Introduction
Spontaneous urticaria (SU) is a common skin
condition characterized by the recurrent appearance of pruritic
wheals, occasionally accompanied by angioedema (1). Analysis of skin biopsies from SU patients
has demonstrated that most inflammatory cells surrounding the small
venules include CD4+ T cells, neutrophils, mast cells,
basophils and eosinophils (2,3). In particular, the release of histamine
and other proinflammatory mediators, due to aberrant activation of
mast cells, is the key pathophysiological event for urticaria
(4). However, the mechanistic insight
into mast cells degranulation remains poorly understood (5).
Recent studies have indicated that Th9 cells, a
subset of T-helper cells, serve a key role in mast cell
accumulation and activation during allergic inflammation (6,7), and are a
major source of interleukin-9 (IL-9). PU.1 (also known as SPI1) is
the key transcription factor through which Th9 cells regulate IL-9
production. More importantly, the study by Schlapbach et al
(8) revealed that most memory Th9
cells were skin-tropic and appeared to possess both autocrine and
paracrine pro-inflammatory abilities (8). Moreover, gene expression analysis of SU
skin lesions had also demonstrated significant upregulation of
cytokine IL-9 signaling pathways (9).
These data overall indicate that Th9 cells serve an important role
in skin inflammatory diseases.
Cytokines have generally been known to be critical
to skin inflammation and regulation of naive T cell differentiation
into distinct effector T cells subsets. For example, IL-4
stimulation leads to Th2 cell polarization, while TGF-β induce
regulatory T cell differentiation (10). However, it has been observed that, in
the absence of IL-6, TGF-β and IL-4 induce Th9 cell generation
(11). Additionally, IL-1β and IL-33
stimulation can induce Th9 cell differentiation by the activation
of nuclear factor-κB (12,13). Despite considerable attention paid to
the research of Th9 cells and related cytokines, their expression
levels and roles in SU patients remain largely unclear.
Thus, in the present study, the authors have made an
effort to understand the contribution of Th9 cells in the
pathogenesis of SU, by comparing Th9 cell populations in the
peripheral blood of patients with SU and healthy controls, along
with analyzing the expression of the transcription factor PU.1.
Moreover, as cytokines have been linked with the regulation of Th9
cell differentiation and function, the authors assessed the plasma
concentrations of cytokines, IL-4, IL-9, IL-17A, IL-33, IL-1β and
transforming growth factor-β1 (TGF-β1). Finally, the correlation
between Th9 cells population and levels of cytokines was
explored.
Materials and methods
Study design
The present study is a case-control study
(EC/2015/005) approved by the Ethics Committee of Binhai County
Hospital (Yancheng, China). The study was conducted in the
Dermatology Clinic at Binhai County Hospital (Yancheng, China)
between November 2015 and March 2016, following obtaining written
informed consent from all patients.
Study participants
The current study comprised 28 healthy volunteers
and 56 patients diagnosed with acute SU (ASU, hives lasting <6
weeks; n=28) or chronic SU (CSU, hives lasting >6 weeks; n=28),
who were all 12 years or older. SU patients were interviewed
regarding the duration and type of urticaria by trained
dermatologists, and the average urticaria activity score was
measured to assess disease severity (typically, 7 days) based on
EAACI/GA(2)LEN/EDF/WAO guidelines
(14). Treatment was suspended for at
least 2 weeks prior to enrolling patients in the study. Patients
with physical, cholinergic, aquagenic, contact and exercise-induced
urticaria were excluded from the study.
Healthy volunteers (n=28) who were blood donors in
the control group were age and sex matched. Any patients receiving
immunosuppressive medication or with any immune system disorder
were excluded. Clinical characteristics of the subjects are
summarized in Table I.
 | Table I.The clinical characteristics of
patients and healthy controls. |
Table I.
The clinical characteristics of
patients and healthy controls.
| Parameters | ASU | CSU | Controls |
|---|
| Age (years) | 36.5±5.6 | 35.6±6.1 | 35.8±7.9 |
| Gender
(female/male) | 16/12 | 17/11 | 16/12 |
| Serum total IgE
(IU/ml) |
226.8±63.9a,b |
82.4±31.2a | 60.7±28.3 |
| Disease severity |
|
|
|
| Mild
(0–2)/day | 8/28 | 7/28 |
|
| Moderate
(3–4)/day | 9/28 | 9/28 |
|
| Severe
(5–6)/day | 11/28 | 12/28 |
|
Blood sample preparation
At the initial visit, 8 ml peripheral blood was
drawn from each subject in a tube with heparin sodium. Out of the 8
ml blood, 1 ml was used for flow cytometric detection of the Th9
cell population within 24 h according to manufacturer's
instructions. Another 2 ml was used for preparation of serum and
the remaining 5 ml was used for the isolation of peripheral blood
mononuclear cells (PBMCs). All blood samples were obtained before
patients received any treatment.
