Introduction
Inflammatory responses elicit a host defense to
external stimuli, including bacterial contamination. Invasion of
pathogens triggers sequential innate immune responses. The main
immune cells that act in the innate immune response include
neutrophils, dendritic cells and macrophages, whose inflammatory
properties involve phagocytic action, antigen presentation, and
production of inflammatory mediators (1). In particular, macrophages initiate and
maintain inflammation via the production of inflammatory mediators,
including nitric oxide (NO), prostaglandin E2
(PGE2), and proinflammatory cytokines (2). An adequate degree of inflammation rescues
the human body from infectious diseases; however, the tight
regulation of inflammatory responses is important, as excessive
inflammatory responses cause severe inflammatory diseases, such as
inflammatory bowel disease, atherosclerosis and rheumatoid
arthritis (3). Therefore, identifying
candidate molecules possessing anti-inflammatory properties is a
valuable strategy for the treatment of severe inflammatory
states.
Nuclear factor-κB (NF-κB) and activator protein 1
(AP-1) transcription factors are major signaling molecules involved
in the regulation of inflammatory mediators (4). NF-κB is a pivotal regulator of
inflammation that controls expression of proinflammatory genes
(5). AP-1, a heterodimeric protein
consisting of members of the Jun and Fos families of DNA-binding
proteins, activates cellular processes, such as inflammation,
proliferation and apoptosis, by binding to the promoter regions of
various genes (6). Thus, AP-1 proteins
are recognized as regulators of cytokine expression and important
modulators of inflammatory diseases.
Many traditional medicines have been used for the
treatment of various inflammatory diseases. Of them, the rhizome of
Anemarrhena asphodeloides Bunge (Asparagaceae) is widely
administered as traditional medicine in China, Korea and Japan for
the treatment of inflammatory disorders, including fever, coughs,
allergies, diabetes, and Alzheimer's disease (7,8). Numerous
previous studies support the use of A. asphodeloides for the
treatment of various inflammatory disorders. Lee Mo Tang, a mixture
of A. asphodeloides and Fritillaria cirrhosa, was
reported to exhibit anti-asthmatic effects via the inhibition of
ovalbumin-induced eosinophil accumulation and Th2-mediated
bronchial hyperresponsiveness in a murine model of asthma (9). Zi Shen Pill, another agent containing
A. asphodeloides, exerted effects on benign prostatic
hyperplasia via inhibition of vascular endothelial growth factor
and basic fibroblast growth factor expression in rats (10). Furthermore, methanol extracts of A.
asphodeloides were reported to inhibit the binding activity of
leukotriene B4 on human neutrophils (11). Certain active components of A.
asphodeloides, including anemarsaponin B, broussonin B,
mangiferin, and timosaponin AIII, have also been reported to
exhibit anti-inflammatory effects in various experimental models,
such as models of learning and memory deficits, ear edema and
colitis mouse models (12–14).
Despite its traditional uses and many studies on the
anti-inflammatory effects of its active components, to the best of
our knowledge, there are no studies regarding the molecular
mechanisms of action of ethanol extracts of A. asphodeloides
(EAA). In the current study, the anti-inflammatory effects of EAA
and mechanisms of action in lipopolysaccharide (LPS)-stimulated
murine macrophage cells were investigated.
Materials and methods
EAA preparation
A 95% ethanol extract (code no. PBC345AS) of A.
asphodeloides was obtained from the Korea Plant Extract Bank
(KPEB Daejeon, South Korea). The concentrated ethanol extract was
manufactured according to the manufacturer's instructions. Briefly,
the rhizomes of A. asphodeloides were dried at room
temperature (RT), treated with ethanol (GR grade), and sonicated
numerous times at 45°C. The extracts were filtrated to remove solid
substances and concentrated with reduced pressure at 45°C. A stock
solution (200 mg/ml) of the extract was prepared in Sigma-Aldrich
dimethoxysulfoxide (DMSO; Merck KGaA, Darmstadt, Germany) and this
was stored at −20°C before use.
Cell culture and reagents
RAW 264.7 macrophages, a mouse monocytic cell line
and HEK 293 cells, a human embryonic kidney cell line, were
purchased from ATCC (Manassas, VA, USA) and maintained in
Dulbecco's modified Eagle's medium (GE Healthcare Bio-Sciences,
Pittsburgh, PA, USA) containing 10% fetal bovine serum (GE
Healthcare Bio-Sciences), 50 U/ml penicillin, and 50 µg/ml
streptomycin (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) at 37°C in a humidified atmosphere containing 5%
CO2. Rabbit anti-inhibitor of κBα (IκBα; cat. no.
