Introduction
Ovarian cancer (OC) is the most common cause of
mortality among women with gynecologic cancer worldwide (1). Although this tumor type may develop at
young ages, the majority of the cases occur in postmenopausal women
(2). Since the disease presents with
non-specific symptoms, approximately 70% of patients with OC are
not diagnosed until the disease has reached an advanced stage
(3). Patients with high-grade OC often
have poor prognosis and a high mortality rate (4). Therefore, early diagnosis of OC is a key
factor in improving patient survival. Currently, tumor markers,
such as the human epididymis protein 4 (HE4) (5) and carbohydrate antigen-125 (CA-125)
(6), and the risk of ovarian
malignancy algorithm (ROMA) and risk malignancy index (RMI)
(7–9) are
important tools for the differential diagnosis of patients with
abdominopelvic masses.
HE4 is a member of the four-disulfide core family
that comprises a heterogeneous group of small acid- and heat-stable
proteins of divergent function (5).
Over the past decade, HE4 has gained widespread use as an effective
tumor marker in the diagnosis of OC. Numerous clinical studies have
demonstrated significant elevations of serum HE4 levels in patients
with gynecological cancer and have confirmed that HE4 levels may be
used as a biomarker for OC with higher specificity than the widely
used CA-125 (4,7,8,10–12). A
previous study observed that HE4 had sensitivity of 72.9% and
specificity of 95% in the differential diagnosis of OC and benign
ovarian masses (13).
Although HE4 is a valuable marker in OC diagnosis,
under certain circumstances, the evaluation of serum HE4 levels may
be problematic when patients suffer from additional conditions
(4). Abnormal HE4 concentrations are
detected in certain nonmalignant diseases, causing difficulties in
the differential diagnosis of OC. Furthermore, age, menopause
status, and smoking habits directly affect serum HE4 levels;
therefore, these conditions should be considered in patients who
present with abnormal HE4 levels. Recent studies reported that
serum HE4 concentrations significantly increase in patients with
chronic kidney disease (CKD), renal failure and heart failure
(14–16). In the study by Nagy et al
(14), increased HE4 levels were
measured in patients with early stage CKD, indicating that the
serum HE4 level is significantly affected by the estimated
glomerular filtration rate (eGFR). Lv et al (17) demonstrated that patients with chronic
renal deficiency exhibited elevated serum HE4 levels that were
significantly higher than those of patients with benign
gynecological diseases. These data indicate that serum HE4
concentrations may be affected by variable demographical factors or
by non-malignant diseases. Thus, serum HE4 levels show a high
false-positive rate in the differential diagnosis of OC, with the
main factor being the presence of CKD (4).
Recent studies have suggested that urine assays are
a non-invasive alternative for the evaluation of HE4 levels.
Urinary HE4 levels in patients with OC are significantly higher
than those in healthy women or patients with benign diseases
(10). The ratio between urinary HE4
and urinary creatinine facilitates the differential diagnosis of
benign and malignant ovarian tumors (18). Furthermore, the combination of HE4 with
CA-125 or eGFR is helpful for discriminating healthy controls from
patients with OC (19).
The question of whether the combined detection of
serum and urinary HE4 levels distinguishes OC from CKD remains
unknown. Therefore, the aim of the present study was to evaluate
the diagnostic efficacy of the combined detection of serum and
urinary HE4 levels in differentiating OC from CKD.
Materials and methods
Ethical approval
The present study was approved by the Medical Ethics
Committee of the Affiliated Hospital of North Sichuan Medical
College (Nanchong, China) and each participant provided written
informed consent.
Recruitment of patients
In the present study, 31 patients with OC (whose
diagnosis was confirmed by postoperative pathological findings), 38
female patients with CKD and 36 healthy control (HC) females were
consecutively recruited from the Affiliated Hospital of North
Sichuan Medical College between August 2014 and July 2016. The mean
age of patients in the OC, CKD, and HC groups was 53±12 years
(range, 16–74 years), 55±13 years (range, 27–76 years), and 52±17
years (range, 24–83 years), respectively. All participants within
each group were age-matched. In the OC group, there were 11 cases
of serous papillary carcinoma, 9 cases of low-grade serous
carcinoma, 6 cases of endometrioid carcinoma, 4 cases of high-grade
serous carcinoma, and 1 case of malignant germ cell tumor.
