprague-Dawley rat embryos (embryonic day 18) acquired from
OrientBio, Inc. (Seongnam, South Korea). Hippocampal neurons were
dissociated from the tissue samples as previously described
(20). Following trituration and
trypsinization, hippocampal cells were resuspended in plating
medium (86.55% Minimum Essential Medium Eagle with Earle's Balanced
Salt Solution, 10% re-filtered and heat inactivated fetal bovine
serum, 0.45% glucose, 100 µM sodium pyruvate, 200 µM glutamine, and
100 mg/l streptomycin and 100 U/ml penicillin). The single-cell
suspension was seeded in 100-mm petri-dishes containing
poly-L-lysine coated coverslips at a density of 5×105/ml
(pre-warmed at 37°C). After 4 h, the cells were maintained in
Neurobasal Medium (21103–049; Invitrogen; Thermo Fisher Scientific,
Inc, Waltham, MA, USA) supplemented with 1% B27, 200 µM glutamine
and 100 mg/l streptomycin, 100 U/ml penicillin in a humidified
atmosphere of 5% CO2 at 37°C. Protocols were performed
in accordance with the national guidelines governing animal care in
South Korea.
Experimental protocol
TMT chloride (0.1–10 µM) was added to the 7-day
primary cultures of in vitro hippocampal neurons for 24 h
with or without ginsenoside Rd (1–40 µg/ml) pre-treatment for 24 h.
Then cells were subjected to cell viability assay.
The entire timescale was 3 weeks. Mice received a
single injection of TMT chloride (2 mg/kg/body weight) dissolved in
sterile saline immediately (intraperitoneally) on day 14, while the
ginsenoside Rd (20 mg/kg/body weight) was intraperitoneally
injected for 21 consecutive days. Control mice received 0.9%
sterile saline injections (intraperitoneally) of the same volume.
The treatment groups were as follows: Saline-treated group (Con;
n=8); TMT chloride-treated animals (TMT; n=10); ginsenoside
Rd-treated group (Rd; n=8); and TMT and ginsenoside Rd co-treated
group (TMT + Rd; n=9).
Assessment of tremor/seizure
severity
Twenty-four hours post-injection, all mice were
scored during a 5-min interval on a tremor/seizure severity scale
(21) as follows: 1, normal behavior;
2, hyper-responsiveness to sound and handling; 3, whole body mild
tremor with normal motor activity; 4, whole body tremor with
extended periods of immobility; 5, rigid posture; 6, forelimb
clonus, rearing and falling; 7, repeated incidence of level 4
behavior; and 8, severe tonic-clonic behavior.
Passive avoidance test
A passive avoidance test was performed at day 21 in
identical compartments. The illuminated compartment (20×20×20 cm)
contained a 100-W bulb, and the floor of the non-illuminated
compartment (20×20×20 cm) was composed of 2-mm stainless steel rods
with 1-cm intervals. These two compartments were separated by a
guillotine door (5×5 cm). For the acquisition trials, mice were
initially placed in the illuminated compartment and the door was
opened 15 sec later. When the mouse entered the non-illuminated
compartment, the door was closed and an electrical foot shock (0.5
mA) of 5 sec duration was delivered through the stainless steel
rods. Twenty-four hours after the acquisition trials, the mice were
placed in the illuminated compartment again for the retention
trials. The time taken for a mouse to enter the non-illuminated
compartment after the door was opened was defined as the
step-through latency time in the retention trials. If a mouse did
not enter the non-illuminated compartment within 300 sec, it was
assumed that the mouse had remembered the single training
trial.
Immunohistochemistry
Coronal sections (8 µm) were sliced using a
microtome and deparaffinized according to standard protocols. To
reveal the morphological changes induced by TMT, Nissl staining of
hippocampal sections was performed. The population and morphology
of neurons and astrocytes in tissue samples were identified
immunocytochemically using anti-glial fibrillary acidic protein
(GFAP) antibody (astroglial cells; cat. no. sc-166458; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA). Sections were placed in
citrate buffer (0.01 M; pH 6.0) and incubated at 80°C for 30 min.
The sections were washed with phosphate-buffered saline (PBS)
twice, for 5 min each time, and blocked with 3% fetal bovine serum
blocking solution for 30 min, which was followed by incubation with
primary anti-body GFAP (dilution, 1:50) overnight at 4°C. The
sections were counterstained with hematoxylin and cover slipped
using histomount mounting solution.
Image acquisition and analysis
A Zeiss Research Microscope was used for
phase-contrast microscopy. Morphometric analyses and quantification
were performed using ImageJ software (version 1.50i) with the
simple neurite tracer plug-in (National Institute of Health,
Bethesda, MD, USA). The lengths of primary dendrites and axons were
analyzed.
The sections were analyzed using an Olympus
microscope equipped with a digital camera (Olympus Corporation,
Tokyo, Japan). The cells were counted by a researcher who was
blinded to the experimental conditions. Every ten sections
throughout the hippocampus were processed for counting (5–6
sections per animal).
