Introduction
Nigella sativa (NS) seed, commonly known as a
black cumin, belongs to the Ranunculaceae family (1). It is an herbaceous plant, which is
composed of several constituents, including moisture, oil,
proteins, carbohydrates, vitamins and minerals (2,3). Among
these, fixed oils and unsaturated fatty acids comprise 30 and 85%
of the ingredients in NS seed (2,4). In
addition, NS seed contains various chemical compounds with
pharmacological properties, including thymoquinone, nigellone,
melanthin, damascenone, p-cymene and pinene (5–7). NS seed has
been traditionally used as a folk medicine in North Africa,
Southeast Asia and Mediterranean countries for many centuries.
Indeed, it is used for the treatment of asthma, bronchitis, cough,
fever and headaches (8). Previous
studies have demonstrated that NS seed has numerous therapeutic
activities, such as anti-inflammatory (9), antioxidant (5,10),
immune-modulatory (11),
cardioprotective (12,13) and hepatoprotective effects (14). However, the effects of NS seed on the
fatigue following exercise have not yet been fully elucidated.
Fatigue is defined as a feeling of extreme physical
or mental tiredness, resulting from severe stress and hard physical
or mental work (15,16). It may be associated with many
activities, such as exercise, aging, tumor growth, multiple
sclerosis and Parkinson's disease (17–19). In
particular, physical fatigue induced by strenuous exercise is
thought to lead to a deterioration in performance, causing a
decrease in muscular power and endurance, as well as in mental
functions (20,21). The underlying mechanism suggests that
strenuous exercise accumulates metabolic products (e.g., reactive
oxygen species (ROS), lipid peroxides and lactic acid), and leads
to oxidative stress, which can potentially contribute to fatigue
(22,23).
Accordingly, many studies have focused on the
development of drugs or therapies with respect to fatigue (24,25). In
addition, considering the limitations of available therapies for
fatigue in modern medicine, potential alternatives from traditional
medicine are worth investing because of their safety, availability,
and ease of administration (26). In
the current study, the authors sought to investigate the
anti-fatigue activities of NS seed extract in rats subjected to the
exhaustive swimming test as a fatigue model.
Materials and methods
Preparation of NS extract
NS seeds were obtained from a local market in
Mymensingh, Bangladesh. The extraction of NS seeds was performed as
previously described (27). Briefly,
the black seeds were washed with running tap water, air-dried and
ground to powder form (0.2–0.3 mm particle size, to ensure
homogeneity). A total of 800 g powdered seeds were extracted in a
Soxhlet apparatus with petroleum ether. The extract obtained was
evaporated to a viscous liquid at 40°C under reduced pressure
(yield of 300 ml, 310.7 g and 39.2%). Gas chromatographic analysis
of the final extract failed to identify significant amounts of
n-hexane and n-heptane (0.1%), which are the major
indicators for this distillate.
Animal models and NS seed extract
administration
All experimental protocols employed herein were
approved by the Committee on the Care of Laboratory Animal
Resources of Chonbuk National University and were conducted in
accordance with the Guide for the Care and Use of Laboratory
Animals published by the US National Institutes of Health
(Bethesda, MA, USA; NIH Publication no. 85–23, revised 1996). A
total of forty male Sprague-Dawley rats (220–250 g, Samtako Bio
Korea Co., Ltd., Daejeon, Korea) were used for all experiments.
Animals were housed in cages maintained at 23±2°C with 50±5%
humidity and subjected to a 12-h light/dark cycle. Food and water
was available ad libitum prior to exercise. The NS extract
was freshly dissolved in either distilled water and orally
administered using a gavage with a dose of 2 g/kg/day. In addition,
NS extract was administered on the 21st day, 1 h prior to the
initiation of swimming exercise. Distilled water was administered
for control group.
Exhaustive swimming
A pool for exhaustive swimming was designed
especially for rats. The system consisted of a glass chamber (70 cm
in height, 60 cm in length and 90 cm in width) filled with water to
a height of 55 cm. The pool was equipped with a heating system and
an air pumping system. To prevent floating during swimming, water
bubbles were produced by tubes connected to the air pumping system.
The temperature of the water within the glass chamber was
maintained at 36±1°C via a thermostatically controlled heater
located at the base of the chamber. The rats were individually
applied to forced-swimming until exhaustion, which was defined as
failure to rise to the surface of the water to breathe for 7 sec
(28).
Analysis of blood and serum
biochemical parameters
Blood was collected from the tail vein just before
swimming and from the caudal vena cava following swimming. A Nova
Stat Profile® pHOx Ultra Analyzer system (Nova
Biomedical, Waltham, MA, USA) was used to measure pH and to
quantify levels of partial pressure of carbon dioxide
(pCO2), the partial pressure of oxygen
(pO2) and oxygen saturation (O2sat).
In addition, the concentrations of glucose, lactate and hemoglobin
(Hb) were measured, along with hematocrit (Hct). Serum was
immediately separated by centrifugation at 1,500 × g for 10
min following swimming and stored at −80°C until biochemical
analysis. A Hitachi 7020 system (Hitachi Corporation, Tokyo, Japan)
was used for analyses of total protein (TP), albumin, lactic
dehydrogenase (LDH) and creatine kinase (CK) levels.