sc-371) and mouse anti-α-tubulin (cat. no. sc-5286) antibodies were
purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
Rabbit anti-inducible NO synthase (iNOS; cat. no. 2982),
anti-cyclooxygenase-2 (COX-2) (cat. no. 4842), anti-p-IκBα
(Ser32/36; cat. no. 9246), anti-p38 (cat. no. 9212), anti-p-p38
(Thr180/Tyr182; cat. no. 9211), anti-extracellular signal-regulated
kinase (ERK; cat. no. 9102), anti-c-Jun N-terminal kinase (JNK;
9252), anti-p-JNK (Thr183/Tyr185; cat. no. 9251) and mouse
anti-p-ERK (Thr202/Tyr204; cat. no. 9106) were purchased from Cell
Signaling Technology, Inc. (Danvers, MA, USA). Goat anti-rabbit
immunoglobulin G (IgG; cat. no. LF-SA8002) and goat anti-mouse IgG
(cat. no. LF-SA8001) secondary antibodies were purchased from
AbFrontier (Young In Frontier Co., Ltd., Seoul, South Korea).
Ez-cytox solution was purchased from Daeil Lab (Seoul, South
Korea). Ready-SET-Go! ELISA kits for the detection of interleukin
(IL)-6 and tumor necrosis factor (TNF)-α were obtained from
Ebioscience, Inc. (Thermo Fisher Scientific, Inc.).
Measurement of cell viability
RAW 264.7 macrophages were pretreated with EAA (10,
20, 50, 100 and 200 µg/ml) for 2 h and further incubated for 24 h
at 37°C in the absence or presence of LPS (1 µg/ml). Following
incubation, Ez-cytox solution (one-tenth of the culture medium) was
added to each well and incubated for 1 h at 37°C. Supernatants were
transferred to fresh 96-well plates and the absorbance was measured
at a wavelength of 450 nm using a Synergy H1 Microplate Reader.
Measurement of NO production
NO production in activated macrophages was measured
according to the method described in our previous study (15). Briefly, cells were seeded in 96-well
plates (4.0×104 cells/well) and incubated at 37°C
overnight. Cells were pretreated with various concentrations of EAA
(10, 20, 50 and 100 µg/ml) for 2 h prior to LPS treatment.
Following stimulation with LPS (1 µg/ml) for 24 h, the supernatants
(100 µl) were transferred to fresh 96-well plates and 100 µl Griess
reagent (1% sulfanilamide, 0.1% N-1-naphthylenediamine
dihydrochloride and 2.5% phosphoric acid) was added to each well.
Nitrite standard solution (NaNO2; 2.5, 5, 10, 25, 50 and
100 µM) was used to generate a standard curve for calculating the
quantity of NO in the supernatants. The absorbance was measured at
a wavelength of 540 nm using a Synergy H1 Microplate Reader (BioTek
Instruments, Inc., Winooski, VT, USA).
Enzyme-linked immunosorbent assay
(ELISA)
Cells were seeded in 96-well plates
(4.0×104 cells/well) and incubated at 37°C overnight.
Cells were pretreated with various concentrations of EAA (10, 20,
50 and 100 µg/ml) for 2 h prior to LPS treatment. Subsequent to
stimulation with LPS (1 µg/ml) for 24 h, the culture supernatants
were collected and diluted according to a predetermined dilution
rate for each proinflammatory cytokine. The production of
proinflammatory cytokines, including IL-6 and TNF-α, was measured
using Ready-SET-Go! ELISA kits for each type of cytokine, according
to the manufacturer's instructions. Briefly, 96-well plates were
coated with coating solution overnight at 4°C, washed with 1X
phosphate-buffered saline (PBS)/Tween-20 (PBST) three times, and
treated with 1X Assay Diluent for 1 h at RT. The wells were emptied
and diluted supernatant and standard solutions were added to each
well. Following incubation (2 h) at RT, the plates were washed with
1X PBST three times and detection antibody solution diluted in 1X
Assay Diluent was added to each well. The plates was washed after 1
h of treatment, horseradish peroxidase (HRP)-streptavidin solution
was added for 30 min and the plates were washed five times with 1X
PBST. Tetramethylbenzidine solution was subsequently added to the
plates and they were incubated for 10 min in the dark. Additional 1
N H3PO4 was added to the wells to terminate
the reaction and the absorbance in each well was measured using a
Synergy H1 Microplate Reader at a wavelength of 450 nm.
Luciferase reporter assays
HEK 293 cells were seeded into a 100-mm dish (70%
confluence on the day of transfection) and transfected with
pNF-κB-luc or pAP-1-luc cis-reporter plasmids (Agilent
Technologies, Inc., Santa Clara, CA, USA), which contains the NF-κB
or AP-1 promoters, respectively [gWIZ-green fluorescent protein
(GFP; Aldevron, Fargo, ND, USA) served as an internal control for
transfection efficiency]. Transfected cells were divided into
12-well plates, incubated at 37°C overnight, and treated with
various concentrations of EAA (10, 20, 50 and 100 µg/ml) in the
presence of phorbol-12-myristate-13-acetate (PMA). After 24 h of
incubation at 37°C, cells were lysed using cell culture lysis
reagent (Promega Corporation, Madison, WI, USA) and luciferase
activity was measured using luciferin as a substrate. The GFP
expression levels were determined using the fluorescence change of
excitation at 485 nm and emission at 525 nm.