According to the OC staging guidelines of the International
Federation of Gynecologists and Obstetricians (20), there were 3 cases of stage I, 8 cases
of stage II, 17 cases of stage III, and 3 cases of stage IV. Eleven
patients were premenopausal, while 20 patients were postmenopausal.
Diagnoses of CKD were reconfirmed by nephrologists according to
Kidney Disease Improving Global Outcomes guidelines (21). The participants in the CKD group
exhibited various types of chronic disease, such as hypertension,
cardiovascular disease, type 2 diabetes mellitus, hyperlipidemia,
autoimmune disease, peripheral artery disease and renal dysfunction
(eGFR<90 ml/min/1.73 m2). Participants in the control
group had normal eGFR values (eGFR>90 ml/min/1.73 m2)
and were free of benign and malignant gynecological diseases.
Sample collection
Serum samples (3 ml) were obtained by venipuncture
and collected into vacuum tubes to clot. Samples were then
centrifuged at 500 × g for 5 min at room temperature. Urine samples
were simultaneously collected for HE4 level detection. All samples
were obtained preoperatively at primary diagnosis. The serum and
urine samples were cryopreserved (−80°C) until HE4 level
analysis.
Measurement of HE4 level
Serum HE4 (S-HE4) and urinary HE4 (U-HE4)
concentrations were measured by electrochemiluminescent immunoassay
on a Cobas 800 e602 (Roche Diagnostics, Shanghai, China). The HE4
cut-off value was 140 pmol/l and the HE4 measurement range was
15–1,500 pmol/l. Samples with HE4 concentrations greater than the
measurement range (1,500 pmol/l) were re-measured following
dilution according to the manufacturer's instructions.
Other variables
The serum CA-125 concentration was measured by
electrochemiluminescent immunoassay on a Cobas 800 e602. The serum
creatinine concentration was measured by enzymatic assay on a
AU5800 AU chemistry autoanalyzer (Beckman Coulter, Inc., Shanghai,
China) and the serum cystatin concentration was measured using a
particle-enhanced turbidimetric immunoassay on the AU5800 AU
chemistry autoanalyzer. The value of eGFR was calculated according
to the CKD epidemiology collaboration equation (22). Additional clinical and demographic
