Introduction
Lichen planus (LP) is a chronic inflammatory disease
that affects the squamous cell layer, including the skin and
mucosal membranes (1). Certain studies
revealed that ~1.9% of the population affected by LP presented with
LP confined to the oral cavity (termed oral LP; OLP) and women were
the susceptible population (63–70% of cases) (2). Degeneration of basal cells and
infiltration of inflammatory cells into the subepithelial layer of
connective tissue are regarded as the pathological characteristics
of LP/OLP (3).
The aetiology and pathogenesis of LP are not fully
understood, although it is widely agreed that immunological
response is important in the process (4). Previous studies indicated that the
subepithelial inflammatory infiltrate cells, which characterize
LP/OLP were predominantly composed of T cells that locally present
in the involved epithelium and under epithelial tissues (5). Most lymphocytes in the lamina propria are
CD4+ T cell clones with helper activity, or
CD4+ T cell clones that lack cytotoxic activity, while
the majority of intraepithelial lymphocytes in LP/OLP are
CD8+ T cells (6–9). Keratinocytes in LP/OLP submit major
histocompatibility complex class II antigens to CD4+ T
cells causing secretion of the cytokines, interleukin-2 (IL-2) and
interferon-γ (INF-γ) that are associated with type 1 T helper
cells. IL-2 and INF-γ in turn activate the CD8+ T cells
leading to keratinocyte apoptosis via tumor necrosis factor-α
(TNF-α) (10). TNF-α promotes the
activation of nuclear factor-κB in subepithelial T cells, and
causes increased expression of proinflammatory cytokines, such as
IL-6 (11–13).
IL-6 is a type of proinflammatory cytokine produced
in LP lesions that exerts effects on humoral and cellular
immunities. It promotes B cell differentiation, stimulates
immunoglobulin G secretion, T cell growth and differentiation, and
cytotoxic T cell differentiation (14). IL-6 is generated by activated
monocytes, macrophages, endothelial cells, and activated T and B
cells that respond to a variety of stimuli, including infection and
trauma (15).
Numerous studies have identified that the IL-6
concentration in the serum of LP patients was higher than that in
the healthy control subjects, particularly in the severe forms of
LP. Furthermore, serum IL-6 concentrations were identified to be
particularly valuable for monitoring disease activity and treatment
response (15,16). However, there is currently a lack of
systematic analysis between IL-6 and LP/OLP. Consequently, a
meta-analysis of all eligible studies was performed in the present
study to obtain a more precise analysis of the association between
IL-6 and LP/OLP.
Materials and methods
Study identification and
selection
To identify correlative literature, bibliographical
searches were performed in the China National Knowledge
Infrastructure (CNKI, http://www.cnki.net), PubMed (http://www.ncbi.nlm.nih.gov/pubmed) and Embase
(http://www.elsevier.com/solutions/embase-biomedical-research)
databases using the following terms: ‘Lichen planus’, ‘oral lichen
planus’ and ‘interleukin-6 (IL_6)’. The upper date was limited to
20 December 2015. The search was focused towards studies that had
been conducted in humans. Full-text articles published in English
and Chinese were included, and the most complete research and
recent study was selected when one author had published various
articles using the same patient data.
Inclusion criteria
The inclusion criteria of the current meta-analysis
were as follows: i) IL-6 and the pathology of LP or OLP were
evaluated; ii) case-control studies; iii) ELISA was used to
determine serum IL-6 levels; iv) contained sufficient data.
Data extraction
The studies were reviewed by two investigators,
disagreement around eligibility were resolved by discussion between
the two investigators. The following details were obtained from
each article: First author's name, publication date, ethnicity,
sample numbers of cases and control subjects, and the IL-6
expression level in each group (mean ± standard deviation; SD).
Statistical analysis
Mean differences (MDs) and SDs were used to
summarize data with continuous outcomes. MDs with 95% confidence
intervals (CIs) were used to determine the strength of the
association between IL-6 and LP/OLP. The pooled MDs for the LP/OLP
associated with IL-6 were calculated. Subgroup analyses were
performed by ethnicity. Heterogeneity assumptions were assessed
using the I2 test and an I2
value <50% indicated a lack of heterogeneity among the studies.