Preparation of total cell lysates
RAW 264.7 macrophages pretreated with EAA were
further stimulated with LPS (1 µg/ml) for the optimal duration for
detecting target proteins [IκBα and transforming growth factor
β-activated kinase 1 (TAK1) for 3 min; mitogen-activated protein
kinases (MAPKs) for 15 min; and iNOS and COX-2 for 24 h]. Following
stimulation for the indicated durations, cells were washed three
times with ice-cold PBS. Lysis buffer (0.5% NP-40, 0.5% Triton
X-100, 150 mM NaCl, 20 mM Tris-HCl (pH 8.0), 1 mM
ethylenediaminetetraacetic acid, 1% glycerol, 1 mM
phenylmethylsulfonyl fluoride, 10 mM NaF and 1 mM
Na3VO4) was added to the washed wells and
collected into microtubes after 10 min. Following centrifugation at
15,814 × g for 30 min at 4°C, the supernatants were prepared in
fresh microtubes.
Immunoblot analysis
After boiling the mixture of lysates and sample
buffers, aliquots of the samples (20 µg) were separated by 10%
sodium dodecyl sulfate-polyacrylamide gel electrophoresis and
transferred to nitrocellulose membranes with transfer buffer [192
mM glycine, 25 mM Tris-HCl (pH 8.8), and 20% methanol (v/v)] at 100
V for 1 h. After blocking with 5% non-fat dried milk, each membrane
was incubated overnight at 4°C with the primary antibodies
(dilution, 1:1,000). Each membrane was then incubated for an
additional 1 h with the secondary peroxidase-conjugated IgG
antibodies (1:5,000). Subsequent to washing five times with 1X
PBST, the target proteins were detected using enhanced
chemiluminescence. Protein levels were quantified by scanning of
the immunoblots and analysis with LabWorks software 4.6 (UVP, LLC,
Upland, CA, USA).
Statistical analysis
The data are represented as the mean ± standard
error of the mean (SEM). Comparisons between multiple experimental
groups were performed using one-way analysis of variance followed
by Dunnett's post-hoc test using GraphPad Prism 3.0 (GraphPad
Software, Inc., San Diego, CA, USA). P<0.05 was considered to
indicate a statistically significant difference. The data from nine
replicates were analyzed, including three independent experiments
with three replicates in each.
Results
Inhibitory effects of EAA on
LPS-induced NO production and expression levels of iNOS and
COX-2
As the inhibitory effect of an anti-inflammatory
agent should be assessed at concentrations that do not exhibit
cytotoxicity, the maximal non-cytotoxic concentration was
determined with a cell viability assay. EAA did not exhibit
cytotoxicity in concentrations up to 100 µg/ml. However,
significant cytotoxicity was observed at 200 µg/ml (Fig. 1A). Thereafter, concentrations of ≤100
µg/ml EAA were used throughout the current study. To evaluate the
anti-inflammatory properties of EAA, the effect of EAA on the
production of NO, a proinflammatory mediator, was measured in
LPS-treated RAW 264.7 cells. As shown in Fig. 1B, EAA inhibited LPS-induced NO
production in a dose-dependent manner. The results indicate that
EAA inhibits LPS-induced NO production in macrophages.
 | Figure 1.Effects of EAA on the production of
inflammatory mediators. RAW 264.7 macrophages were pre-treated with
EAA (10, 20, 50, 100 and 200 µg/ml) for 2 h, and were incubated in
the presence or absence of LPS (1 µg/ml) for an additional 24 h.
(A) Cell viability was determined using EZ-Cytox solution. Cell
viability data are presented as the mean ± SEM and analyzed using
one-way ANOVA. *P<0.01 vs. LPS-untreated or -treated control
groups. (B) NO levels in the supernatants were measured using
Griess reagent. NO levels were calculated with a standard curve
using nitrite standard solution (NaNo2). The relative
production of NO in the EAA-treated compared with the LPS-treated
group is presented as the mean ± SEM and analyzed using one-way
ANOVA. *P<0.001 vs. LPS-untreated control groups.
aP<0.05 and bP<0.01 vs. LPS-treated
groups. Western blot analysis was performed with total cell lysates
and the expression levels of (C) iNOS and (D) COX-2 were detected
using specific antibodies. The results were normalized to tubulin
(loading control). The relative expression of iNOS in the
EAA-treated compared with the LPS-treated group is presented as the
mean ± SEM and analyzed using one-way ANOVA. *P<0.001 vs.