Western blotting
Hippocampi were dissected and homogenized in protein
extraction buffer (PRO-PREP™ 17081; iNtRON Biotechnology, Seongnam,
South Korea). The supernatant was collected following
centrifugation at 9,740 × g for 30 min at 4°C. Following
quantification, the samples (20 µg protein per lane) were subjected
to preparative sodium dodecyl sulfate-polyacrylamide gel
electrophoresis in a 10% gel and electrophoretically transferred
onto polyvinylidene fluoride (PVDF) membranes (EMD Millipore,
Billerica, MA, USA) using a trans-blot device (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) at a constant current of 15
V overnight at 4°C. The PVDF membranes were soaked in 5% skimmed
milk in PBS solution for 2 h at room temperature to block
non-specific binding, rinsed in PBS with Tween-20 (PBST), and
incubated with B-cell lymphoma 2 (Bcl-2; cat. no. 2876), bcl-2-like
protein 4 (Bax; cat. no. 5023S) and caspase-3 (cat. no. 9664S)
antibodies (diluted 1:1,000 in 5% skimmed milk in TBST; Cell
Signaling Technology, Inc., Danvers, MA, USA) overnight at 4°C. The
membranes were then washed three times (10 min each time) in PBST
and incubated for 2 h with goat anti-rabbit immunoglobulin G
secondary antibody (dilution, 1:5,000; cat. no. sc-2030; Santa Cruz
Biotechnology, Inc.). Western blot analysis for GAPDH, the loading
control (dilution, 1:4,000; cat. no. sc-25778; Santa Cruz
Biotechnology, Inc.), was performed using the same procedure. The
blots were quantified using ImageJ image analysis software (version
1.50i). Band intensity values were expressed as a percentage of the
control average.
Statistical analysis
All statistical analyses were conducted using SPPSS