Analysis of glycogen contents in liver
and gastrocnemius muscle (g. muscle) tissues
The liver and g. muscle were cut, weighed and
homogenized in cold perchloric acid. The homogenate was centrifuged
for 15 min at 15,000 × g at 4°C. The supernatant was
carefully decanted. A standard glycogen (Sigma; St.Louis, MO, USA)
and tissue extract were mixed with iodine-potassium iodide reagent
for binding iodine to glycogen. The mixtures were measured by the
SpectraMax M3 ELISA reader (Molecular Devices, LLC, Sunnyvale, CA,
USA) at 460 nm wavelength.
Analysis of antioxidant defense
Levels of malondialdehyde (MDA) in serum, liver and
g. muscle were measured with an OXI-TEK TBARS assay kit (Enzo Life
Sciences Inc., Farmingdale, NY, USA). Reaction products were
quantitated by measuring the absorbance at 532 nm according to the
manufacturer's protocol. The levels of superoxide dismutase (SOD)
in the serum, liver and g. muscle were quantitated using a SOD
activity kit (cat. no. ADI-900-157, Enzo Life Sciences Inc.) by
measuring the absorbance of the reaction products at 450 nm. The
total glutathione (tGSH) and oxidized glutathione (GSSG) levels
were measured using a glutathione (total) detection kit (cat. no.
ADI-900-160) from Enzo Life Sciences Inc. by measuring the
absorbance of the reaction products at 405 nm. The concentration of
reduced glutathione (GSH) was calculated using the formula
(GSH=tGSH-GSSG), which was provided in the kit protocol. The redox
ratio was calculated using the formula GSH:GSSG=(tGSH-2GSSG)/GSSG,
as described previously (29).
Statistical analysis
All data are reported as mean ± standard error of
the mean. Statistical significance was analyzed using the Student's
t test or, where applicable, two-way analysis of variance
with Bonferroni post-hoc analysis for multiple group comparisons
using GraphPad Prism software (version, 5.03; GraphPad Software
Inc., La Jolla, CA, USA), and P<0.05 was considered
statistically significant.
Results
Effects of NS extract on the
exhaustive swimming on rats
To evaluate the effect of NS seed extract on
exercise durability, the exhaustive swimming test was performed
using rats, which were pre-treated with either distilled water as a
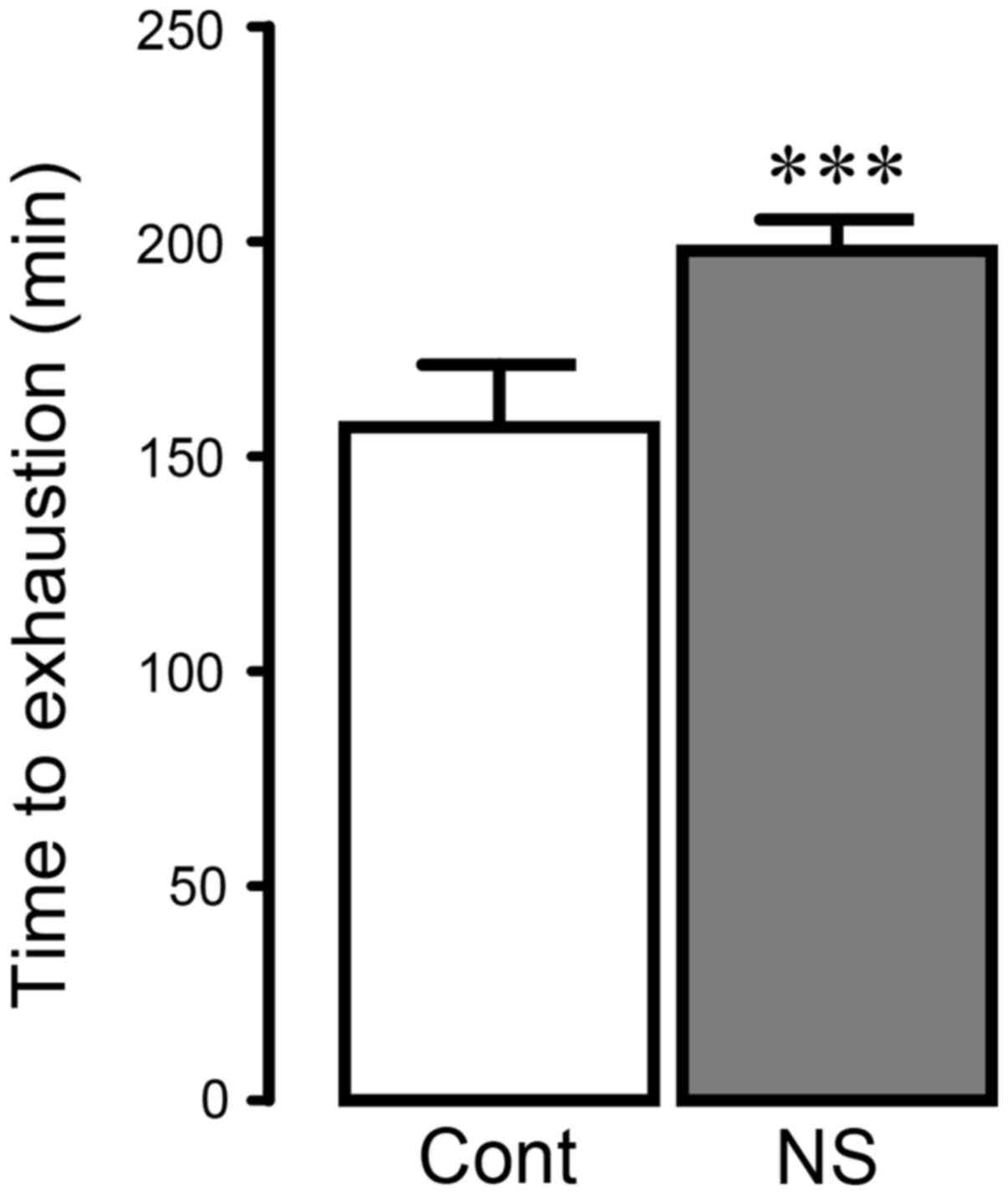
control or NS extracts for 21 days. As demonstrated in Fig. 1, the swimming time to exhaustion was
significantly increased in the NS-treated group compared with
control group (P<0.001; 24.6% increase vs. control). These data
indicated that NS seed extract may prolong the swimming time in
rats.