LPS-untreated control groups. aP<0.05,
bP<0.01, and cP<0.001 vs. LPS-treated
groups. EAA, ethanol extracts of the rhizome of A.
asphodeloides; LPS, lipopolysaccharide; SEM, standard error of
the mean; ANOVA, analysis of variance; NO, nitric oxide; iNOS,
inducible NO synthase; COX-2, cyclooxygenase-2. |
Subsequently, the effect of EAA on the protein
expression levels of iNOS, an NO synthesizing enzyme, and COX-2, an
enzyme responsible for the production of PGE2, was
evaluated in order to investigate the transcriptional regulation of
proinflammatory mediators. As shown in Fig. 1C and D, the immunoblot analysis with
iNOS- and COX-2-specific antibodies revealed an inhibitory effect
of EAA on LPS-induced iNOS and COX-2 expression levels in a
dose-dependent manner. These data indicate that NO and
PGE2 production are tightly regulated at the protein
level by EAA.
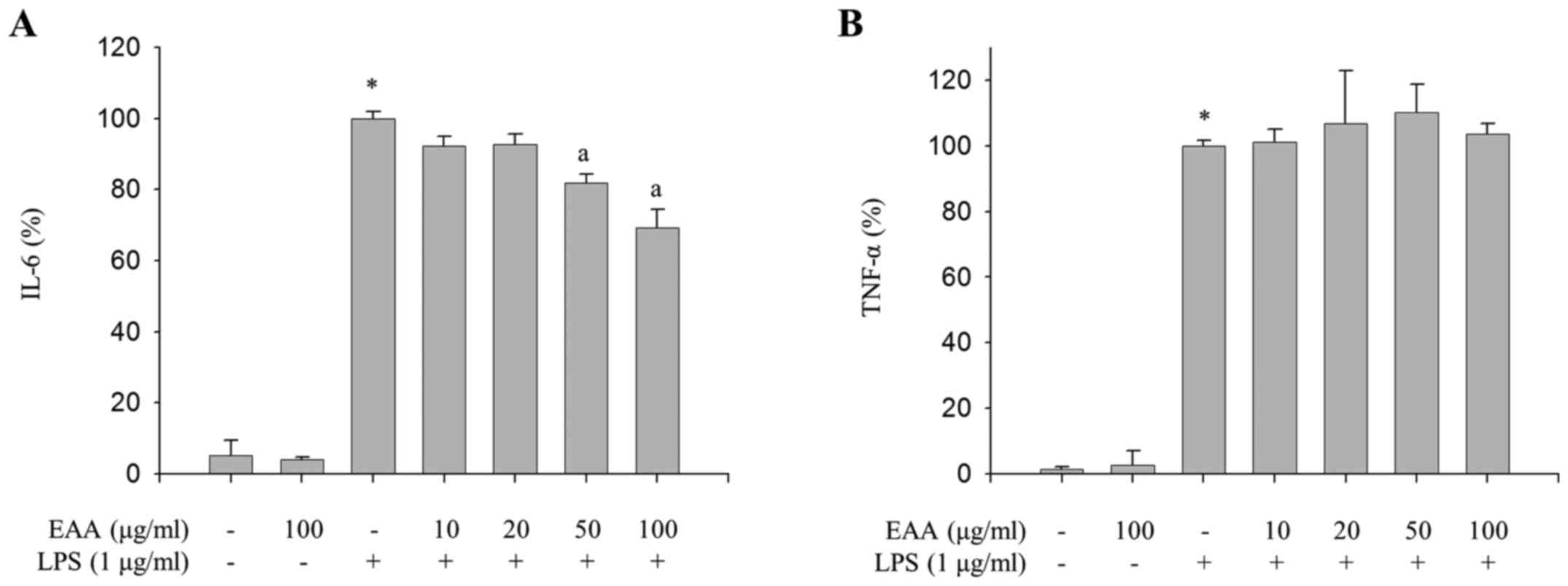
Inhibitory effects of EAA on
LPS-induced production of IL-6
As the excessive production of inflammatory
mediators, including IL-1β, IL-6 and TNF-α, as well as NO and
PGE2, in macrophages is accompanied by severe
inflammation (16), the effect of EAA
on the production of proinflammatory cytokines in LPS-treated RAW
264.7 macrophages were measured to investigate the additional
anti-inflammatory properties of EAA. As presented in Fig. 2A, EAA inhibited LPS-induced production
of IL-6. However, LPS-induced TNF-α production was not alleviated
by EAA (Fig. 2B). These results
indicate that EAA inhibits the production of IL-6, whereas TNF-α
production is not regulated by EAA in LPS-treated macrophages.