characteristics, and patient laboratory data were obtained by
review of medical records.
Statistical analysis
As all of the continuous variables had skewed
distributions, the median and range were used to describe these
variables. Differences between groups were evaluated using the
Kruskal-Wallis test and Mann-Whitney U test. A receiver operating
characteristic (ROC) curve was constructed to assess specificity,
sensitivity, and the area under the curve (AUC) with a 95%
confidence interval (CI). The optimal cut-off value for diagnosis
was selected by maximizing Youden's index (the sum of sensitivity
and specificity) and minimizing the overall error [square root of
the sum (1-sensitivity)2+(1-specificity)2].
P<0.05 was considered to indicate a statistically significant
difference and data analyses were performed using SPSS 17.0
statistical software (SPSS, Inc., Chicago, IL, USA).
Results
Comparisons of variables among the OC,
CKD and HC groups
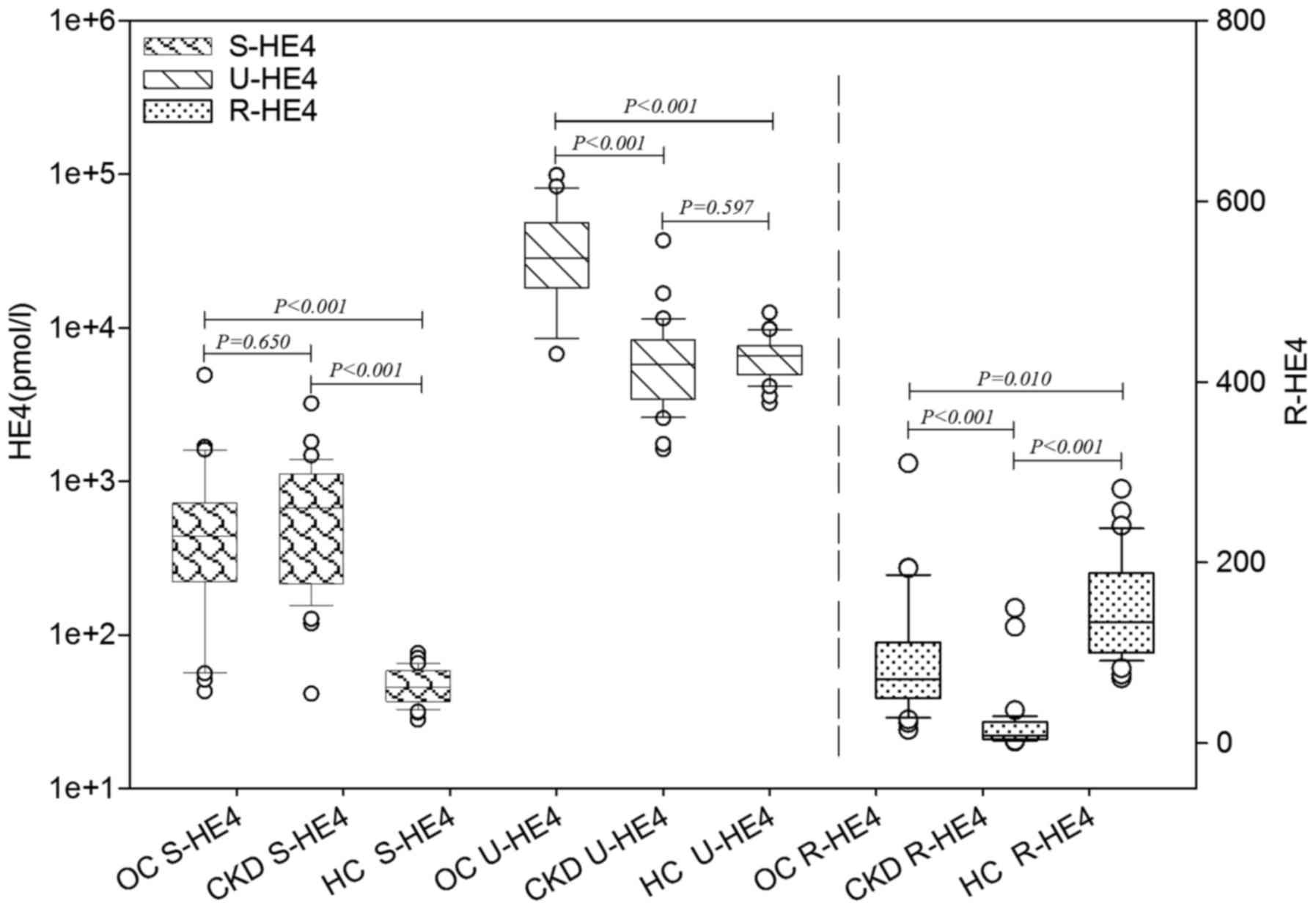
S-and U-HE4 levels and ratios of urinary-to-serum
HE4 (R-HE4) levels were analyzed in the OC, CKD and HC groups. As
presented in Table I and Fig. 1, S-HE4 levels in the OC and CKD groups
were significantly higher than those in the HC group (P<0.001).
No significant difference in S-HE4 levels was identified between
the OC and CKD groups. U-HE4 levels in the OC group were
significantly higher than those in the CKD and HC groups
(P<0.001). No significant difference in U-HE4 levels was
identified between the CKD and HC groups. The R-HE4 was observed to
be significantly different between the OC, CKD and HC groups
(P=0.010). The R-HE4 in the OC group was significantly higher than
that in the CKD group (P<0.001) and significantly lower than
that in the HC group (P<0.001). Furthermore, the serum levels of
creatinine and cystatin C in the CKD group were significantly
higher than those in the OC and HC groups (P<0.001). The eGFR
value in the CKD group was significantly lower than that in the OC
and HC groups (P<0.001).
 | Table I.Comparison of variables among the OC,
CKD and HC groups. |
Table I.
Comparison of variables among the OC,
CKD and HC groups.