Effects of NS seed extract on
hemodynamic parameters following exhaustive swimming
Exhaustive swimming resulted in significant
decreases of pH (P<0.001), pO2 (P<0.001)
and O2sat levels (P<0.001), but an increase of
pCO2 level (P<0.001), when compared with the
pre-swimming control group. However, these changes were
significantly attenuated by pre-treatment with NS seed extract
compared with the post-swimming control group (P<0.01). Hb and
Hct levels were slightly increased (P>0.05) following swimming
in the control group. Notably, in pre-swimming and post-swimming of
NS-pre-treated groups, these values had a tendency to increase
compared with pre-swimming-control group, even though values in
post-swimming group with pre-treatment of NS extract were not
significantly changed compared with post-swimming control group
(P>0.05; Table I). Finally, TP was
markedly elevated in both control and NS-pre-treated groups
following exhaustive swimming, when compared with pre-swimming
control group. Moreover, there was no obvious difference in albumin
levels between control and NS-treated groups following exhaustive
swimming (Table I). These data
indicated that NS seed extract could maintain the blood homeostasis
following exhaustive swimming.
 | Table I.Effects of NS extract on blood
biochemical parameters after forced-swimming. |
Table I.
Effects of NS extract on blood
biochemical parameters after forced-swimming.
|
| Control | NS |
|---|
|
|
|
|
|---|
|
| Pre-swimming | Post-swimming | Pre-swimming | Post-swimming |
|---|
| pH | 7.30±0.03 |
7.09±0.03c | 7.30±0.02 |
7.13±0.03e |
| Hb (g/dl) | 12.5±0.5 |
13.0±0.4a |
15.5±0.4d | 15.9±0.4 |
| Hct (%) | 42±1 | 43±1a | 45±1d | 46±1 |
|
pCO2 (mmHg) | 44.8±2.5 |
78.7±4.9c | 42.0±2.4 |
68.2±3.0e |
|
pO2 (mmHg) | 58.6±3.0 |
24.7±1.7c |
67.9±2.9a |
37.9±2.78e |
| O2Sat
(%) | 81.6±2.2 |
33.0±4.9c | 85.3±3.5 |
46.4±3.9e |
| TP (g/dl) | 5.0±0.2 |
6.4±0.3b | 4.7±0.2 |
5.6±0.3d |
| Albumin (g/dl) | 2.9±0.2 | 3.1±0.2 | 2.6±0.1 | 3.0±0.1 |
Effects of NS seed extract on
fatigue-related serum biomarkers after exhaustive swimming
Fatigue can be evaluated by several important
biochemical indicators, including glucose, lactate, LDH and CK.
Exhaustive swimming caused depleted glucose, as an energy source,
and the accumulation of lactate, LDH and CK (30). Thus, the authors examined the levels of
fatigue-related blood biomarkers to test the anti-fatigue effect of
NS seed extract. The exhaustive swimming led to a significant
decrease in serum glucose level (from 127 to 103.2 mg/dl following
exercise; P<0.001) but significant increases in lactate (3.5 to
5.5 mmol/l; P<0.001), LDH (202.4 to 811.6 IU/l; P<0.001) and
CK (405.6 to 1288.4 IU/l; P<0.001) levels (Fig. 2) compared with the pre-swimming-control
group. However, pre-treatment with NS extract significantly
protected these exercise-induced alterations after exhaustive
swimming (Fig. 2). The serum glucose
level was significantly increased in the NS-treated group compared
with control group following exhaustive swimming (P<0.01).