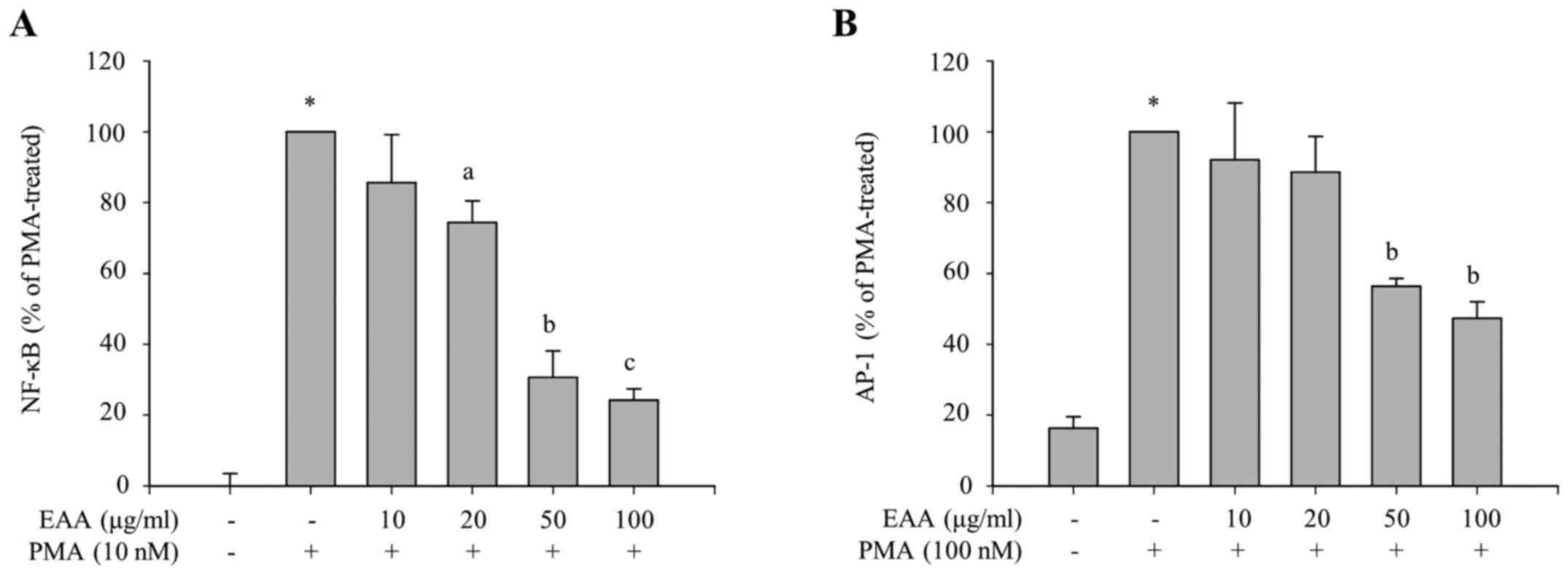
Selective inhibition of the NF-κB and
p38 pathways by EAA
To identify which transcription factors are involved
in the inhibitory effects of EAA on the production of
proinflammatory mediators, the transcriptional activities of NF-κB
and AP-1, major transcription factors in the inflammatory
responses, were measured using luciferase reporter assays. HEK 293
cells were treated with various concentrations of EAA in the
presence of PMA, which induces transcriptional activation of NF-κB-
or AP-1-dependent genes. The luciferase reporter gene is placed
under the control of NF-κB or AP-1 transcriptional activity. As
presented in Fig. 3A and B,
PMA-induced luciferase activities, which are regulated by NF-κB or
AP-1 transcription factor, respectively, were reduced by EAA
treatment. This indicates that EAA exerts anti-inflammatory
responses via the inhibition of NF-κB and AP-1 transcriptional
activities.
LPS-induced NF-κB activation in macrophages is
predominantly mediated by IκBα phosphorylation at Ser-32/36,
followed by IκBα degradation and the translocation of released
cytoplasmic p50 and p65 complex to the nucleus (17). Phosphorylation in the activation loops
of MAPKs leads to their activation, which results in
transcriptional activation of AP-1, a transcription factor that
binds to the promoter regions of inflammatory mediator genes
(4). EAA-mediated IκBα phosphorylation
levels in LPS-treated RAW 264.7 cells were measured to assess
whether EAA regulates NF-κB activation. The p-IκBα levels were
reduced and the IκBα levels were increased by EAA treatment in a
dose-dependent manner (Fig. 4A),
indicating that EAA inhibits IκBα phosphorylation at Ser-32/36 and,
thus, reduces degradation of IκBα. Subsequently, the
phosphorylation levels in the activation loops of three MAPKs (ERK,
JNK and p38) were measured by EAA in LPS-treated RAW 264.7 cells.
As shown in Fig. 4B, EAA selectively
inhibits the phosphorylation of p38 without altering the total p38
levels, whereas ERK and JNK phosphorylation was not regulated by
EAA. These results indicate that EAA exhibits its anti-inflammatory
properties in macrophages by inhibiting the activation of the major
inflammatory signaling pathways, NF-κB and p38.