Therefore, the pooled odds ratio estimate of each study was
calculated using the fixed-effects model (17) or the random-effects model was used
(18). All statistical analyses for
the current study were performed using RevMan 5.3 software
(Cochrane Collaboration; http://www.cochrane-net.org/revman).
Results
Study characteristics
A total of eight studies involving 299 LP/OLP cases
and 231 control subjects met the inclusion criteria and were
analyzed (12,13,19–24). The authors and year of publication,
country, ethnicity of the study populations, and subtypes of OLP
and LP were included. The number of cases and control subjects in
each study are presented in Table I.
Of the eight publications, five comparisons were performed in Asian
individuals (13,20,22–24) and three were in Caucasian individuals
(19,12,21). One
study was performed among LP patients (21), five were performed in OLP patients
(12,20,22–24), while two were performed in mixed LP
disease (the patients that have both skin and oral mucosal lesions)
(13,19). Of all the OLP patients, four studies
referred to erosive OLP (EOLP) and none EOLP (NEOLP) (13,22–24), one study referred to EOLP (20) and one to NEOLP (12).
 | Table I.Characteristics of the eight
case-control studies included in the current meta-analysis. |
Table I.
Characteristics of the eight
case-control studies included in the current meta-analysis.
|
|
|
|
|
| Age (year) |
|
|
|
|---|
|
|
|
|
|
|
|
|
|
|
|---|
| Author, year | Country study was
performed in | Ethnicity of
population | Disease | Type | Case | Control | Cases/controls
(n) | Analysis
Method | (Refs.) |
|---|
| Abdel-Haq,
2014 | Poland | Caucasian | LP, OLP | – | 52±15 | 41±13 | 56/56 | ELISA | (19) |
| Zhang, 2008 | China | Asian | LP, OLP | Mixed LP | 54±17 | 40±16 | 30/30 | ELISA | (13) |
| Gu, 2004 | America | Caucasian | OLP | NEOLP | 61.4±9.0 | 62.5±8.8 | 10/30 | ELISA | (12) |
| Goel, 2015 | India | Asian | OLP | EOLP | 46.95±11.96 | – | 42/10 | ELISA | (20) |
| Toruniowa,
1995 | Poland | Caucasian | LP | – | – | – | 20/14 | ELISA | (21) |
| Zhang, 2004 | China | Asian | OLP | Mixed OLP | 40±10 | 39±11 | 60/30 | ELISA | (22) |
| Wang, 2014 | China | Asian | OLP | Mixed OLP | 45.8±13.4 | 44.8±12.8 | 50/50 | ELISA | (23) |
| Su, 2014 | China | Asian | OLP | Mixed OLP | – | – | 31/31 | ELISA | (24) |
Meta-analysis
The heterogeneity for the eight comparisons were
analyzed (Fig. 1). The I2
value was 99% (P<0.00001), indicating the presence of
heterogeneity. Therefore, the random-effects model was selected to
synthesize the data and the MD was 16.24 (95% CI=9.84–22.64). The
outcomes indicate that the serum IL-6 concentrations were
statistically higher in the LP patients when compared with those in
the healthy group.
Subgroup analysis
Subgroup analyses were performed according to
ethnicity (Fig. 2). A significantly
increased level of IL-6 (MD=26.48, 95% CI=11.73–41.23; P=0.0004)
was identified among Asian individuals, while no significant
increase among Caucasian individuals was observed (MD=1.01, 95%
CI=−0.78–2.79; P=0.27).