version 20 (IBM SPSS, Armonk, NY, USA). P≤0.05 was considered to
indicate a statistically significant difference. When a
statistically significant overall group effect was found, multiple
comparisons were made using Fisher protected least significant
different post hoc tests to compare the individual groups.
Results
TMT induces apoptosis of primary
hippocampal neurons in culture
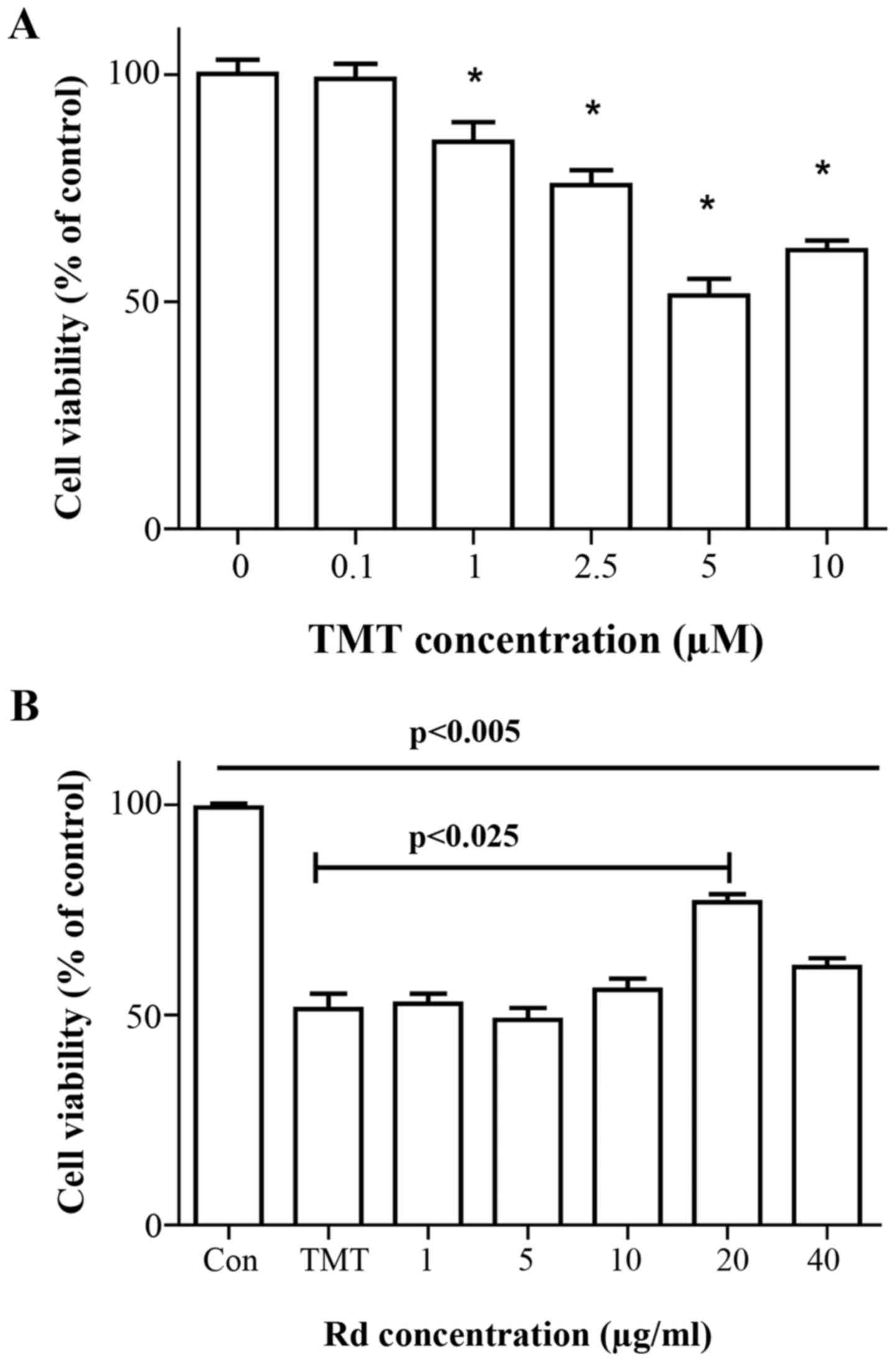
No morphological changes were observed in the
neurons following exposure to 0–1 µM TMT for 24 h, as revealed by
phase contrast microscopy. Application of greater TMT
concentrations (0, 2.5, 5.0 and 10.0 µM) induced a dose-dependent
increase of neuronal degeneration manifested by neurite
fragmentation and regression, as well as somal rounding and
shrinkage (data not shown). Dose-dependent effects of TMT on
neuronal viability are presented in Fig.
1A. To further analyze the protective effects of Rd on
TMT-induced cytotoxicity, the percentage of apoptotic cells with
obvious morphological changes following exposure to 5 µM TMT for 24
h was quantified. To optimize the effective concentration,
hippocampal neurons were treated with different concentrations of
ginsenoside Rd. Ginsenoside Rd treatment (in the range of 10–40 µM)
significantly increased the cell viability (including neurite
outgrowth) in a dose-dependent manner, whereas no significant
effects were noted following treatment with 1 or 5 µM ginsenoside
Rd. An optimal concentration of 20 µg/ml pre-treatment for 24 h was
proposed herein (Fig. 1B). Together,
these data indicate that apoptotic cell death induced by TMT was
efficiently prevented by ginsenoside Rd pre-treatment.
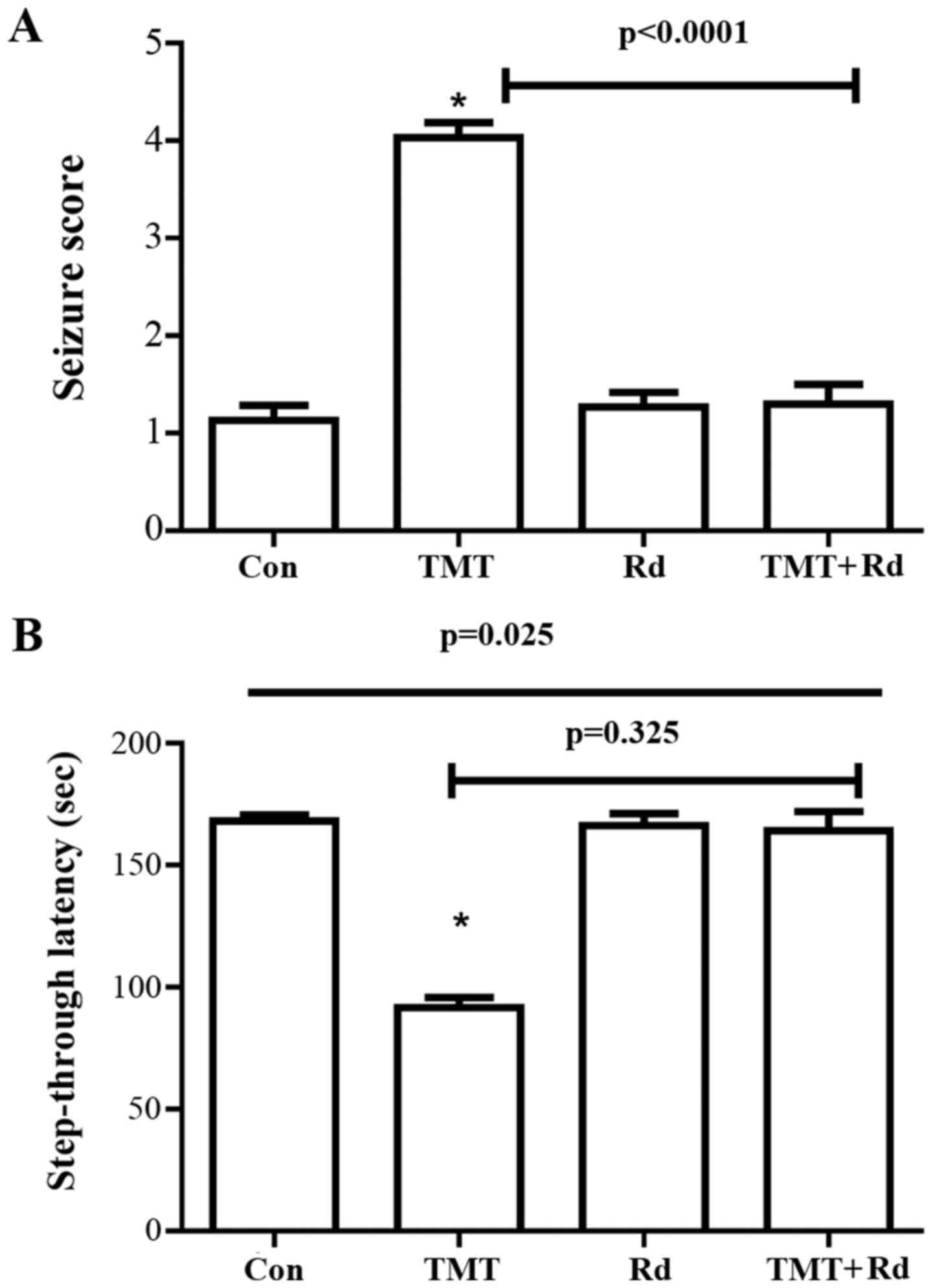
TMT-induced behavior changes