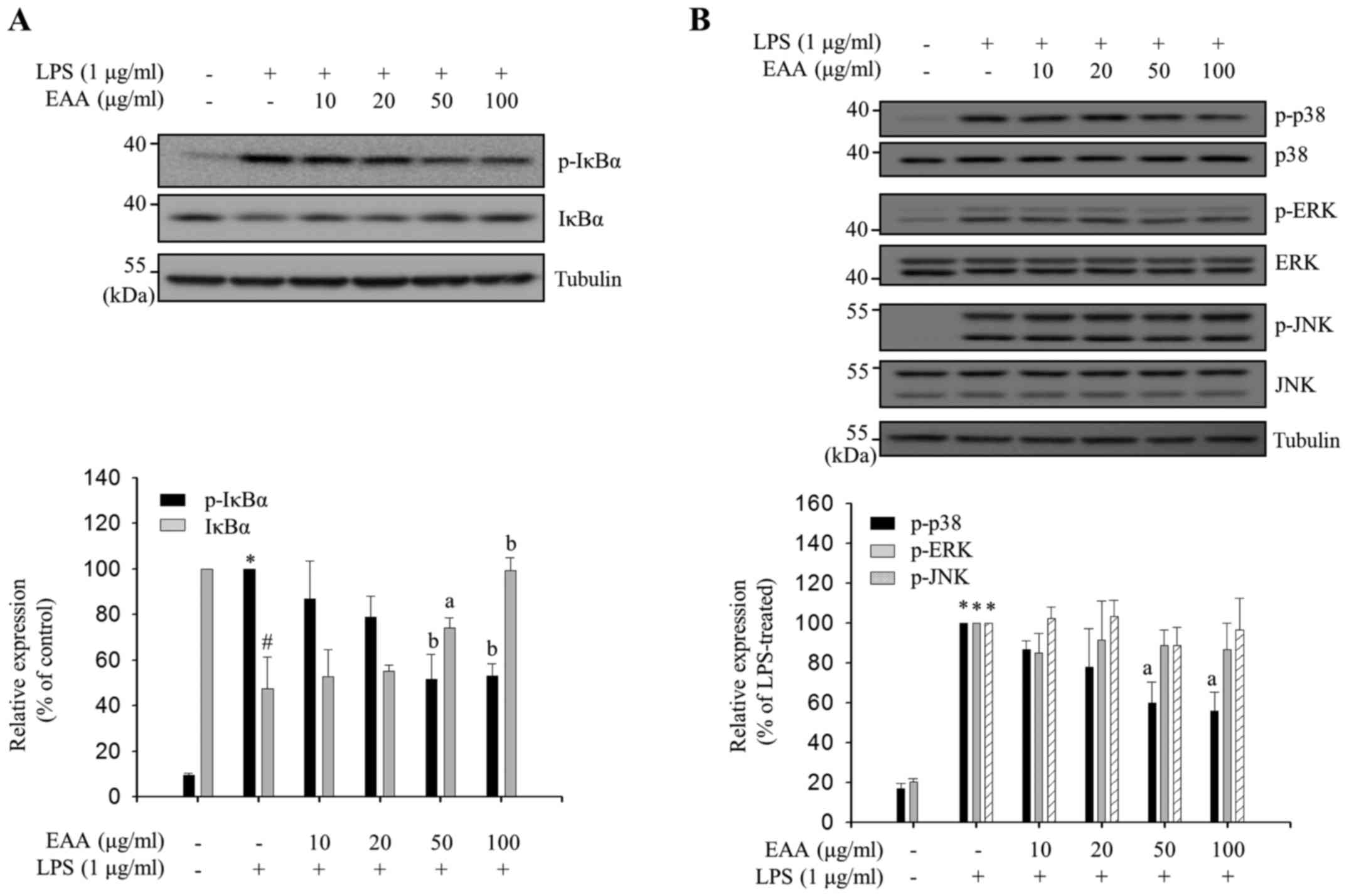 | Figure 4.Inhibitory effects of EAA on
inflammatory signaling pathways. RAW 264.7 macrophages were
pre-treated with EAA (10, 20, 50 and 100 µg/ml) for 2 h, and
incubated with LPS (1 µg/ml) for the indicated times. Total cell
lysates were prepared following LPS stimulation for (A) 3 and (B)
15 min. Western blot analysis was performed using the appropriate
antibodies. The expression levels and phosphorylation levels of
IκBα, p38, ERK and JNK were measured using enhanced
chemiluminescence solutions. The results are presented following
normalization to endogenous tubulin level. the relative IκBα level
compared with the non-treated group and p-IκBα, p-p38, p-ERK and
p-JNK levels compared with the LPS-treated group are presented as
the mean ± standard error of the mean. Data were analyzed using
one-way analysis of variance. #P<0.01 and *P<0.001
vs. LPS-untreated control groups. aP<0.05 and
bP<0.01 vs. LPS-treated groups. EAA, ethanol extracts
of the rhizome of A. asphodeloides; LPS, lipopolysaccharide;
IκBα, inhibitor of κBα; ERK, extracellular regulated kinase; JNK,
c-Jun N-terminal kinase; p, phosphorylated. |
Discussion
iNOS triggers its effector molecule, NO, which is a
free radical that is synthesized from l-arginine and results in
cellular damage at sites of inflammation (18). COX-2 catalyzes the production of
PGE2 from the lipid arachidonic acid, which ultimately
induces inflammation and fever (19).