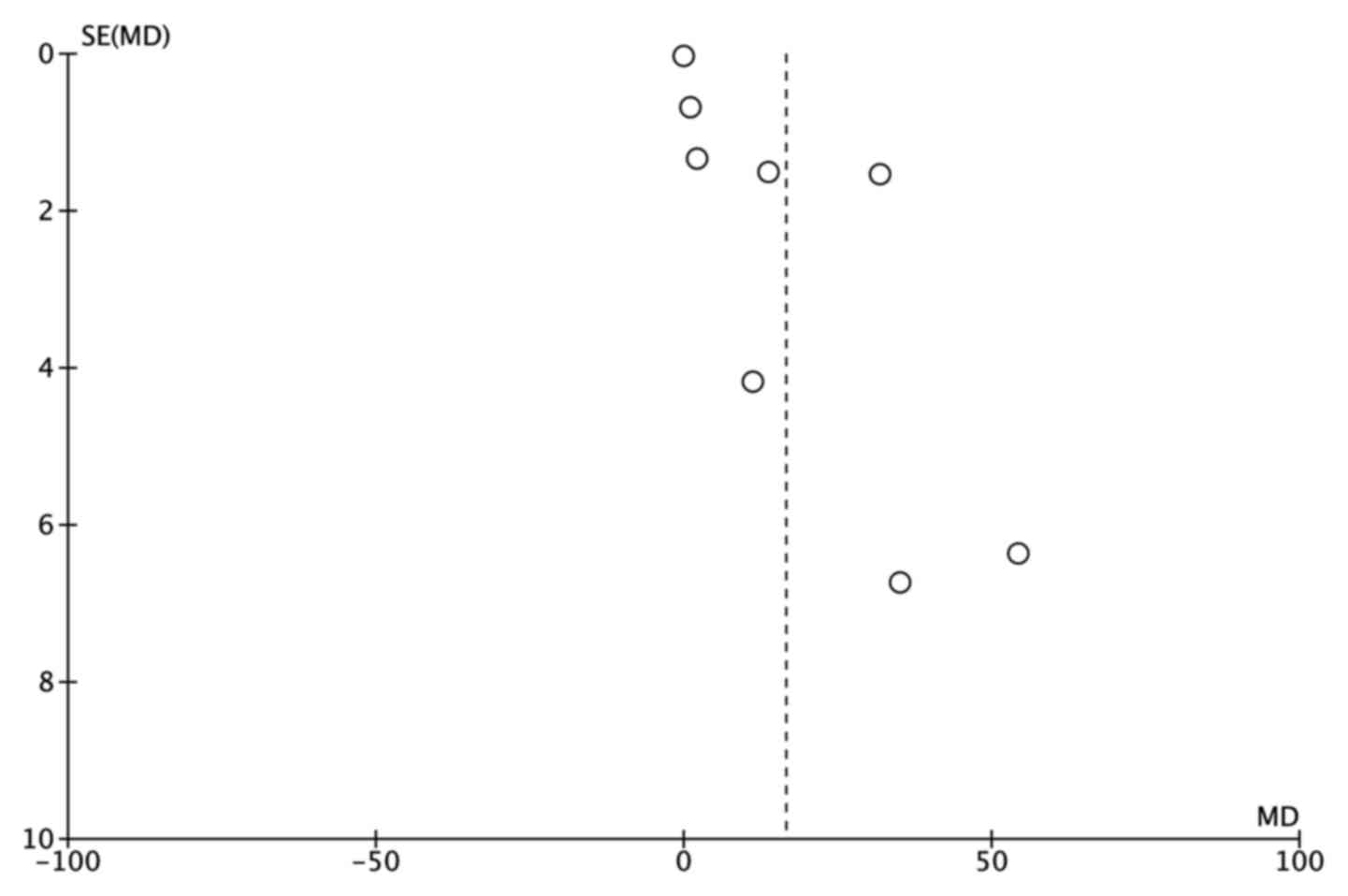
Publication bias
A funnel plot was constructed and Egger's test was
performed to access the publication bias of the studies. The funnel
plots (Fig. 3) demonstrated marked
asymmetry. These results indicate that there was publication bias
in the current meta-analysis.
Discussion
IL-6 is a type of cytokine that has multiple
functions. Numerous types of cell, including macrophage cells,
neutrophils and fibroblasts synthesize IL-6 in response to
stimulation, such as infection and trauma (25,26). IL-6
cell signals are sent through a receptor expressed in various types
of cells that stimulate numerous biological progresses, including
antibody and autoantibody production, T cell activation, B cell
differentiation and acute-phase protein augmentation (27,28). As a
result of the IL-6 versatility, the differences in synthesizing and
releasing IL-6 may regulate the susceptibility, development, and
progression of autoimmune and inflammatory diseases due to the
individual variability (29–32). According to a previous study, many
common oral diseases, such as periodontal diseases, LP and oral
cancer are associated with IL-6 deregulation (33).