| Variable | OC (n=31) | CKD (n=38) | HC (n=36) |
|---|
| S-HE4 (pmol/l) | 439
(43–4927)a | 670.1
(41–3212)a | 45.65 (28–76) |
| U-HE4 (pmol/l) | 28,560
(6752–98740)a,b | 5790
(1,621–37,024) | 6,573
(3,220–12,542) |
| R-HE4 | 71.091
(14.7–309.7)a,b | 8.7
(1.7–149.5)a | 134.4
(71.9–281.3) |
| Creatinine
(µmol/l) | 56.7
(37.9–95.3)b | 279.9
(69.8–980.2)a | 48.2 (34.2–76.3) |
| eGFR
(ml/min/1.73m2) | 102.9
(52.2–127.3)b | 18.2
(3.0–78.3)a | 116.8
(70.1–138.3) |
| Serum cystatin C
(mg/l) | 0.71
(0.5–1.31)b | 2.91
(1.15–7.66)a | 0.66 (0.38–0.94) |
Diagnostic performance of HE4 in
differentiating OC from HC
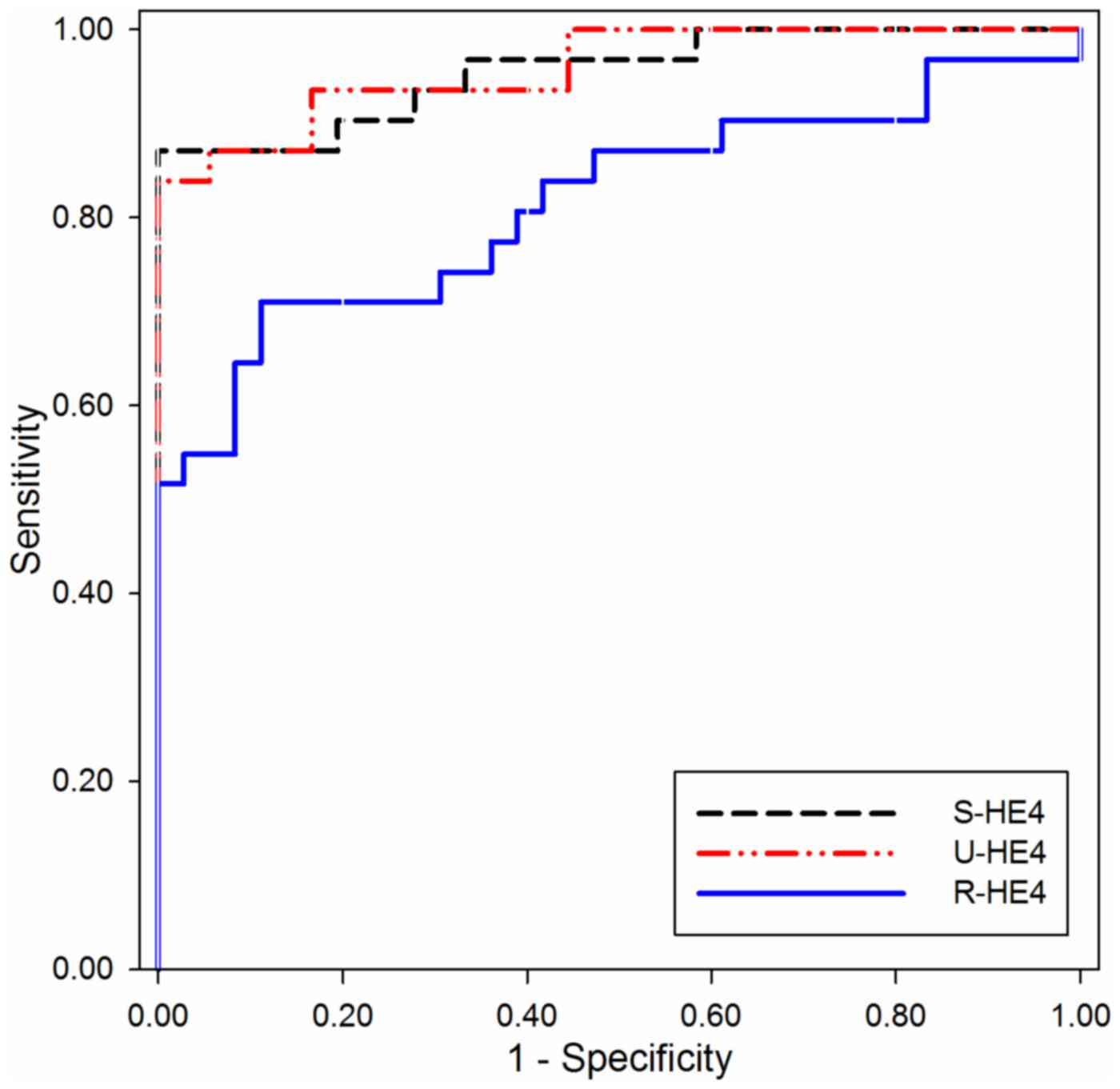
ROC curves were constructed to investigate the
diagnostic performance of S- and U-HE4 levels and R-HE4 for
distinguishing OC from HC. As shown in Fig. 2 and Table
II, the AUC of S-HE4 was 0.955 (95% CI, 0.907–1.004), which
provided an optimal cut-off value of 88.65 pmol/l, sensitivity of
87.1% and specificity of 100.0% in distinguishing OC from HC. The
AUC value of U-HE4 was 0.959 (95% CI, 0.915–1.003), which provided
an optimal cut-off value of 14,116 pmol/l, sensitivity of 83.9%,
and specificity of 100.0% in distinguishing OC from HC. Consistent
with the results of S- and U-HE4 levels, the R-HE4 was useful for
differentiating OC from HC. The AUC of R-HE4 was 0.815 (95% CI,
0.705–0.926), which provided an optimal cut-off value of 96.15,
sensitivity of 71.0% and specificity of 88.9% in separating OC from
HC.