Within 24 h of TMT exposure, mice exhibited clinical
signs of tremor and seizure activity that were attenuated by
ginsenoside Rd treatment (Fig. 2A).
The seizure score of the TMT group was significantly greater
(P<0.0001) than that of the control. The seizure score was
significantly reduced in the TMT+Rd mice (P<0.0001) when
compared with the TMT mice. Furthermore, the Rd alone group did not
differ from the control group.
Significant overall group effects were observed in
the passive avoidance test (P=0.025) subsequent to a three-week
treatment. In the passive avoidance test (Fig. 2B), although TMT-treated mice exhibited
significantly decreased step-through latency (P=0.009) compared
with the control group mice, no significant differences were noted
in the TMT+Rd (20 mg/kg; P=0.325) and Rd (P=0.124) groups when
compared with the control group.
TMT induces neuronal loss in the
hippocampus and astroglial activation
To investigate the contribution of ginsenoside Rd on
the protection of hippocampal neurons, mice were treated with
ginsenoside Rd (20 mg/kg body weight) for 21 days. As revealed by
Nissl staining (Fig. 3A-D), treatment
with TMT induced the loss of hippocampal Cornu Ammonis 1 (CA1)
subregion cells, although this was not observed in the Rd+TMT
group. As demonstrated in Fig. 3E, the
number of pyramidal neurons in the CA1 subregions was determined.
The neuronal loss indicated the marked toxicity of TMT in the
hippocampus.
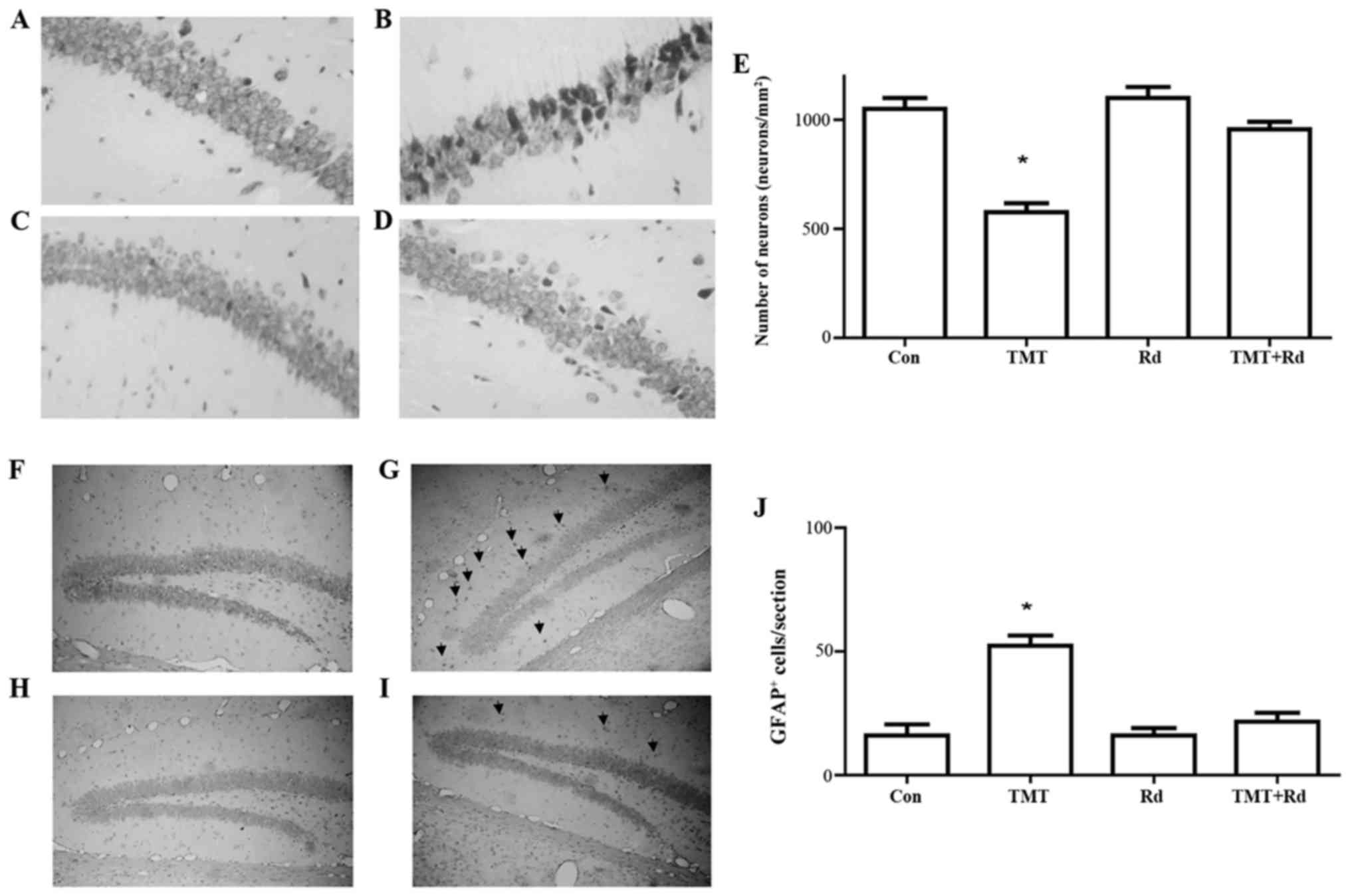 | Figure 3.Treatment with TMT induced hippocampal
subregion CA1 neuron loss and astrocyte activation. Nissl staining
for coronal sections from the (A) control, (B) TMT, (C) Rd and (D)
TMT + Rd groups. Original magnification, ×100. (E) The number of