Conversely, increased levels of serum lactate, LDH and CK, due to
exhaustive swimming, were significantly attenuated by pre-treatment
of NS extract (lactate, P<0.05; LDH and CK, P<0.001; Fig. 2). Taken together, the authors also
demonstrated the anti-fatigue effects of NS extract on exhaustive
swimming from the analysis of typical fatigue-related indicators
(e.g., glucose, lactate, LDH and CK).
 | Figure 2.Effects of NS extract on serum
biomarkers related to fatigue. Serum levels of (A) glucose, (B)
lactate, (C) LDH and (D) CK were measured following the collection
of blood just prior to and following swimming. The Cont group were
treated with distilled water, while the experimental group was
treated with NS extract. Data are expressed as the means ± standard
error of the mean (n=10 per group). *P<0.05, **P<0.01 and
***P<0.001, measured via Bonferroni post hoc test following
two-way analysis of variance vs. the pre-swimming Cont;
#P<0.05, ##P<0.01 and
###P<0.001, measured via Student's t-test
between post-swimming groups. NS, Nigella sativa; LDH,
lactate dehydrogenase; CK, creatine kinase; Cont, control group;
NS, NS-treated group. |
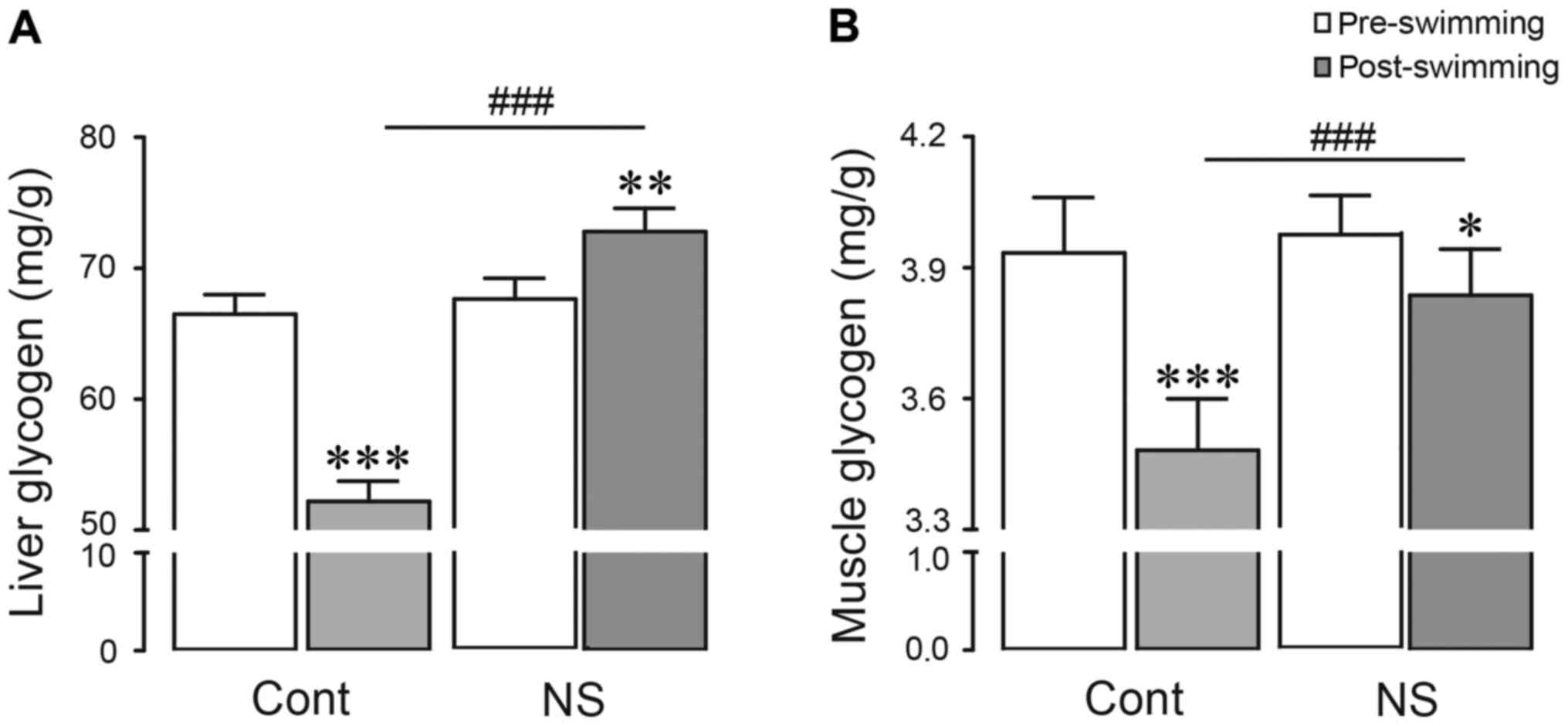
Effect of NS extract on glycogen
contents in liver and muscle
Glycogen content, which is main storage form of
glucose, is an integral determining factor in fatigue following
exhaustive swimming. Many studies have reported that glycogen is
significantly depleted in both liver and muscles during exhaustive
swimming (31). In the present study,
following exhaustive swimming, glycogen levels in liver and g.
muscle were significantly diminished compared with
pre-swimming-control group (21.5 and 11.5% decreases in liver and
g. muscle vs. control; P<0.001, respectively; Fig. 3). However, in NS extract-treated group,
glycogen content was restored in both liver and g. muscle (39.4 and
10.2% increases in liver and g. muscle vs. swimming-control;
P<0.001, respectively). In particular, the liver glycogen in
NS-pre-treated-swimming group was significantly increased compared
with control group (9.5% increase vs. control; P<0.05; Fig. 3A). These results demonstrated that NS
extract administration may reserve glycogen in liver and g.
muscle.