Detection of Th9 cell population in
peripheral blood
For Th9 cell detection, peripheral blood cells (100
µl) were activated with phorbol-12-myristate-13-acetate (50 ng/ml)
and ionomycin (1 µg/ml) in the presence of 1 µg/ml brefeldin (all
from Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for 5 h at
37°C. Following activation, the samples were stained with
fluorescein isothiocyanate-labeled anti-CD3 (cat. no. 555339), and
phycoerythrin-labeled anti-CD4 (cat. no. 555347) monoclonal
antibodies for 30 min (BD Biosciences, San Jose, CA, USA) at room
temperature according to the manufacturer's instructions. This was
followed by the lysis of red blood cells (BD Biosciences) and
fixation with BD Cytofix™ fixing buffer (BD Biosciences). Next,
cells were permeabilized by adding BD Perm/Wash™ buffer (BD
Biosciences) and incubated with PerCP-cy5.5 labeled anti-IL-9 (cat.
no. 561461; 1:10; BD Biosciences) and PEcy7 labeled anti-IL-17
antibodies (cat. no. 25717942; 1:10; eBioscience, Inc.; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) for 30 min at room
temperature. Finally, the labeled cells were suspended in 200 µl
phosphate-buffered saline and immediately analyzed with CellQuest
Pro (BD Biosciences). CD4+ T cells with the
CD4+IL-9+IL-4−IL-17−
phenotype represented the Th9 cell population.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
PBMCs purified by Ficoll-Hypaque density gradient
centrifugation method (Shanghai Westang Bio-Tech Co., Ltd.,
Shanghai, China) were used to isolate total RNA using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.). cDNA
synthesis and amplification was done using isolated RNA with the
cDNA Reverse Transcription kit (Applied Biosystems; Thermo Fisher
Scientific, Inc.) and QuantiTect SYBR Green PCR kit (Invitrogen;
Thermo Fisher Scientific, Inc.), respectively. The following primer
sequences were used for gene specific amplification: PU.1 sense,
5′-TGAGAAGGACAGGGAGCCAA-3′ and antisense,
5′-GAGAAGCTGAGTGCCATGCA-3′; β-actin sense,
5′-TGGCACCCAGCACAATGAA-3′ and antisense,
5′-CTAAGTCATAGTCCGCCTAGAAGCA-3′. The reaction mix was run with each
20 µl reaction containing ~50 ng cDNA, 0.3 µM sense and antisense
primers on a thermal cycler [7500 PCR system (Applied Biosystems;
Thermo Fisher Scientific, Inc.)] with following conditions: 1 min
at 95°C, followed by 40 cycles at 95°C for 15 sec and 60°C for 1
min. The relative mRNA levels of the PU.1 gene were calculated by
the 2−ΔΔCq method (15).
Cytokine analysis
To detect cytokines, serum samples were collected
from 2 ml peripheral blood and immediately stored at −80°C, until
cytokine analysis. The detection of cytokines IL-4, IL-17A, IL-9,
TGF-β1, IL-1β and IL-33 was performed according to the
manufacturer's protocol (Bio-Rad Laboratories, Inc., Hercules, CA,
USA), and their concentrations were determined using Luminex 200
(Luminex Corporation, Austin, TX, USA). Each sample was measured
twice and the mean value was used for statistical analysis. The
minimum detectable concentrations of cytokines IL-9, IL4, IL-17A,
TGF-β1, IL-1β and IL-33 in the present assay were 6.17, 13.10,
9.21, 6.12, 3.12 and 2.16 pg/ml, respectively.
Statistical analysis
Statistical analysis was performed using Stata
(version, 7.0; (StataCorp LLC, College Station, TX, USA) software.
The skewed data were expressed as median (M, 25–75 percentiles).
Statistical significance between three and two groups was
determined by the Kruskai-Wallis test and a two-tailed Mann-Whitney
U test, respectively. The normal distribution of data was presented
as mean ± standard deviation and statistical significance in two
groups was compared using the Student's t-test. Linear correlations
were calculated by Spearman coefficients. P<0.05 was considered
to indicate a statistically significant difference.