However, improper upregulation of iNOS and COX-2 by proinflammatory
stimuli has been associated with the pathophysiology of certain
types of inflammatory disorders, including sepsis and arthritis
(20). Numerous studies have
demonstrated that natural products that inhibit iNOS and COX-2
expression levels could be valuable phytomedicines for the
treatment of severe inflammatory states (21–23). In the
present study, it was demonstrated that EAA attenuates LPS-induced
iNOS and COX-2 expression levels in RAW264.7 macrophages without
causing cytotoxic effects (Fig. 1).
These results indicate that EAA is a promising candidate for an
anti-inflammatory phytomedicine.
In addition to iNOS and COX-2, activated macrophages
produce pro-inflammatory cytokines, such as TNF-α, IL-1β and IL-6
(24). These proinflammatory cytokines
stimulate an increase in blood flow and permeability of
capillaries, lead to infiltration of immune cells and cause
inflammatory responses (24). Notably,
EAA reduced the production of IL-6, but not TNF-α, in LPS-induced
RAW264.7 macrophages (Fig. 2),
indicating that EAA may suppress LPS-induced inflammation by
selectively inhibiting IL-6 expression levels. IL-6 has a vital
role in the earliest stages of inflammation; when its activation
persists, acute inflammation becomes chronic inflammation (25). Furthermore, IL-6 is an endogenous
mediator of LPS-induced fever (26).
Therefore, selective inhibition of IL-6 production by EAA may
alleviate the acute response to LPS, thereby reducing LPS-induced
inflammation.
In the current study, EAA suppressed LPS-induced
phosphorylation of IκBα and p38 (Fig.
4), although it did not affect ERK and JNK phosphorylation.
Selective regulation of major inflammatory signal transductions by
EAA may be accomplished by targeting individual kinases upstream of
NF-κB and MAPK. LPS binding to toll-like receptor 4 (TLR4) on the
surface of macrophages leads to the recruitment of accessory
molecules and subsequent activation of TAK1, an upstream kinase
that regulates NF-κB and MAPK signal transduction (27). Following TAK1 activation, NF-κB is
regulated by IκB kinases (IKKs), and each MAPK is activated by its
specific upstream kinase (27).
Therefore, EAA may selectively regulate NF-κB and p38 or their
specific upstream kinases, IKKs and MKK3/6, respectively, but not
TAK1 or TLR4 accessory molecules.
In conclusion, the current results indicate that EAA
may serve as a potent anti-inflammatory phytomedicine via the
inhibition of NF-κB and p38, and subsequent production of
inflammatory mediators, including iNOS, COX-2 and IL-6. Although
the current study clarified the anti-inflammatory effects of EAA
and its underlying mechanisms of action in murine macrophages,
further studies using experimental animal models of inflammation
are required to provide further support of EAA as a valuable
candidate for the treatment of severe inflammatory states.
Acknowledgements
The present study was supported by the National
Research Foundation of Korea (NRF) grant funded by the Ministry of
Education, Science and Technology (NRF-2016R1A6A3A11931134).
References
|
1
|
Nowarski R, Gagliani N, Huber S and
Flavell RA: Innate immune cells in inflammation and cancer. Cancer
Immunol Res. 1:77–84. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wu LC, Fan NC, Lin MH, Chu IR, Huang SJ,
Hu CY and Han SY: Anti-inflammatory effect of spilanthol from
Spilanthes acmella on murine macrophage by down-regulating
LPS-induced inflammatory mediators. J Agric Food Chem.
56:2341–2349. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cutolo M: Macrophages as effectors of the
immunoendocrinologic interactions in autoimmune rheumatic diseases.