LP is a chronic inflammatory mucocutaneous disorder
with unknown etiology (34). Clinical
and immunohistochemical studies strongly support the role of immune
deregulation in the pathogenesis of LP. Dysregulation specifically
involves the cellular immune system and a complex cytokine network
(35–37). Cytokines are a type of low-weight
soluble protein produced by different cells in the innate and
adaptive immune system. They transmit signals through receptors on
the cell surface to active, differentiated or maturate immune
cells, and are important in the activation and modulation of the
immune system (38). Therefore, levels
of ILs in lesions, saliva and serum of LP patients are considered
to be a credible indicator of therapeutic reactions on a molecular
basis (15,36,39).
Certain studies identified an increased serum IL-6
level in LP patients when compared with control groups (24,40,41). In addition, a decreased serum IL-6
concentration was identified in LP patients subsequent to therapy
(36). Furthermore, it has been found
that higher salivary IL-6 levels may primarily be due to
keratinocytes, tissue-infiltrating mononuclear cells and
fibroblasts of LP lesions. The elevated serum IL-6 concentration
may primarily due to peripheral blood mononuclear cells (PBMCs) and
endothelial cells. The locally secreted IL-6 may diffuse into blood
capillaries or be drained into the lymphatic vessels, which finally
empty into the blood circulation. Together, the locally secreted
IL-6 and the systemically produced IL-6 by PBMCs and endothelial
cells are the possible reason for the elevated serum IL-6
concentration in LP patients (15).
In the current study, a distinct association between
the concentrations of IL-6 and LP was identified during the total
combined analysis. These results indicated that the IL-6 serum
levels were significantly higher in patients with LP/OLP than in
the control group patients. When stratified according to ethnicity,
the Asian patients demonstrated significantly increased serum
levels of IL-6, while no significant increase was observed among
the Caucasian individuals.
There were certain limitations of the present
meta-analysis. Firstly, although the publications were carefully
searched, definite criteria were set, and strict data extraction
and analysis were performed to minimize the likelihood of
heterogeneity, it existed in almost every comparison. Only
published studies were included in the current analysis, while the
negative or non-significant findings were ignored. This may have
contributed to the publication bias. However, the limited number of
studies included in the investigation together with the small
sample sizes may have resulted in an insufficient ability to
evaluate a statistically significant effect. Furthermore, subgroup
analysis eliminated age, gender, and other factors due to the
impossibility of extracting relevant data from primary
publications. In addition, certain associated studies may have been
neglected, as only publications written in Chinese and English were
selected from the chosen databases. Consequently, the outcome of
the current meta-analysis should be explicated carefully. Future
studies that incorporate age, gender, and other factors may provide
further information for confirming the underlying mechanism of IL-6
in LP.
Despite the above-mentioned limitations, the current
study indicates that IL-6 is associated with the pathogenesis of
LP. The concentration level of IL-6 was increased in LP patients
from the Asian population, although no significant associations
were observed in Caucasian populations. Large sample studies using
standardized, unbiased methods and well-matched controls are
required in the future.
Acknowledgements
The present study was supported by the Natural
Science Foundation of Shandong Province (grant no. ZR2015HM019) and
the Science and Technology Development Plan of Shandong Province
(grant no. 2010GSF10270).
References
|
1
|
Carrozzo M, de Uboldi Capei M, Dametto E,
Fasano ME, Arduino P, Broccoletti R, Vezza D, Rendine S, Curtoni ES
and Gandolfo S: Tumor necrosis factor-alpha and interferon-gamma
polymorphisms contribute to susceptibility to oral lichen planus. J
Invest Dermatol. 122:87–94. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Silverman S Jr and Bahl S: Oral lichen
planus update: Clinical characteristics, treatment response, and
malignant transformation. Am J Dent. 10:259–263. 1997.PubMed/NCBI
|
|
3
|
Boyd AS and Nelder KH: Lichen planus. J Am
Acad Dermatol. 25:593–619. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Payeras MR, Cherubini K, Figueiredo MA and
Salum FG: Oral lichen planus: Focus on etiopathogenesis. J Oral
Biol. 58:1057–1069. 2013. View Article : Google Scholar
|
|
5
|
Lodi G, Scully C, Carrozzo M, Griffiths M,
Sugerman PB and Thongprasom K: Current controversies in oral lichen
planus: Report of an international consensus meeting. Part 2.