Results
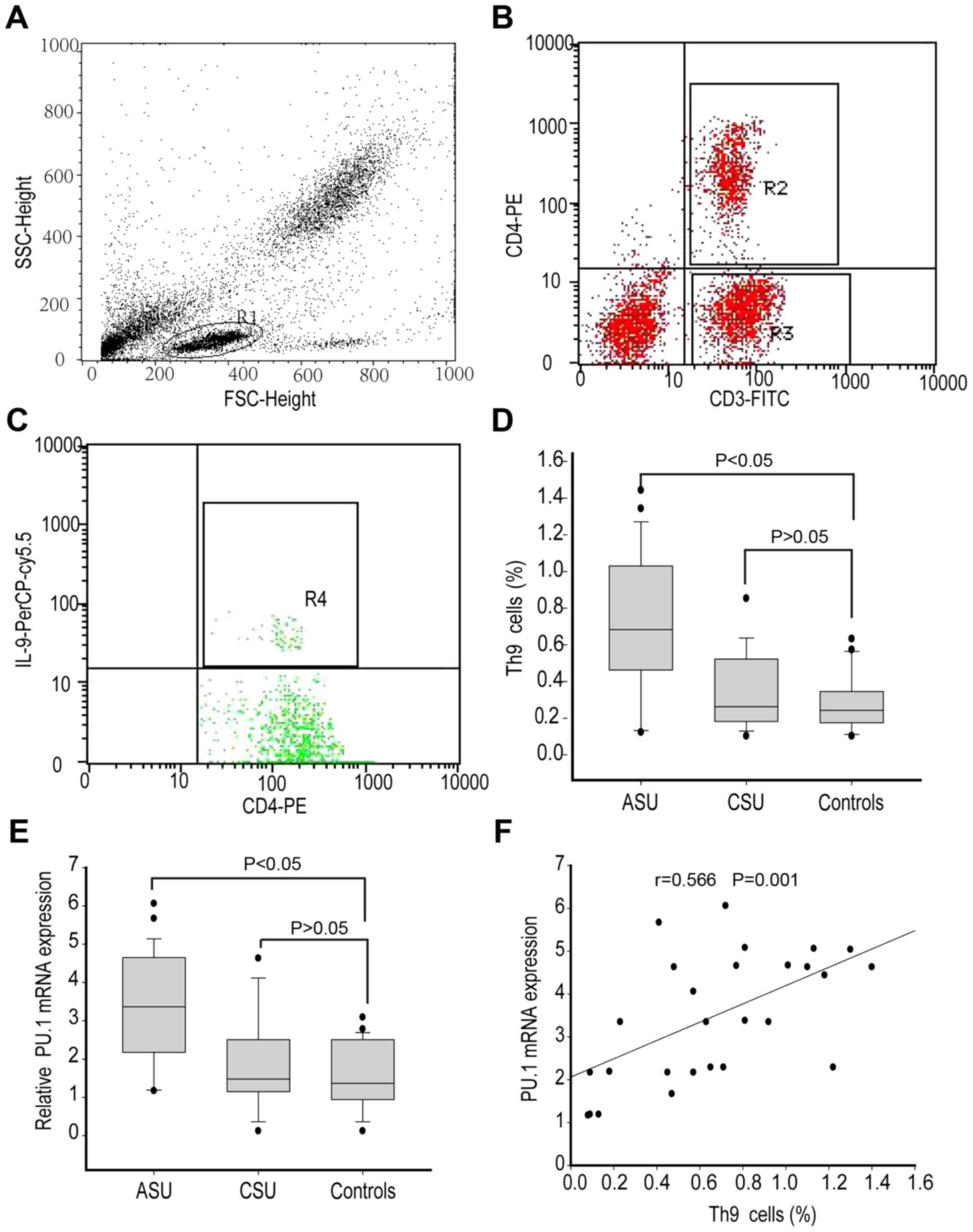
ASU patients displayed increased Th9
cell population
Changes in Th9 cell population have been reported in
patients with allergic skin diseases (16). Here, the authors assessed the
percentage of Th9 cells (Fig. 1A-C) in
peripheral blood isolated from ASU, CSU and healthy control
subjects. The data demonstrated that ASU patients had a higher
percentage of Th9 cells (median 0.65%, range 0.43–0.97%; P<0.05)
when compared to CSU patients (median 0.21%, range 0.13–0.44%) and
healthy controls (median 0.20%, range 0.13–0.32%) as indicated in
Fig. 1D. However, the percentage of
Th9 cells indicated similarities between CSU patients and healthy
controls (P>0.05; Fig. 1D).
Next, the authors assessed the mRNA levels of PU.1,
which is the primary transcription factor involved in Th9-mediated
function. RT-qPCR analysis suggested that the expression of PU.1
mRNA was significantly increased in the PBMCs isolated from ASU
patients (median 3.44, range 2.23–4.85; P<0.05) in comparison to
CSU patients (median 1.41, range 1.13–2.52) and healthy controls
(median 1.32, range 0.98–2.36), as presented in Fig. 1E. However, CSU patients and healthy
controls did not present any significant difference. Moreover, the
percentage of Th9 cells also demonstrated a positive correlation
with PU.1 mRNA levels (r=0.566, P<0.05) in ASU patients
(Fig. 1F).