pyramidal neurons in the CA1 subregion of the hippocampus was
determined. Each bar represents the mean ± standard error of the
mean. Glial fibrillary acidic protein immunostaining for coronal
sections from the (F) control, (G) TMT, (H) Rd and (I) TMT + Rd
groups. The arrows indicate positive cells. Original magnification,
×60. (J) Quantification of GFAP. Each bar represents the mean ±
standard error of the mean. *P<0.05 vs. Con. TMT, trimethyltin;
CA1, Cornu Ammonis 1; Con, control; Rd, ginsenoside Rd. |
GFAP immunocytochemical staining demonstrated that
TMT induced a marked increase of GFAP immunoreactivity in the
entire hippocampus, with the most significant expression observed
in the astrocytes of the dentate gyrus (Fig. 3F-I). The morphology of astrocytes and
intensive astrofilament staining indicated hypertrophic activity of
astroglia. As can be seen in Fig. 3J,
the TMT group had significantly more GFAP+ cells than
the saline-treated control group (P<0.004). In contrast to this
observation, the Rd and TMT+Rd groups were not significantly
different from the controls (P=0.34).
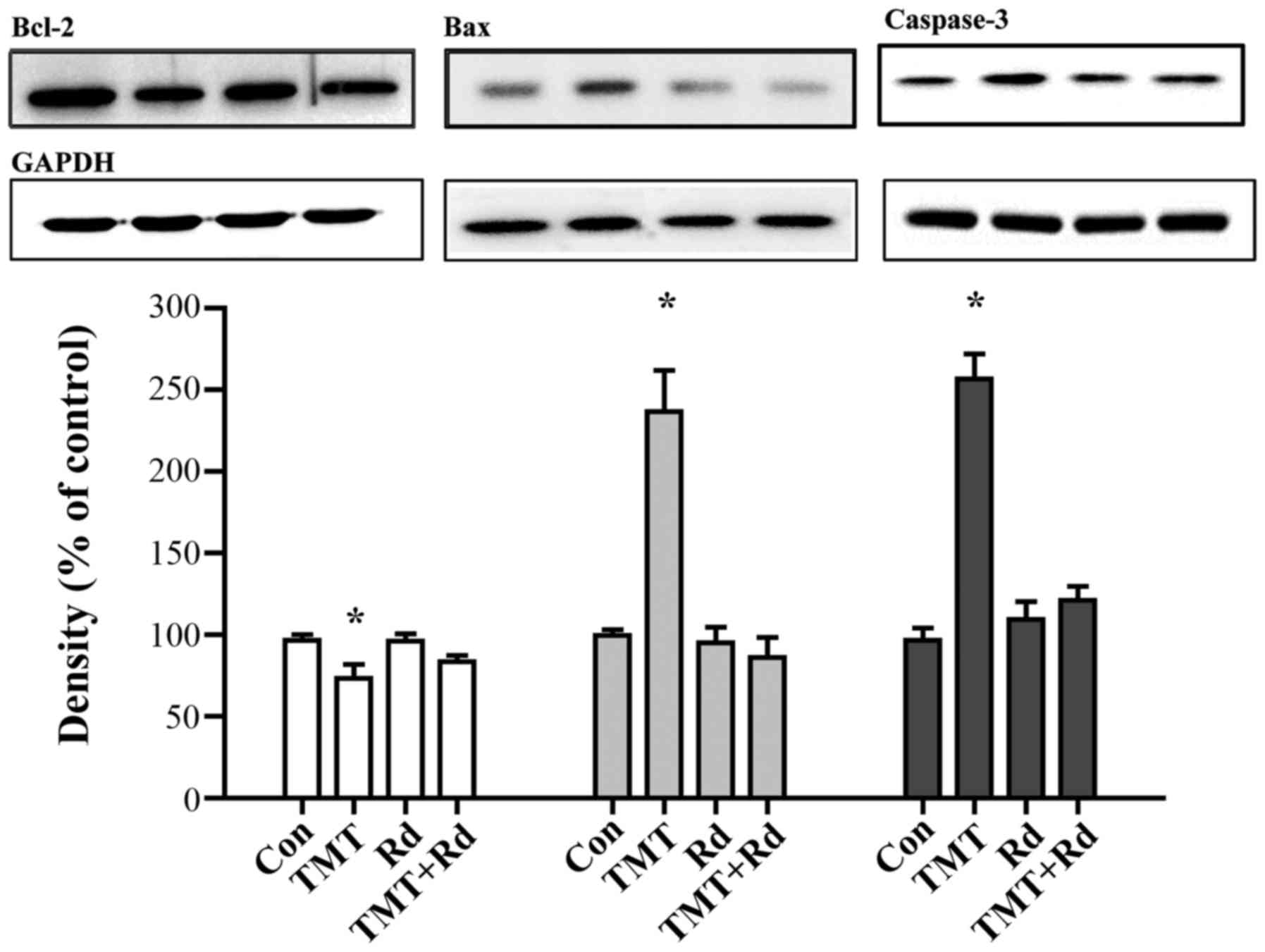
Ginsenoside Rd alters apoptosis
induced by TMT in the hippocampus
Induction of apoptosis, the cellular waste disposal
process, was evaluated by the protein expression of Bcl-2, Bax, and
caspases-3. The expression level of Bcl-2 was identified to be
significantly reduced by TMT when compared with that of the control
group (P<0.05). By contrast, the expression levels of Bax and
caspase-3 were significantly increased in the TMT group
(P<0.05), indicating that apoptosis was induced in the
hippocampus. Notably, treatment with ginsenoside Rd reversed the