Clinical management and malignant transformation. Oral Surg Oral
Med Oral Pathol Oral Radiol Endod. 100:164–178. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Matthews JB, Scully CM and Potts AJ: Oral
lichen planus: An immunoperoxidase study using
monoclonal-antibodies to lymphocyte subsets. Br J Dermatol.
111:587–595. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ishii T: Immunohistochemical demonstration
of T cell subsets and accessory cells in oral lichen planus. J Oral
Pathol. 16:356–361. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kilpi AM, Rich AM, Radden BG and Reade PC:
Direct immumofluorescence in the diagnosis of oral mucosal
diseases. Int J Oral Maxillofac Surg. 17:6–10. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sugerman PB, Savage NW and Seymour GJ:
Phenotype and suppressor activity of T-lymphocyte clones extracted
from lesions of oral lichen planus. Br J Dermatol. 131:319–324.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Roopashree MR, Gondhalekar RV, Shashikanth
MC, George J, Thippeawamy SH and Shukla A: Pathogenesis of oral
lichen planus-a review. J Oral Pathol Med. 39:729–734. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sugerman PB, Satterwhite K and Bigby M:
Autocytotoxic T-cell clones in lichen planus. Br J Dermatol.
142:449–456. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gu GM, Martin MD, Darveau RP, Truelove E
and Epstein J: Oral and serum IL-6 levels in oral lichen planus
patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod.
98:637–678. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang Y, Lin M, Zhang S, Wang Z, Jiang L,
Shen J, Bai J, Gao F, Zhou M and Chen Q: NF-kappaB-dependent
cytokines in saliva and serum from patients with oral lichen
planus: A study in an ethnic Chinese population. Cytokine.
41:144–149. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hibi M, Nakajima K and Hirano T: IL-6
cytokine family and signal transduction: A model of the cytokine
system. J Mol Med (Berl). 74:1–12. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun A, Chia JS, Chang YF and Chiang CP:
Serum interleukin-6 level is a useful marker in evaluating
therapeutic effects of levamisole and Chinese medicinal herbs on
patients with oral lichen planus. J Oral Pathol Med. 31:196–203.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rhodus NL, Cheng B, Bowles W, Myers S,
Miller L and Ondery F: Proinflammatory cytokine levels in saliva
before and after treatment of (erosive) oral lichen planus with
dexamethasone. Oral Dis. 12:112–116. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mantel N and Haenszel W: Statistical
aspects of the analysis of data from retrospective studies of
disease. J Natl Cancer Inst. 22:719–748. 1959.PubMed/NCBI
|
|
18
|
DerSimonian R and Laird N: Meta-analysis
in clinical trials. Contemp Clin Trials. 45:139–145. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Abdel-Haq A, Kusnierz-Cabala B, Darczuk D,
Sobuta E, Dumnicka P, Wojas-Pelc A and Chomyszyn-Gajewska M:
Interleukin-6 and neopterin levels in the serum and saliva of
patients with lichen planus and oral lichen planus. J Oral Pathol
Med. 43:734–739. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Goel S, Marwah A, Kaushik S, Garg VK and
Gupta S: Role of serum interleukin-6 in deciding therapy for
multidrug resistant oral lichen planus. J Clin Exp Dent.
7:e477–e482. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Toruniowa B, Krasowska D, Kozioł M,
Ksiazek A and Pietrzak A: Serum levels of IL-6 in Mycosis
Fungoides, Psoriasis, and lichen planus. Ann N Y Acad Sci.
762:432–434. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang Z, Zhang Q, Wang Y and Zhang W:
Correlations between serum levels of interleukin-2, interleukin-4,
interleukin-6 and clinical sympotoms in patients with oral lichen
planus. J Dent Prev Treat. 12:96–98. 2004.(In Chinese).
|
|
23
|
Wang YM, Fu S, Zhou B, Wang C and Zhou J:
Analysis of Th1/Th2 in the use of hormone therapy in patients with
OLP when T cell response patterns and correlation. Chin J Front Med
Sci (Electronic Version). 6:30–33. 2014.(In Chinese).
|
|
24
|
Kui SU and Hong Xu: The effects of
thymopeptides enteric-coated tablets on oral lichen planus and the
expression of related cytokines. J Pract Stomatol. 30:429–431.