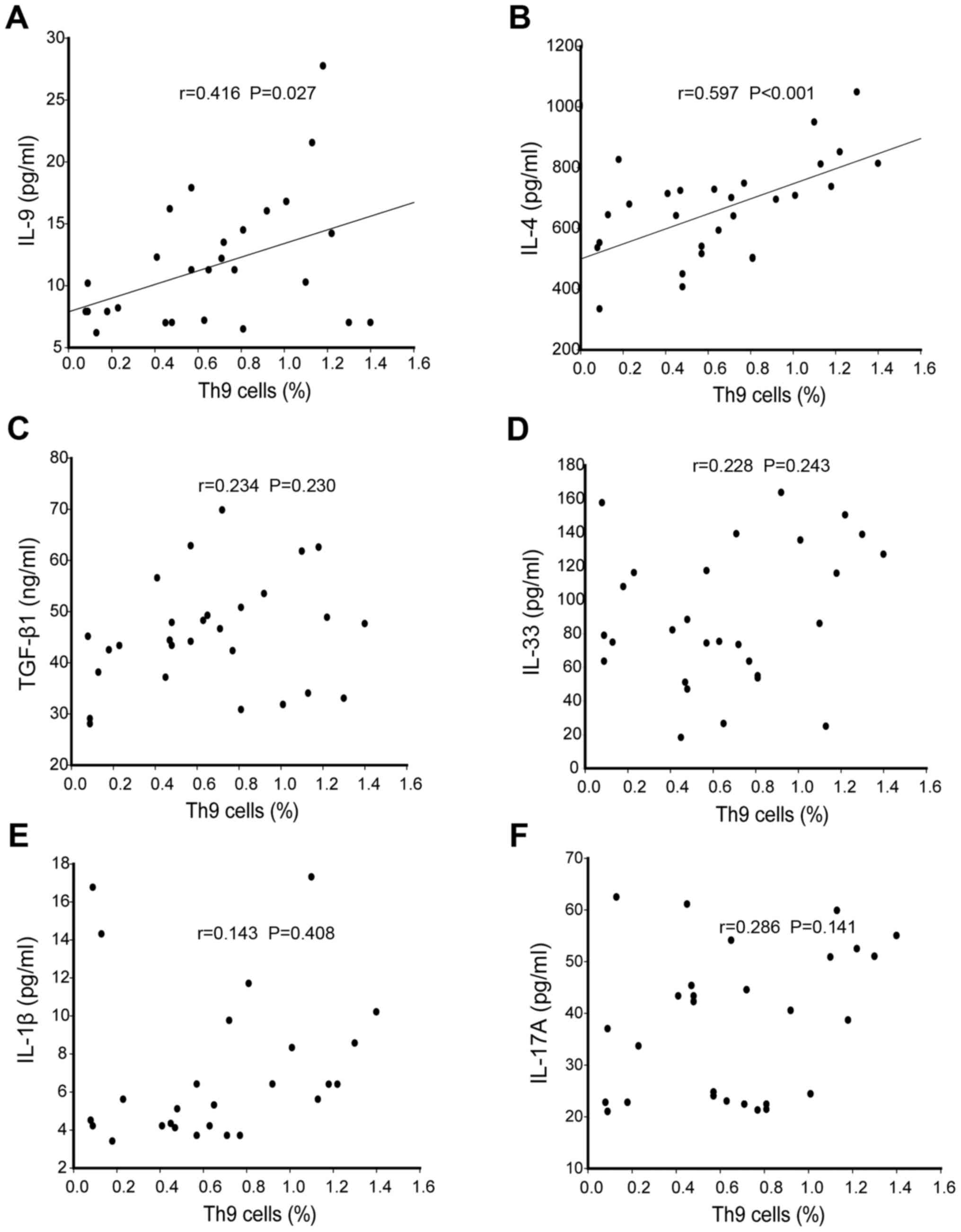
Comparison of Th9 related cytokines in
SU patients
As cytokines can affect Th9 cells differentiation
and function, serum concentrations of cytokines IL-9, IL-33, IL-4,
TGF-β1, IL-1β and IL-17A were measured. It was observed that ASU
patients had higher levels of IL-9 and IL-4 (P<0.05) compared to
CSU patients and healthy controls, although there was no
significant difference between them (P>0.05), as identified in
Fig. 2A and B. However, TGF-β1
presented higher levels in both ASU and CSU patients as compared to
healthy controls (P<0.05; Fig. 2C),
whereas no significant differences were observed in IL-33, IL-1β
and IL-17A levels between these three groups (P>0.05; Fig. 2D-F).