decrease of Bcl-2 and increase of Bax and caspase-3 expression
levels, indicating a neuronal protective effect (Fig. 4).
Discussion
The current study demonstrated that exposure to TMT
alters the physical condition (tremor/seizure test) and spatial
recognition memory (passive avoidance test), which are associated
with hippocampal dysfunction. Consistent with functional deficits,
significant neuron loss and astroglial activation were observed in
the hippocampus of treated mice. In addition, this cytotoxicity was
confirmed in the primary hippocampal neuron culture system.
Notably, there was a pronounced protective effect of ginsenoside Rd
in the TMT exposure models. Pre-treatment with 20 µg/ml ginsenoside
Rd for 24 h markedly reduced cell death (~25%) compared with TMT
treatment alone, indicating that ginsenoside Rd protected against
TMT-induced apoptosis.
Animals exposed to TMT suffer spontaneous seizures
and elevation of neuronal excitability (22,23). In the
present study, whole body tremor, with extended periods of
immobility, was observed in mice that received TMT injections.
Additionally, the toxic interaction of TMT with the hippocampus and
other limbic brain regions is responsible for affecting spatial
learning and memory. Deficits in passive avoidance behavior result
from TMT exposure (24,25), which is consistent with the
observations in the current study. To explain any potential
protection against these functional decrements, the current study
hypothesized that a specific protection of ginsenoside Rd would
adversely impact hippocampal impairment. Significant reduction in
tremor seizures, and improved learning and memory ability suggest
that ginsenoside Rd attenuates toxicity herein.