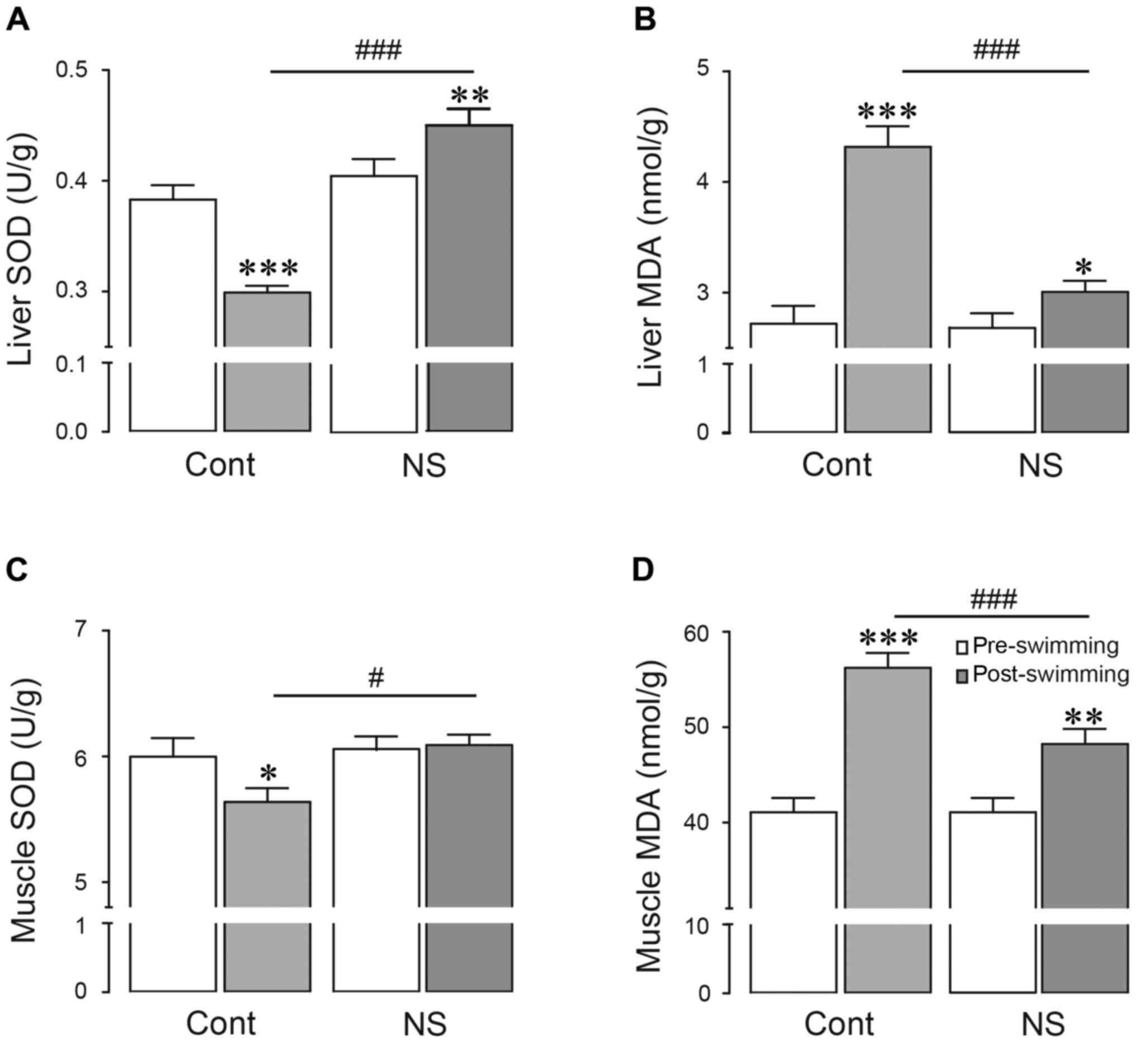
Effects of NS seed extract on
antioxidant parameters in serum, liver and g. muscle
Oxidative stress occurs following exhaustive
swimming, and subsequently may lead to pathology and clinical
symptoms of fatigue (32). Therefore,
the authors investigated the antioxidant activity of NS extract
following exhaustive swimming by examining the SOD, MDA and serum
redox ratio (GSH/GSSG) values as typical oxidative stress related
parameters. Serum SOD level was significantly decreased in the
swimming control-group, when compared with the control group (4.9%
decrease vs. control; P<0.05). As expected, in the NS
extract-pre-treated group, serum SOD level was significantly
increased compared with the swimming control-group following
exhaustive swimming (5.8% increase vs. swimming-control; P<0.01;
Fig. 4A). Serum MDA level was
dramatically increased following exhaustive swimming (28.1%
increase vs. control; P<0.001). However, pre-treatment with NS
extract inhibited the serum MDA level following exhaustive swimming
(15.8% decrease vs. swimming-control; P<0.001; Fig. 4B). Similarly, the serum redox ratio
(GSH:GSSG) was significantly decreased in the swimming-control
group, when compared with the control group (10.8% decrease vs.