 | Figure 2.Analysis of Th9 related cytokines in
SU patients and control groups. Plasma levels of Th9 related
cytokines (A) IL-9, (B) IL-4, (C) TGF-β1, (D) IL-33, (E) IL-1β and
(F) IL-17A. Box plots represent the median (25–75 percentile) and
dots represents outliers. Statistical significance was determined
by Kruskai-Wallis test Th9, T helper cell 9; SU, spontaneous
urticaria; IL, interleukin; TGF-β1, transforming growth factor-β1;
ASU, acute spontaneous urticaria; CSU, chronic spontaneous
urticaria. |
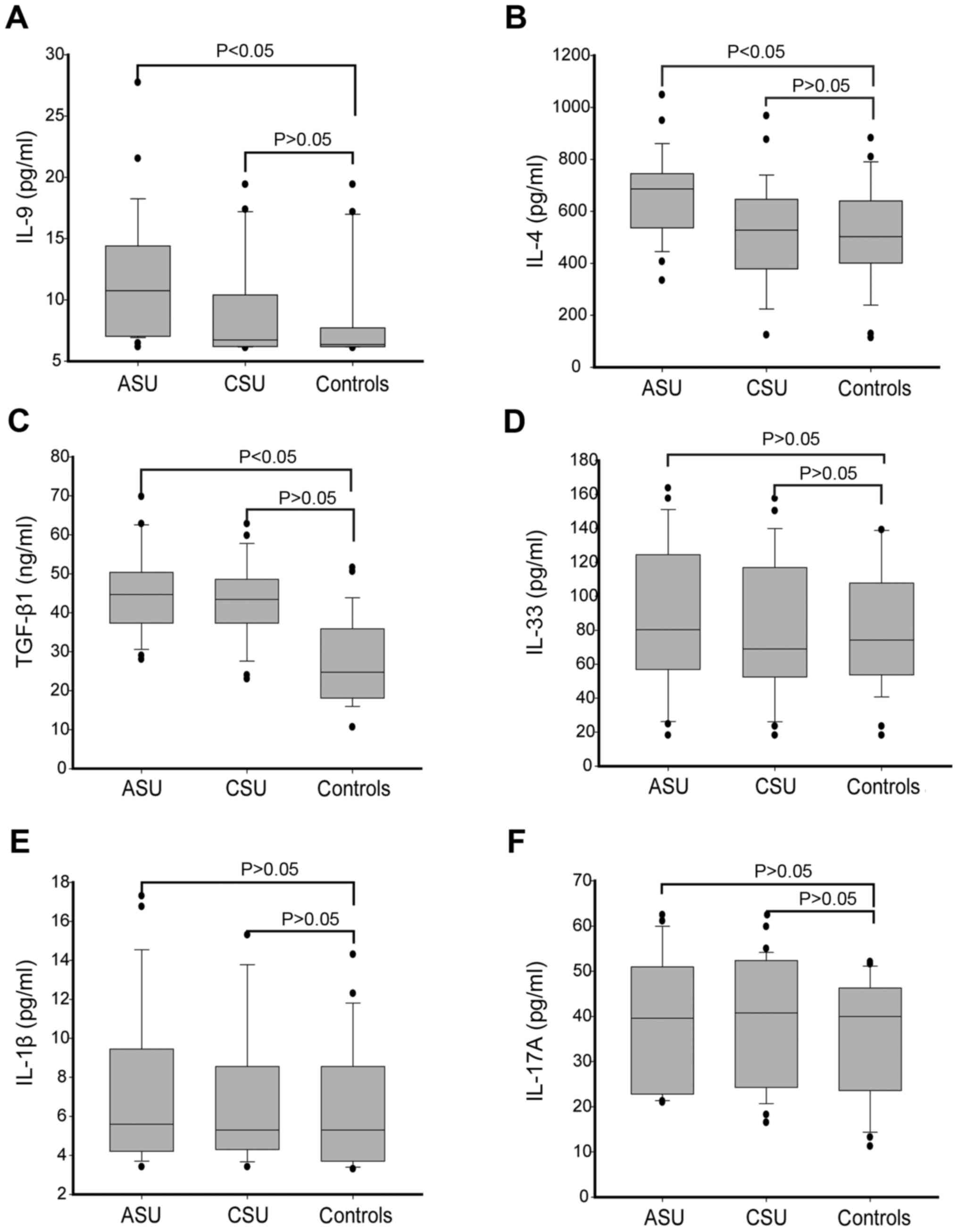
Th9 cell percentage and serum
cytokines IL-4 and IL-9 levels demonstrated a positive correlation
in ASU patients
Next, the authors examined whether there was any
correlation between the percentage of Th9 cells and the
concentrations of cytokines TGF-β1, IL-4 IL-9, IL-1β, IL-33 and
IL-17A in the serum of ASU patients. Interestingly, a positive
correlation was observed between the percentage of Th9 cells and
the concentration of IL-9 (r=0.644, P<0.05) and IL-4 (r=0.444,
P<0.05) in serum, as presented in Fig.
3A and B. However, Th9 cell percentage did not demonstrate any
correlation with the concentrations of TGF-β1, IL-1β, IL-17A and
IL-33 cytokines in the serum of ASU patients (P>0.05; Fig. 3C-F).