 | Table II.Diagnostic performance of HE4 in
differentiating patients with ovarian cancer from healthy control
subjects. |
Table II.
Diagnostic performance of HE4 in
differentiating patients with ovarian cancer from healthy control
subjects.
| Variable | Serum HE4 | Urine HE4 | R-HE4 |
|---|
| Area under the
curve | 0.955 | 0.959 | 0.815 |
| P-value | <0.001 | <0.001 | <0.001 |
| 95% CI | 0.907–1.004 | 0.915–1.003 | 0.705–0.926 |
| Cut-off value | 88.65 | 14116 | 96.15 |
| Sensitivity (%) | 87.1 | 83.9 | 71.0 |
| Specificity (%) | 100.0 | 100.0 | 88.9 |
| Youden's index
(%) | 87.1 | 83.9 | 59.9 |
Diagnostic performance of HE4 in
differentiating OC from CKD
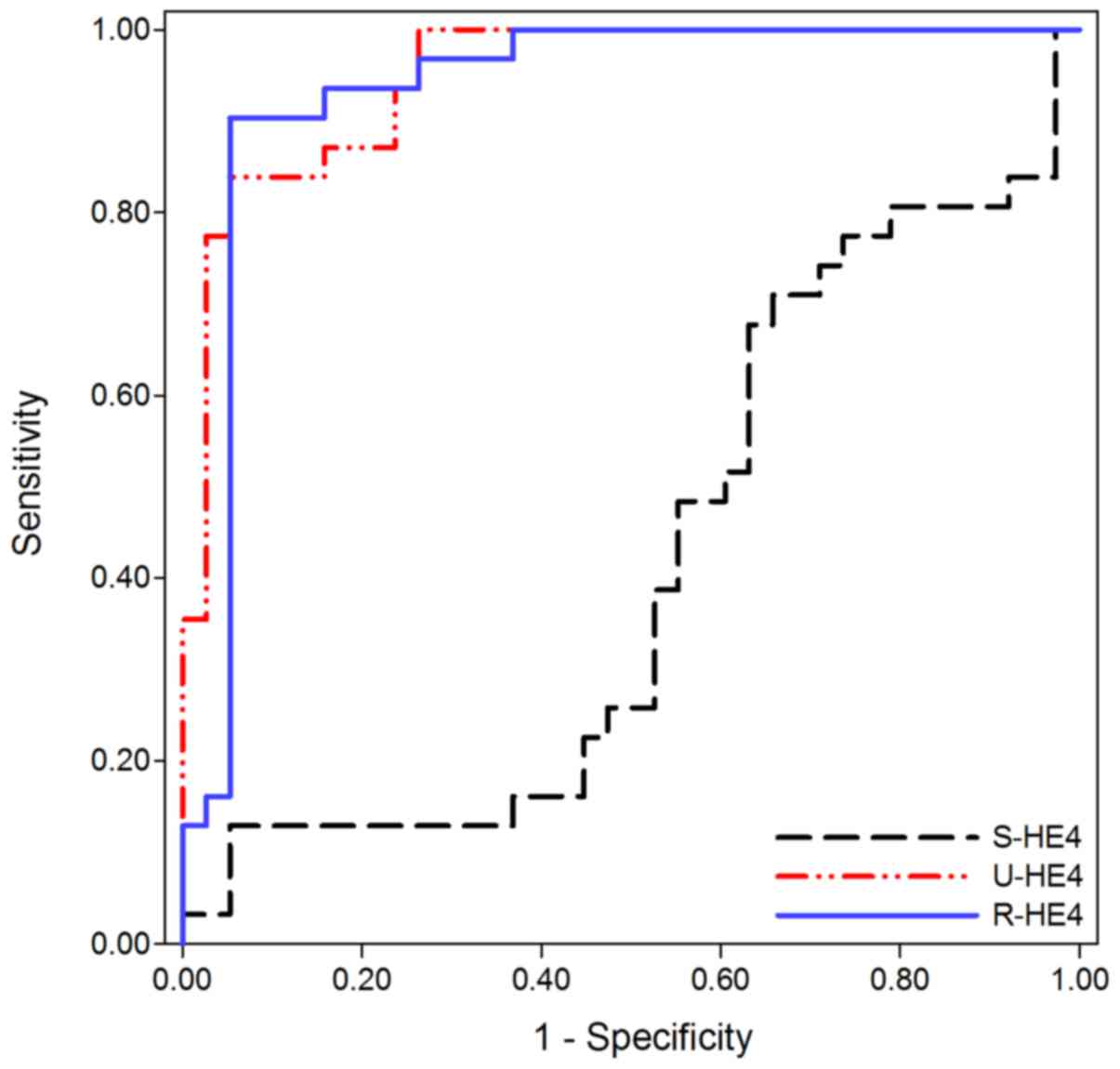
As previously stated, no significant difference in
S-HE4 levels were observed between OC and CKD. Consequently, S-HE4
levels did not assist with differentiating OC from CKD (AUC=0.416;
95% CI, 0.277–0.555, 12.9% sensitivity and 94.7% specificity).
Conversely, U- and R-HE4 levels were useful in differentiating OC
from CKD. As presented in Fig. 3 and