The impact of TMT on neurons was assessed in the
current study using a primary hippocampal neuron culture and mouse
models. In the present study, TMT induced primary hippocampal
neuron cell death in a dose-dependent manner. In the animal model,
TMT treatment developed extensive lesions in the CA. Furthermore,
apparent neuronal loss was observed in the CA1 subregion in the
hippocampus, in the present study, which was confirmed by Nissl
staining. The present results are consistent with those of previous
studies (5,26), indicating that hippocampal
vulnerability is typical in TMT exposure. Furthermore, TMT-induced
neuronal loss is associated with the activation of astrocytes and
GFAP expression levels are altered in the hippocampus following TMT
treatment. Various studies have established the active role of
astrocytes in TMT-induced neurodegeneration. Certain studies
indicated that reactive astrocytes express a trophic response, such
as nerve growth factor and tropomyosin receptor kinase A to TMT
exposure (27). Certain studies
proposed an involvement of P2X2 purinergic receptor signaling
induction from astroglial (28). In
addition, the role of astroglia in preventing TMT-induced neuronal
cell death by modulating oxidative stress was also proposed
(29). Notably, the present data
identified similar GFAP expression levels between the control group
and the TMT+Rd group. Thus, it may indicate that ginsenoside Rd
prevented the TMT-induced neuronal toxicity, rather than
detoxication. Previous findings suggest a direct involvement of
mitochondrial function in response to TMT (30,31). The
present study hypothesized that the effectors, Bcl-2 and Bax, in
the intrinsic (mitochondrial mediated) apoptotic pathway were
modulated by ginsenoside Rd treatment, indicating a potential
molecular mechanism.
In conclusion, the present study demonstrated the
deleterious effect of TMT on neurons, which resulted in hippocampal
dysfunction. Treatment with ginsenoside Rd may prevent the toxicity
in in vitro and in vivo models. These results
therefore indicate that ginsenoside Rd may be administered as a
potential neuroprotective agent.
Acknowledgements
The present study was supported by the Korean
Society of Ginseng and Korean Ginseng Corporation (2013).
References
|
1
|
Dorman DC: An integrative approach to
neurotoxicology. Toxicol Pathol. 28:37–42. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ha JS, Jin DE, Park SK, Park CH, Seung TW,
Bae DW, Kim DO and Heo HJ: Antiamnesic Effect of Actinidia arguta
Extract Intake in a Mouse Model of TMT-Induced Learning and Memory
Dysfunction. Evid Based Complement Alternat Med. 2015:8764842015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lattanzi W, Corvino V, Di Maria V,
Michetti F and Geloso MC: Gene expression profiling as a tool to
investigate the molecular machinery activated during hippocampal
neurodegeneration induced by trimethyltin (TMT) administration. Int
J Mol Sci. 14:16817–16835. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim J, Yang M, Kim J, Song L, Lee S, Son
Y, Kang S, Bae CS, Kim JC, Kim SH, et al: Developmental and
degenerative modulation of brain-derived neurotrophic factor
transcript variants in the mouse hippocampus. Int J Dev Neurosci.
38:68–73. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bouldin TW, Goines ND, Bagnell RC and
Krigman MR: Pathogenesis of trimethyltin neuronal toxicity.
Ultrastructural and cytochemical observations. Am J Pathol.
104:237–249. 1981.PubMed/NCBI
|
|
6
|
Park HJ, Lee MS, Shim HS, Lee GR, Chung
SY, Kang YM, Lee BJ, Seo YB, Kim KS and Shim I: Fermented
Saccharina japonica (Phaeophyta) improves neuritogenic activity and
TMT-induced cognitive deficits in rats. Algae. 31:73–84. 2016.
View Article : Google Scholar
|
|
7
|
Shim HS, Park HJ, Ahn YH, Her S, Han JJ,
Hahm DH, Lee H and Shim I: Krill-Derived Phosphatidylserine
Improves TMT-Induced Memory Impairment in the Rat. Biomol Ther
(Seoul). 20:207–213. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kaur S and Nehru B: Alteration in
glutathione homeostasis and oxidative stress during the sequelae of
trimethyltin syndrome in rat brain. Biol Trace Elem Res.
153:299–308. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gasparova Z, Stara V, Janega P, Navarova
J, Sedlackova N, Mach M and Ujhazy E: Pyridoindole
antioxidant-induced preservation of rat hippocampal pyramidal cell
number linked with reduction of oxidative stress yet without
influence on cognitive deterioration in Alzheimer-like
neurodegeneration. Neuro Endocrinol Lett. 35:454–462.
2014.PubMed/NCBI
|
|
10
|
Kim HJ, Kim P and Shin CY: A comprehensive
review of the therapeutic and pharmacological effects of ginseng
and ginsenosides in central nervous system. J Ginseng Res. 37:8–29.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Peng L, Sun S, Xie LH, Wicks SM and Xie
JT: Ginsenoside Re: Pharmacological effects on cardiovascular
system. Cardiovasc Ther. 30:e183–e188. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim MY and Cho JY:
20S-dihydroprotopanaxadiol, a ginsenoside derivative, boosts innate
immune responses of monocytes and macrophages. J Ginseng Res.
37:293–299. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang Y, Kan H, Yin Y, Wu W, Hu W, Wang M
and Li W and Li W: Protective effects of ginsenoside Rg1 on chronic
restraint stress induced learning and memory impairments in male
mice. Pharmacol Biochem Behav. 120:73–81. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou Y, Li HQ, Lu L, Fu DL, Liu AJ, Li JH
and Zheng GQ: Ginsenoside Rg1 provides neuroprotection against
blood brain barrier disruption and neurological injury in a rat
model of cerebral ischemia/reperfusion through downregulation of
aquaporin 4 expression. Phytomedicine. 21:998–1003. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang Y, Li X, Wang X, Lau W, Wang Y, Xing
Y, Zhang X, Ma X and Gao F: Ginsenoside Rd attenuates myocardial
ischemia/reperfusion injury via Akt/GSK-3β signaling and inhibition
of the mitochondria-dependent apoptotic pathway. PLoS One.