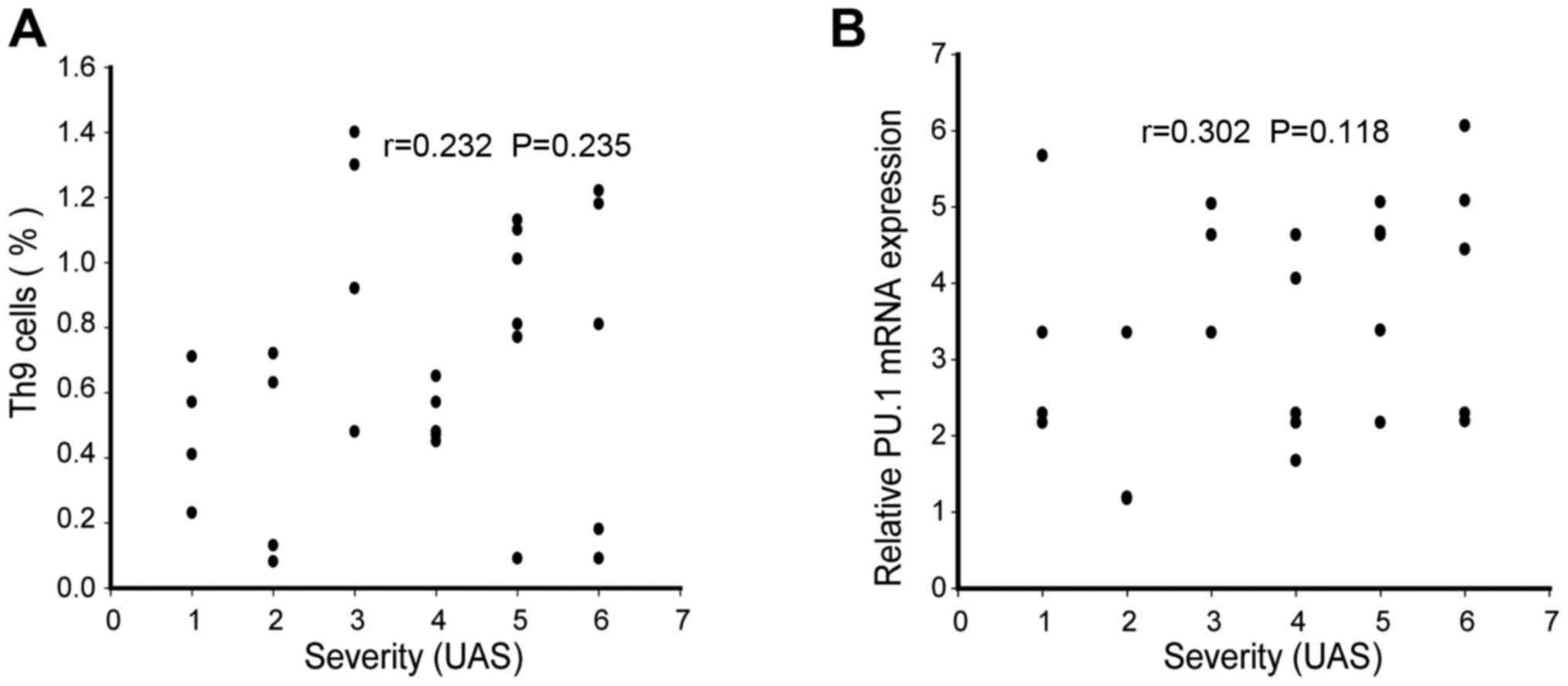
Th9 cell percentage and PU.1 mRNA
expression did not present correlation with disease severity in ASU
patients
Finally, the correlation between urticaria activity
score and the levels of Th9 cells and PU.1 mRNA expression was
tested in ASU patients. The current analysis revealed that disease
severity indicated no correlation with the percentage of Th9 cells
(r=0.232, P>0.05,) and PU.1 mRNA expression (r=0.302, P>0.05)
in the peripheral blood (Fig. 4A and
B).
Discussion
The present study identified that ASU patients
displayed significantly increased frequency of Th9 cells in
peripheral blood than CSU patients and healthy controls. This
observation was consistent with RT-qPCR results, which demonstrated
higher mRNA expression of the PU.1 transcription factor, primarily
responsible for Th9 cell-mediated regulation of IL-9 production. In
addition, there were increased levels of Th9-related cytokines,
such as TGF-β1, IL-9 and IL-4 in the peripheral blood of ASU
patients, which has been suggested to serve an important role in
Th9 development (11).
PU.1 is a transcription factor implicated in the
regulation of Th9 cells function by directly binding to IL-9 loci
(17). The observation of an increased
percentage of Th9 cells along with higher expression of PU.1 mRNA
and IL-9 cytokine in the peripheral blood of ASU patients suggested
that, Th9 is functionally important in ASU. The study by Ma et
al (16) also confirmed the
pathogenic role of Th9 cells in atopic dermatitis based on similar
findings. Furthermore, Th9 cell percentage positively correlated
with IL-9 levels. As it has been suggested previously that Th9
cells appear to be important cellular sources of IL-9, which
contributes to mast cell proliferation (6), it seems logical that Th9 cells may serve
an important role in SU, which involves mast cell regulation.