control; P<0.05), but was increased in the exercise-NS
extract-treated group (39.1% increase vs. swimming-control;
P<0.001; Fig. 4C).
 | Figure 4.Effects of NS extract on serum
antioxidant parameters. Serum levels of (A) SOD and (B) MDA were
measured by enzyme-linked immunosorbent assay after collecting
blood just prior to swimming or following swimming. Tissues were
treated with distilled water (control) or NS extract. (C) The redox
ratio (GSH/GSSG) was calculated following measuring serum GSH and
GSSG. Data are expressed as the means ± standard error of the mean
(n=10 per group). *P<0.05, **P<0.01 and ***P<0.001,
measured via Bonferroni post hoc test following two-way analysis of
variance vs. the pre-swimming Cont.; #P<0.05,
##P<0.01 and ###P<0.001, measured via
Student's t-test between post-swimming groups. NS,
Nigella sativa; SOD, superoxide dismutase; MDA,
malondialdehyde; GSH, reduced glutathione; GSSG, oxidized
glutathione; Cont, control group; NS, NS-treated group. |
In addition, SOD levels in liver and g. muscle were
significantly decreased compared with the control group following
exhaustive swimming (21.9 and 6.0% decreases in liver and g. muscle
vs. control; P<0.001, respectively; Fig. 5A and C). Notably, SOD levels in both
tissues were significantly increased by pre-treatment of NS extract
compared with the swimming-control-group (50.5 and 8.0% increases
in liver and g. muscle vs. swimming-control; P<0.001,
respectively; Fig. 5A and C). MDA
levels in liver and g. muscle were significantly increased by
pre-treatment of NS extract compared with the control group
following exhaustive swimming (58.7 and 36.9% increases, in liver
and g. muscle vs. control; P<0.001, respectively). However, when
the NS extract was pre-treated, MDA levels in both tissues were
significantly inhibited compared with exercise-control group (30.4
and 14.2% decreases in liver and g. muscle vs. swimming-control;
P<0.001, respectively; Fig. 5B and
D). These data suggested that NS extract may have a beneficial
role against oxidative stress to alleviate physical fatigue
following exercise.
Discussion
NS seeds possess many pharmacological activities,
and are especially well known for their potent antioxidant effects.
Many previous studies have reported that NS seeds may reduce
toxicity in a number of diseases including diabetes, neural
disease, renal disease, cardiovascular disease and cancer, due to
their antioxidant activities from both in vitro and in
vivo approaches (6,33–36).
Therefore, in the present study, the authors focused on determining
the protective effects of NS seeds against exhaustive
swimming-induced fatigue.
To evaluate the anti-fatigue effects of NS extract
in rats, the authors performed the exhaustive swimming, which has
been commonly used as a fatigue model (37). The results demonstrated that
pre-treatment of NS extract significantly increased the swimming
time to exhaustion (Fig. 1). Further,
in hemodynamic parameters, NS extract may attenuate decreased
values of pO2 and O2sat and decrease
values of pCO2, resulting in impaired oxygen
supply during fatigue (Table I). Thus,
the presented results indicated that NS extract may improve the
swimming capacity against the fatigue.
Main causes of fatigue after exhaustive swimming are
the depletion of energy sources and dysregulation of anti-oxidant
defenses system (30). Regarding the
depletion of energy sources, many studies have been demonstrated
that energy sources, such as glucose and glycogen are depleted
during exhaustive swimming, which in turn cause to physical fatigue
(38). Here, it was observed that
glycogen contents in both the liver and g. muscle were restored
upon administration of NS extract (Figs.
2A and 5). In addition, under
normal conditions, ATP, as an energy source, is produced by
glycolysis (conversion of glycogen into glucose), which is, in
turn, broken down into pyruvate (39).
However, the muscles obtain energy from anaerobic glycolysis
(conversion of pyruvate to lactate) during intense exercise. This
intense exercise leads to accumulation of lactate, and lactate then
drops the blood and muscle pH, and consequently, fatigue is
occurred (40). The results
demonstrated that levels of serum glucose, lactate and LDH (key
enzyme for lactate production) were increased following exhaustive
swimming. Notably, these increases were dramatically attenuated
when NS extract pre-treated (Fig.
2B-D). In addition, it was demonstrated that glycogen contents
in liver and g muscle tissues were dramatically preserved when
pre-treated NS extract following exhaustive swimming (Fig. 3). Therefore, the authors suggested that
NS extracts may contribute to fatigue retardation through
preservation of glycogen content and reduction of lactate
accumulation.