Ann N Y Acad Sci. 876:32–42. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Adcock IM: Transcription factors as
activators of gene transcription: AP-1 and NF-kappa B. Monaldi Arch
Chest Dis. 52:178–186. 1997.PubMed/NCBI
|
|
5
|
Karin M and Ben-Neriah Y: Phosphorylation
meets ubiquitination: The control of NF-[kappa]B activity. Annu Rev
Immunol. 18:621–663. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wisdom R: AP-1: One switch for many
signals. Exp Cell Res. 253:180–185. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Park SY, Choi YH and Lee W: Dangnyohwan
improves glucose utilization and reduces insulin resistance by
increasing the adipocyte-specific GLUT4 expression in Otsuka
Long-Evans Tokushima Fatty rats. J Ethnopharmacol. 115:473–482.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang Y, Dan Y, Yang D, Hu Y, Zhang L,
Zhang C, Zhu H, Cui Z, Li M and Liu Y: The genus Anemarrhena Bunge:
A review on ethnopharmacology, phytochemistry and pharmacology. J
Ethnopharmacol. 153:42–60. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yeum HS, Lee YC, Kim SH, Roh SS, Lee JC
and Seo YB: Fritillaria cirrhosa, Anemarrhena asphodeloides,
Lee-Mo-Tang and cyclosporine a inhibit ovalbumin-induced eosinophil
accumulation and Th2-mediated bronchial hyperresponsiveness in a
murine model of asthma. Basic Clin Pharmacol Toxicol. 100:205–213.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun H, Li TJ, Sun LN, Qiu Y, Huang BB, Yi
B and Chen WS: Inhibitory effect of traditional Chinese medicine
Zi-Shen Pill on benign prostatic hyperplasia in rats. J
Ethnopharmacol. 115:203–208. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee HJ and Ryu JH: Hinokiresinol: A novel
inhibitor of LTB4 binding to the human neutrophils. Planta Med.
65:3911999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Garcia D, Delgado R, Ubeira FM and Leiro
J: Modulation of rat macrophage function by the Mangifera indica L.
extracts Vimang and mangiferin. Int Immunopharmacol. 2:797–806.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim JY, Shin JS, Ryu JH, Kim SY, Cho YW,
Choi JH and Lee KT: Anti-inflammatory effect of anemarsaponin B
isolated from the rhizomes of Anemarrhena asphodeloides in
LPS-induced RAW 264.7 macrophages is mediated by negative
regulation of the nuclear factor-kappaB and p38 pathways. Food Chem
Toxicol. 47:1610–1617. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lu WQ, Qiu Y, Li TJ, Tao X, Sun LN and
Chen WS: Timosaponin B-II inhibits pro-inflammatory cytokine
induction by lipopolysaccharide in BV2 cells. Arch Pharm Res.
32:1301–1308. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cho YC, Ju A, Kim BR and Cho S:
Anti-inflammatory effects of Crataeva nurvala Buch. Ham. are
mediated via inactivation of ERK but not NF-κB. J Ethnopharmacol.
162:140–147. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Feghali CA and Wright TM: Cytokines in
acute and chronic inflammation. Front Biosci. 2:d12–d26. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Karin M and Delhase M: The I kappa B
kinase (IKK) and NF-kappa B: Key elements of proinflammatory
signalling. Semin Immunol. 12:85–98. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Connelly L, Palacios-Callender M, Ameixa
C, Moncada S and Hobbs AJ: Biphasic regulation of NF-kappa B
activity underlies the pro- and anti-inflammatory actions of nitric
oxide. J Immunol. 166:3873–3881. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cuccurullo C, Mezzetti A and Cipollone F:
COX-2 and the vasculature: Angel or evil? Curr Hypertens Rep.
9:73–80. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Asehnoune K, Strassheim D, Mitra S, Kim JY
and Abraham E: Involvement of reactive oxygen species in Toll-like
receptor 4-dependent activation of NF-kappa B. J Immunol.
172:2522–2529. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Camacho-Barquero L, Villegas I,
Sánchez-Calvo JM, Talero E, Sánchez-Fidalgo S, Motilva V and
Alarcón de la Lastra C: Curcumin, a Curcuma longa constituent, acts
on MAPK p38 pathway modulating COX-2 and iNOS expression in chronic
experimental colitis. Int Immunopharmacol. 7:333–342. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Garcia-Mediavilla V, Crespo I, Collado PS,
Esteller A, Sánchez-Campos S, Tuñón MJ and González-Gallego J: The
anti-inflammatory flavones quercetin and kaempferol cause
inhibition of inducible nitric oxide synthase, cyclooxygenase-2 and
reactive C-protein, and down-regulation of the nuclear factor
kappaB pathway in Chang Liver cells. Eur J Pharmacol. 557:221–229.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tiwari M, Dwivedi UN and Kakkar P:
Tinospora cordifolia extract modulates COX-2, iNOS, ICAM-1,
pro-inflammatory cytokines and redox status in murine model of
asthma. J Ethnopharmacol. 153:326–337. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sweet MJ and Hume DA: Endotoxin signal
transduction in macrophages. J Leukoc Biol. 60:8–26.
1996.PubMed/NCBI
|
|
25
|
Gabay C: Interleukin-6 and chronic
inflammation. Arthritis Res Ther. 8 Suppl 2:S32006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
LeMay LG, Vander AJ and Kluger MJ: The
effects of pentoxifylline on lipopolysaccharide (LPS) fever, plasma
interleukin 6 (IL 6) and tumor necrosis factor (TNF) in the rat.
Cytokine. 2:300–306. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kawai T and Akira S: The role of
pattern-recognition receptors in innate immunity: Update on
Toll-like receptors. Nat Immunol. 11:373–384. 2010. View Article : Google Scholar : PubMed/NCBI
|