However, surprisingly, a correlation between an increased
proportion of Th9 cells and disease severity in ASU patients was
not observed. The tentative explanation may be that Th9 cells were
skin-tropic or skin-resident, as described previously (8).
In addition, the association between the plasma
TGF-β1 levels and the risk of SU has been reported in a previous
study (18). Patients with CSU
displayed TGF-β genetic variability, which leads to increased
production of TGF-β (19,20). In agreement with these findings, the
present data indicated that ASU patients had significantly high
plasma levels of TGF-β1 and IL-4, which may account for increased
Th9 development (11).
IL-33, the other epithelial cytokine, and a newly
recognized member of the IL-1 family, is a multifunctional protein.
It has been reported to bind to the cell membrane receptor ST2 and
promote Th2 responses in T cells, mast cells, eosinophils,
basophils and innate lymphoid cell populations (21). Consistent with a previous report
(22), the present study confirmed
that all three groups had similar levels of IL-33, and thus
indicated that a Th2 response may not be important in SU
pathogenesis. However, IL-1β has been presented to induce Th9
differentiation (13), but similar
levels between SU patients and controls were also observed.
Another cytokine, IL-9, following stimulation by
Jak1, can also induce Th17 cell proliferation (23). Th17 cytokines, such as IL-17A-F, are
believed to be crucially involved in the pathogenesis of some
autoimmune diseases (24). However,
there are some discrepancies between the results of the levels of
IL-17 in ASU and CSU patients reported in different studies
(19,25,26). These
data, however, demonstrated no significant difference in IL-17A
levels between ASU, CSU patients and healthy controls.
Importantly, the current study has a few
limitations. Firstly, due to the limitation of the study protocol,
it could not be determined whether the increase in Th9 cell
population was primary or secondary to other changes, such as
TGF-β1 and IL-4 in the peripheral blood. Secondly, the sample size
of patients with SU and healthy controls was very small. Thirdly,
it has been shown that ~30–50% of patients with CSU produce
circulating antibodies, while most cases of ASU are associated with
viral infections or allergens (5,27). Thus, in
future studies, the authors intend to investigate the effect of Th9
cells underlying each of these precipitating factors.
In conclusion, the present study demonstrated that
the percentage of Th9 cells in the peripheral blood of ASU patients
was markedly increased when compared to healthy controls.
Furthermore, ASU patients exhibited an increased Th9 related
cytokines, such as TGF-β1 and IL-4. These results indicated that
the increased levels of Th9 cells may serve a role in the
pathogenesis of SU.
Acknowledgements
The present study was supported by a grant from
Medical and Health Technology Development Program in Yancheng City,
China (grant no. YK2015065).
Glossary
Abbreviations
Abbreviations:
|
SU
|
spontaneous urticaria
|
|
PBMCs
|
peripheral blood mononuclear cells
|
|
TGF-β
|
transforming growth factor-β
|
|
IL
|
interleukin
|
|
PBS
|
phosphate-buffered saline
|
|
Th
|
T helper cells
|
|
UAS
|
urticarial activity score
|
References
|
1
|
Godse K, Rajagopalan M, Girdhar M,
Kandhari S, Shah B, Chhajed PN, Tahiliani S, Shankar DS, Somani V
and Zawar V: Position statement for the use of omalizumab in the
management of chronic spontaneous urticaria in Indian patients.
Indian Dermatol Online J. 7:6–11. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Caproni M, Volpi W, Macchia D, Giomi B,
Manfredi M, Campi P, Cardinali C, D'Agata A and Fabbri P:
Infiltrating cells and related cytokines in lesional skin of
patients with chronic idiopathic urticaria and positive autologous
serum skin test. Exp Dermatol. 12:621–628. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kay AB, Ying S, Ardelean E, Mlynek A, Kita
H, Clark P and Maurer M: Elevations in vascular markers and
eosinophils in chronic spontaneous urticarial weals with low-level
persistence in uninvolved skin. Br J Dermatol. 171:505–511. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jafilan L and James C: Urticaria and
Allergy-Mediated Conditions. Prim Care. 42:473–483. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fine LM and Bernstein JA: Urticaria
Guidelines: Consensus and Controversies in the European and
American Guidelines. Curr Allergy Asthma Rep. 15:302015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sehra S, Yao W, Nguyen ET, Glosson-Byers
NL, Akhtar N, Zhou B and Kaplan MH: TH9 cells are required for
tissue mast cell accumulation during allergic inflammation. J
Allergy Clin Immunol. 136:433–40.e1. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Licona-Limón P, Henao-Mejia J, Temann AU,
Gagliani N, Licona-Limón I, Ishigame H, Hao L, Herbert DR and
Flavell RA: Th9 cells drive host immunity against gastrointestinal
worm infection. Immunity. 39:744–757. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schlapbach C, Gehad A, Yang C, Watanabe R,
Guenova E, Teague JE, Campbell L, Yawalkar N, Kupper TS and Clark
RA: Human TH9 cells are skin-tropic and have autocrine and
paracrine proinflammatory capacity. Sci Transl Med. 6:219ra82014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Patel OP, Giorno RC, Dibbern DA, Andrews
KY, Durairaj S and Dreskin SC: Gene expression profiles in chronic
idiopathic (spontaneous) urticaria. Allergy Rhinol (Providence).