Table III, the AUC of U-HE4 levels
was 0.948 (95% CI, 0.900–0.996), which provided an optimal cut-off
value of 13,586 pmol/l, sensitivity of 83.9%, and specificity of
94.7% in distinguishing OC from CKD. Similarly, the AUC of R-HE4
was 0.935 (95% CI, 0.869–1.001), which provided an optimal cut-off
value of 36.85, sensitivity of 90.3%, and specificity of 94.7% in
distinguishing OC from CKD.
 | Table III.Diagnostic performance of HE4 in
differentiating patients with ovarian cancer from patients with
chronic kidney disease. |
Table III.
Diagnostic performance of HE4 in
differentiating patients with ovarian cancer from patients with
chronic kidney disease.
| Variable | Serum HE4 | Urine HE4 | R-HE4 |
|---|
| Area under the
curve | 0.416 | 0.948 | 0.935 |
| P-value | 0.232 | <0.001 | <0.001 |
| 95% CI | 0.277–0.555 | 0.900–0.996 | 0.869–1.001 |
| Cut-off value | 1530 | 13586 | 36.85 |
| Sensitivity
(%) | 12.9 | 83.9 | 90.3 |
| Specificity
(%) | 94.7 | 94.7 | 94.7 |
| Youden's index
(%) | 7.6 | 78.6 | 85.1 |
Diagnostic performance of HE4 in