2014.
|
|
25
|
Kishimoto T, Akira S, Narazaki M and Taga
T: Interleukin-6 family of cytokines and Gp130. Blood.
86:1243–1254. 1995.PubMed/NCBI
|
|
26
|
Matsuki Y, Yamamoto T and Hara K:
Detection of inflammatory cytokine messenger-RNA (MRNA)-expressing
cells in human inflamed gingiva by combined in situ hybridization
and immunohistochemistry. Immunology. 76:42–47. 1992.PubMed/NCBI
|
|
27
|
Hirano T, Matsuda T, Turner M, Miyasaka N,
Buchan G, Tang B, Sato K, Shimizu M, Maini R, Feldmann M, et al:
Excessive production of interleukin 6⁄B cell stimulatory factor-2
in rheumatoid arthritis. Eur J Immunol. 18:1797–1801. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ridker PM, Cushman M, Stampfer MJ, Tracy
RP and Hennekens CH: Inflammation, aspirin, and the risk of
cardiovascular disease in apparently healthy men. N Engl J Med.
336:973–979. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Moreau P, Harousseau JL, Wijdenes J,
Morineau N, Milpied N and Bataille R: A combination of
anti-interleukin 6 murine monoclonal antibody with dexamethasone
and high-dose melphalan induces high complete response rates in
advanced multiple myeloma. Br J Haematol. 109:661–664. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Park MC, Lee SW, Choi ST, Park YB and Lee
SK: Serum leptin levels correlate with interleukin-6 levels and
disease activity in patients with ankylosing spondylitis. Scand J
Rheumatol. 36:101–106. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Packard RR and Libby P: Inflammation in
atherosclerosis: From vascular biology to biomarker discovery and
risk prediction. Clin Chem. 54:24–38. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nishimoto N: Interleukin-6 as a
therapeutic target in candidate inflammatory diseases. Clin
Pharmacol Ther. 87:483–487. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nibali L, Fedele S, D'Aiuto F and Donos N:
Interleukin-6 in oral diseases: A review. Oral Dis. 18:236–243.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Agha-Hosseini F, Mirzaii-Dizgah I, Mikaili
S and Abdollahi M: Increased salivary lipid peroxidation in human
subjects with oral lichen planus. Int J Dent Hyg. 7:246–250. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hu JY, Zhang J, Cui JL, Liang XY, Lu R, Du
GF, Xu XY and Zhou G: Increasing CCL5/CCR5 on CD4+ T cells in
peripheral blood of oral lichen planus. Cytokine. 62:141–145. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Agha-Hosseini F and Mirzaii-Dizgah I: p53
as a neoplastic biomarker in patients with erosive and plaque like
forms of oral lichen planus. J Contemp Dent Pract. 14:1–3. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Agha-Hosseini F, Mirzaii-Dizgah I,
Farmanbar N and Abdollahi M: Oxidative stress status and DNA damage
in saliva of human subjects with oral lichen planus and oral
squamous cell carcinoma. J Oral Pathol Med. 41:736–740. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Postal M and Appenzeller S: The role of
Tumor Necrosis Factor-alpha (TNF-α) in the pathogenesis of systemic
lupus erythematosus. Cytokine. 56:537–543. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nibali L, Fedele S, D'Aiuto F and Donos N:
Interleukin-6 in oral disease: A review. Oral Dis. 18:236–243.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yamamoto T, Yoneda K, Ueta E and Osaki T:
Serum cytokines, interleukin-2 receptor, and soluble intercellular
adhesion molecule-1 in oral disorders. Oral Surg Oral Med Oral
Pathol. 78:727–735. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Karagouni EE, Dotsika EN and Sklavounou A:
Alteration in peripheral blood mononuclear cell function and serum
cytokines in oral ichen planus. J Oral Pathol Med. 23:28–35. 1994.
View Article : Google Scholar : PubMed/NCBI
|