6:101–110. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Caza T and Landas S: Functional and
Phenotypic Plasticity of CD4(+) T Cell Subsets. BioMed Res Int.
2015:5219572015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Veldhoen M, Uyttenhove C, van Snick J,
Helmby H, Westendorf A, Buer J, Martin B, Wilhelm C and Stockinger
B: Transforming growth factor-beta ‘reprograms’ the differentiation
of T helper 2 cells and promotes an interleukin 9-producing subset.
Nat Immunol. 9:1341–1346. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Blom L, Poulsen BC, Jensen BM, Hansen A
and Poulsen LK: IL-33 induces IL-9 production in human CD4+ T cells
and basophils. PLoS One. 6:e216952011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Anuradha R, George PJ, Hanna LE,
Chandrasekaran V, Kumaran P, Nutman TB and Babu S: IL-4-, TGF-β-,
and IL-1-dependent expansion of parasite antigen-specific Th9 cells
is associated with clinical pathology in human lymphatic
filariasis. J Immunol. 191:2466–2473. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zuberbier T, Asero R, Bindslev-Jensen C,
Canonica G Walter, Church MK, Giménez-Arnau A, Grattan CE, Kapp A,
Merk HF, Rogala B, et al: EAACI/GA(2)LEN/EDF/WAO guideline:
definition, classification and diagnosis of urticaria. Allergy.
64:1417–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ma L, Xue HB, Guan XH, Shu CM, Zhang JH
and Yu J: Possible pathogenic role of T helper type 9 cells and
interleukin (IL)-9 in atopic dermatitis. Clin Exp Immunol.
175:25–31. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Goswami R and Kaplan MH: Gcn5 is required
for PU.1-dependent IL-9 induction in Th9 cells. J Immunol.
189:3026–3033. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tavakol M, Movahedi M, Amirzargar AA,
Aryan Z, Bidoki AZ, Heidari K, Soltani S, Gharagozlou M,
Aghamohammadi A, Nabavi M, et al: Association of interleukin 10 and
transforming growth factor β gene polymorphisms with chronic
idiopathic urticaria. Acta Dermatovenerol Croat. 22:239–245.
2014.PubMed/NCBI
|
|
19
|
Daschner A, Rodero M, de Frutos C, Valls
A, Vega F, Blanco C and Cuéllar C: Different serum cytokine levels
in chronic vs. acute Anisakis simplex sensitization-associated
urticaria. Parasite Immunol. 33:357–362. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Papadopoulos J, Karpouzis A, Tentes J and
Kouskoukis C: Assessment of Interleukins IL-4, IL-6, IL-8, IL-10 in
Acute Urticaria. J Clin Med Res. 6:133–137. 2014.PubMed/NCBI
|
|
21
|
Matta BM, Lott JM, Mathews LR, Liu Q,
Rosborough BR, Blazar BR and Turnquist HR: IL-33 is an
unconventional Alarmin that stimulates IL-2 secretion by dendritic
cells to selectively expand IL-33R/ST2+ regulatory T cells. J
Immunol. 193:4010–4020. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Metz M, Krull C and Maurer M: Histamine,
TNF, C5a, IL-6, −9, −18, −31, −33, TSLP, neopterin, and VEGF are
not elevated in chronic spontaneous urticaria. J Dermatol Sci.
70:222–225. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Elyaman W, Bradshaw EM, Uyttenhove C,
Dardalhon V, Awasthi A, Imitola J, Bettelli E, Oukka M, van Snick
J, Renauld JC, et al: IL-9 induces differentiation of TH17 cells
and enhances function of FoxP3+ natural regulatory T cells. Proc
Natl Acad Sci USA. 106:pp. 12885–12890. 2009; View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Patel DD and Kuchroo VK: Th17 Cell Pathway
in Human Immunity: Lessons from Genetics and Therapeutic
Interventions. Immunity. 43:1040–1051. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Atwa MA, Emara AS, Youssef N and Bayoumy
NM: Serum concentration of IL-17, IL-23 and TNF-α among patients
with chronic spontaneous urticaria: Association with disease
activity and autologous serum skin test. J Eur Acad Dermatol
Venereol. 28:469–474. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Azor MH, dos Santos JC, Futata EA, de
Brito CA, Maruta CW, Rivitti EA, da Silva Duarte AJ and Sato MN:
Statin effects on regulatory and proinflammatory factors in chronic
idiopathic urticaria. Clin Exp Immunol. 166:291–298. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Leru P: Urticaria - an allergologic,
dermatologic or multidisciplinary disease? Rom J Intern Med.
51:125–130. 2013.PubMed/NCBI
|