Secondly, the other important factor in fatigue is
dysregulation of the antioxidant defense system (30). Exhaustive swimming may release reactive
oxygen species (ROS) due to increased oxygen consumption, resulting
in the development of fatigue. Therefore, MDA (an oxidative
degradation product of cell membrane lipids) and SOD (major
component of anti-oxidant defense system) are considered to be
physiological markers relevant for fatigue. In the present study,
the results revealed that elevated MDA levels and decreased levels
of SOD, as well as the serum redox ratio (GSH:GSSG) in liver and g.
muscle tissues were completely reversed by pre-treatment of NS
extract (Fig. 4). These findings
suggested that anti-fatigue effect of NS extract is involved in the
modulation of oxidative stress following exhaustive swimming.
In conclusion, the current study demonstrated that
NS may be able to alleviate the physical fatigue by inhibiting
energy depletion and oxidative stress. The authors further propose
that NS may be a potential strategy for prevention and treatment of
physical fatigue.
Acknowledgements
The current research was supported by the research
funds of Korean Ministry of Science (grant no. 2011-0013872) and
the Brain Korea Plus program. The authors would like to thank
Proofreading Service Center of Chonbuk National University and
e-World Editing Ltd. for proofreading of the manuscript.
References
|
1
|
Hajhashemi V, Ghannadi A and Jafarabadi H:
Black cumin seed essential oil, as a potent analgesic and
antiinflammatory drug. Phytother Res. 18:195–199. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Amin B and Hosseinzadeh H: Black cumin
(Nigella sativa) and its active constituent, thymoquinone: An
overview on the analgesic and anti-inflammatory effects. Planta
Med. 82:8–16. 2016.PubMed/NCBI
|
|
3
|
Abdel-Fattah AM, Matsumoto K and Watanabe
H: Antinociceptive effects of Nigella sativa oil and its major
component, thymoquinone, in mice. Eur J Pharmacol. 400:89–97. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Houghton PJ, Zarka R, de las Heras B and
Hoult JR: Fixed oil of Nigella sativa and derived thymoquinone
inhibit eicosanoid generation in leukocytes and membrane lipid
peroxidation. Planta Med. 61:33–36. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Burits M and Bucar F: Antioxidant activity
of Nigella sativa essential oil. Phytother Res. 14:323–328. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Singh S, Das SS, Singh G, Schuff C, de
Lampasona MP and Catalán CA: Composition, in vitro antioxidant and
antimicrobial activities of essential oil and oleoresins obtained
from black cumin seeds (Nigella sativa L.). Biomed Res Int.
2014:9182092014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kacem R and Meraihi Z: Effects of
essential oil extracted from Nigella sativa (L.) seeds and its main
components on human neutrophil elastase activity. Yakugaku Zasshi.
126:301–305. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Woo CC, Kumar AP, Sethi G and Tan KH:
Thymoquinone: Potential cure for inflammatory disorders and cancer.
Biochem Pharmacol. 83:443–451. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ghannadi A, Hajhashemi V and Jafarabadi H:
An investigation of the analgesic and anti-inflammatory effects of
Nigella sativa seed polyphenols. J Med Food. 8:488–493. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cikman O, Ozkan A, Aras AB, Soylemez O,
Alkis H, Taysi S and Karaayvaz M: Radioprotective effects of
Nigella sativa oil against oxidative stress in liver tissue of rats
exposed to total head irradiation. J Invest Surg. 27:262–266. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Assayed ME: Radioprotective effects of
black seed (Nigella sativa) oil against hemopoietic damage and
immunosuppression in gamma-irradiated rats. Immunopharmacol
Immunotoxicol. 32:284–296. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ebru U, Burak U, Yusuf S, Reyhan B, Arif
K, Faruk TH, Emin M, Aydin K, Atilla II, Semsettin S and Kemal E:
Cardioprotective effects of Nigella sativa oil on cyclosporine
A-induced cardiotoxicity in rats. Basic Clin Pharmacol Toxicol.
103:574–580. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nagi MN and Mansour MA: Protective effect
of thymoquinone against doxorubicin-induced cardiotoxicity in rats:
A possible mechanism of protection. Pharmacol Res. 41:283–289.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mahmoud MR, El-Abhar HS and Saleh S: The
effect of Nigella sativa oil against the liver damage induced by
Schistosoma mansoni infection in mice. J Ethnopharmacol. 79:1–11.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Moriura T, Matsuda H and Kubo M:
Pharmacological study on Agkistrodon blomhoffii blomhoffii BOIE. V.
anti-fatigue effect of the 50% ethanol extract in acute
weight-loaded forced swimming-treated rats. Biol Pharm Bull.