differentiating CKD from HC
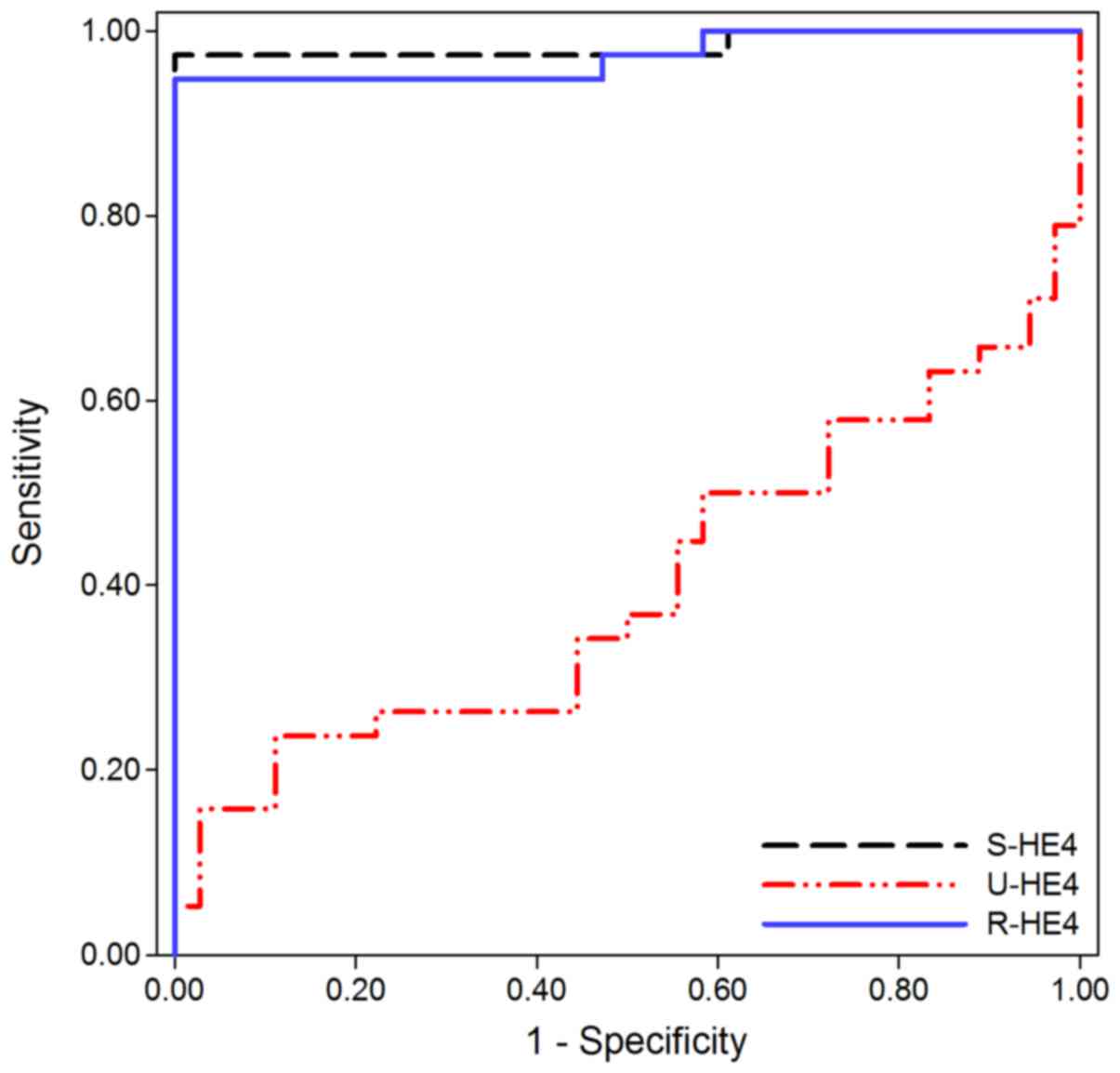
ROC curves were used to investigate the diagnostic
performance of S- and U-HE4 levels and R-HE4 in distinguishing CKD
from HC. As shown in Fig. 4 and
Table IV, the AUC of S-HE4 levels was
0.984 (95% CI, 0.952–1.016), which provided an optimal cut-off
value of 94.15 pmol/l, sensitivity of 97.4%, and specificity of
100.0% in distinguishing CKD from HC. Conversely, U-HE4 levels were
of little diagnostic value for differential diagnosis between CKD
and HC (AUC=0.399, 95% CI, 0.265–0.532, 15.8% sensitivity and 97.2%
specificity). Similar to the results for S-HE4, the AUC of R-HE4
was 0.972 (95% CI, 0.933–1.011), which provided an optimal cut-off
value of 48.54, sensitivity of 94.7%, and specificity of 100.0% in
distinguishing CKD from HC.
 | Table IV.Diagnostic performance of HE4 in
differentiating chronic kidney disease patients from healthy
control subjects. |
Table IV.
Diagnostic performance of HE4 in
differentiating chronic kidney disease patients from healthy
control subjects.