8:e709562013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang C, Du F, Shi M, Ye R, Cheng H, Han
J, Ma L, Cao R, Rao Z and Zhao G: Ginsenoside Rd protects neurons
against glutamate-induced excitotoxicity by inhibiting ca(2+)
influx. Cell Mol Neurobiol. 32:121–128. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li G, Zhang XX, Lin L, et al: Preparation
of Ginsenoside Rg3 and Protection against H2O2-Induced Oxidative
Stress in Human Neuroblastoma SK-N-SH Cells. J Chem.
2014:8485712014. View Article : Google Scholar
|
|
18
|
Li N, Zhou L, Li W, Liu Y, Wang J and He
P: Protective effects of ginsenosides Rg1 and Rb1 on an Alzheimer's
disease mouse model: A metabolomics study. J Chromatogr B Analyt
Technol Biomed Life Sci. 985:54–61. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fang F, Chen X, Huang T, Lue LF, Luddy JS
and Yan SS: Multi-faced neuroprotective effects of Ginsenoside Rg1
in an Alzheimer mouse model. Biochim Biophys Acta. 1822:286–292.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kaech S and Banker G: Culturing
hippocampal neurons. Nat Protoc. 1:2406–2415. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Funk JA, Gohlke J, Kraft AD, McPherson CA,
Collins JB and Harry G Jean: Voluntary exercise protects
hippocampal neurons from trimethyltin injury: Possible role of
interleukin-6 to modulate tumor necrosis factor receptor-mediated
neurotoxicity. Brain Behav Immun. 25:1063–1077. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ishida N, Akaike M, Tsutsumi S, Kanai H,
Masui A, Sadamatsu M, Kuroda Y, Watanabe Y, McEwen BS and Kato N:
Trimethyltin syndrome as a hippocampal degeneration model: Temporal
changes and neurochemical features of seizure susceptibility and
learning impairment. Neuroscience. 81:1183–1191. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Janigro D and Costa LG: Effects of
trimethyltin on granule cells excitability in the in vitro rat
dentate gyrus. Neurotoxicol Teratol. 9:33–38. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Choi GN, Kim JH, Kwak JH, Jeong C-H, Jeong
HR, Lee U and Heo HJ: Effect of quercetin on learning and memory
performance in ICR mice under neurotoxic trimethyltin exposure.
Food Chem. 132:1019–1024. 2012. View Article : Google Scholar
|
|
25
|
Kaur S, Chhabra R and Nehru B: Ginkgo
biloba extract attenuates hippocampal neuronal loss and cognitive
dysfunction resulting from trimethyltin in mice. Phytomedicine.
20:178–186. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Geloso MC, Vercelli A, Corvino V, Repici
M, Boca M, Haglid K, Zelano G and Michetti F: Cyclooxygenase-2 and
caspase 3 expression in trimethyltin-induced apoptosis in the mouse
hippocampus. Exp Neurol. 175:152–160. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Koczyk D and Oderfeld-Nowak B: Long-term
microglial and astroglial activation in the hippocampus of
trimethyltin-intoxicated rat: Stimulation of NGF and TrkA
immunoreactivities in astroglia but not in microglia. Int J Dev
Neurosci. 18:591–606. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Latini L, Geloso MC, Corvino V, Giannetti
S, Florenzano F, Viscomi MT, Michetti F and Molinari M:
Trimethyltin intoxication up-regulates nitric oxide synthase in
neurons and purinergic ionotropic receptor 2 in astrocytes in the
hippocampus. J Neurosci Res. 88:500–509. 2010.PubMed/NCBI
|
|
29
|
Gunasekar PG, Mickova V, Kotyzova D, Li L,
Borowitz JL, Eybl V and Isom GE: Role of astrocytes in trimethyltin
neurotoxicity. J Biochem Mol Toxicol. 15:256–262. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Qu M, Zhou Z, Chen C, Li M, Pei L, Chu F,
Yang J, Wang Y, Li L, Liu C, et al: Lycopene protects against
trimethyltin-induced neurotoxicity in primary cultured rat
hippocampal neurons by inhibiting the mitochondrial apoptotic
pathway. Neurochem Int. 59:1095–1103. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang L, Li L, Prabhakaran K, Borowitz JL
and Isom GE: Trimethyltin-induced apoptosis is associated with
upregulation of inducible nitric oxide synthase and Bax in a
hippocampal cell line. Toxicol Appl Pharmacol. 216:34–43. 2006.
View Article : Google Scholar : PubMed/NCBI
|