19:62–66. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim KM, Yu KW, Kang DH, Koh JH, Hong BS
and Suh HJ: Anti-stress and anti-fatigue effects of fermented rice
bran. Biosci Biotechnol Biochem. 65:2294–2296. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cochrane GD, Rizvi S, Abrantes AM,
Crabtree B, Cahill J and Friedman JH: The association between
fatigue and apathy in patients with either Parkinson's disease or
multiple sclerosis. Parkinsonism Relat Disord. 21:1093–1095. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Theoharides TC: Atopic conditions in
search of pathogenesis and therapy. Clin Ther. 35:544–547. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kluger BM, Rakowski D, Christian M, Cedar
D, Wong B, Crawford J, Uveges K, Berk J, Abaca E, Corbin L and
Garvan C: Randomized, controlled trial of acupuncture for fatigue
in parkinson's disease. Mov Disord. 31:1027–1032. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hao G, Zhang C, Cao W and Hao J: Effects
of intragastric administration of five oyster components on
endurance exercise performance in mice. Pharm Biol. 52:723–728.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang XL, Ren F, Huang W, Ding RT, Zhou QS
and Liu XW: Anti-fatigue activity of extracts of stem bark from
Acanthopanax senticosus. Molecules. 16:28–37. 2011. View Article : Google Scholar
|
|
22
|
Alessio HM: Exercise-induced oxidative
stress. Med Sci Sports Exerc. 25:218–224. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee SP, Mar GY and Ng LT: Effects of
tocotrienol-rich fraction on exercise endurance capacity and
oxidative stress in forced swimming rats. Eur J Appl Physiol.
107:587–595. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Castro-Marrero J, Sáez-Francàs N, Santillo
D and Alegre J: Treatment and management of chronic fatigue
syndrome/myalgic encephalomyelitis: All roads lead to Rome. Br J
Pharmacol. 174:345–369. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Korsen M, Kunz R, Schminke U, Runge U,
Kohlmann T and Dressel A: Dalfampridine effects on cognition,
fatigue, and dexterity. Brain Behav. 7:e005592016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tharakan B, Dhanasekaran M and Manyam BV:
Antioxidant and DNA protecting properties of anti-fatigue herb
Trichopus zeylanicus. Phytother Res. 19:669–673. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Le PM, Benhaddou-Andaloussi A, Elimadi A,
Settaf A, Cherrah Y and Haddad PS: The petroleum ether extract of
Nigella sativa exerts lipid-lowering and insulin-sensitizing
actions in the rat. J Ethnopharmacol. 94:251–259. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Oh TW, Oh TW and Ohta F: Dose-dependent
effect of capsaicin on endurance capacity in rats. Br J Nutr.
90:515–520. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Behr J, Maier K, Degenkolb B, Krombach F
and Vogelmeier C: Antioxidative and clinical effects of high-dose
N-acetylcysteine in fibrosing alveolitis. Adjunctive therapy to
maintenance immunosuppression. Am J Respir Crit Care Med.
156:1897–1901. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Norheim KB, Jonsson G and Omdal R:
Biological mechanisms of chronic fatigue. Rheumatology (Oxford).
50:1009–1018. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shang H, Cao S, Wang J, Zheng H and
Putheti R: Glabridin from Chinese herb licorice inhibits fatigue in
mice. Afr J Tradit Complement Altern Med. 7:17–23. 2009.PubMed/NCBI
|
|
32
|
Wu C, Chen R, Wang XS, Shen B, Yue W and
Wu Q: Antioxidant and anti-fatigue activities of phenolic extract
from the seed coat of Euryale ferox Salisb. and identification of
three phenolic compounds by LC-ESI-MS/MS. Molecules.
18:11003–11021. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Adam GO, Rahman MM, Lee SJ, Kim GB, Kang
HS, Kim JS and Kim SJ: Hepatoprotective effects of Nigella sativa
seed extract against acetaminophen-induced oxidative stress. Asian
Pac J Trop Med. 9:221–227. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Magdy MA, Hanan el-A and Nabila el-M:
Thymoquinone: Novel gastroprotective mechanisms. Eur J Pharmacol.
697:126–131. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hosseinzadeh H, Parvardeh S, Asl MN,
Sadeghnia HR and Ziaee T: Effect of thymoquinone and Nigella sativa
seeds oil on lipid peroxidation level during global cerebral
ischemia-reperfusion injury in rat hippocampus. Phytomedicine.
14:621–627. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Al Wafai RJ: Nigella sativa and
thymoquinone suppress cyclooxygenase-2 and oxidative stress in
pancreatic tissue of streptozotocin-induced diabetic rats.
Pancreas. 42:841–849. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tang W, Zhang Y, Gao J, Ding X and Gao S:
The anti-fatigue effect of 20(R)-ginsenoside Rg3 in mice by
intranasally administration. Biol Pharm Bull. 31:2024–2027. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang L, Zhang HL, Lu R, Zhou YJ, Ma R, Lv
JQ, Li XL, Chen LJ and Yao Z: The decapeptide CMS001 enhances
swimming endurance in mice. Peptides. 29:1176–1182. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Westerblad H, Bruton JD and Katz A:
Skeletal muscle: Energy metabolism, fiber types, fatigue and
adaptability. Exp Cell Res. 316:3093–3099. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cairns SP: Lactic acid and exercise
performance: Culprit or friend? Sports Med. 36:279–291. 2006.
View Article : Google Scholar : PubMed/NCBI
|