| Variable | Serum HE4 | Urine HE4 | R-HE4 |
|---|
| Area under the
curve | 0.984 | 0.399 | 0.972 |
| P-value | <0.001 | 0.133 | <0.001 |
| 95% CI | 0.952–1.016 | 0.265–0.532 | 0.933–1.011 |
| Cutoff value | 97.15 | 10062 | 48.54 |
| Sensitivity
(%) | 97.4 | 15.8 | 94.7 |
| Specificity
(%) | 100.0 | 97.2 | 100.0 |
| Youden's index
(%) | 97.4 | 13.0 | 94.7 |
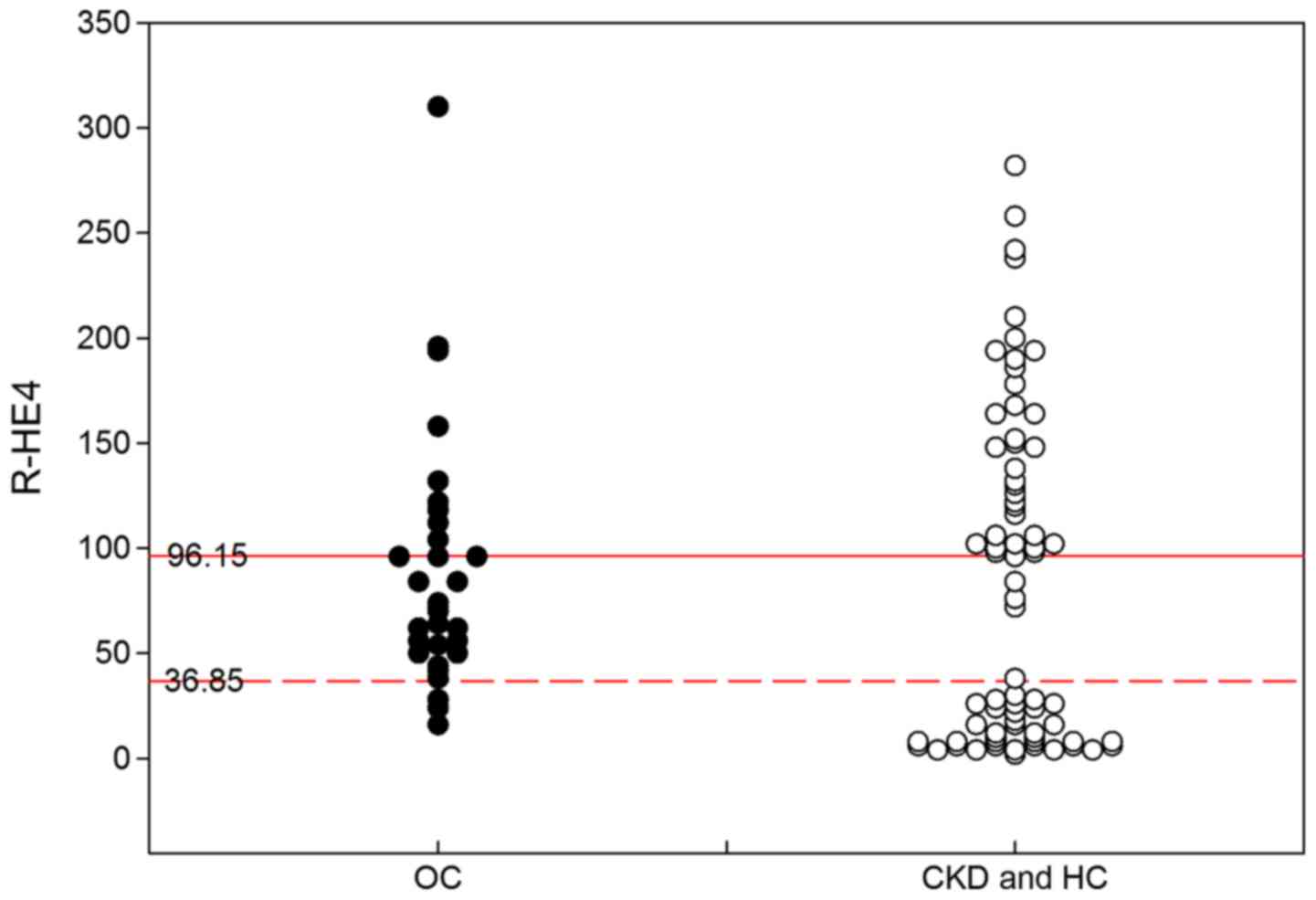
Diagnostic performance of R-HE4 in
differentiating OC patients from non-ovarian cancer objects
As previously described, the R-HE4 was useful for
differentiating OC from CKD and HC with the optimal cut-off values
of 36.85 and 96.15, respectively. The diagnostic performance of
R-HE4 was further analyzed based on the diagnosis interval of 36.85
to 96.15. As shown in Fig. 5, the
R-HE4 provided a sensitivity value of 82.6% and a specificity value
of 85.4% in differentiating OC patients from non-ovarian cancer
objects (including CKD and HC).
Discussion
To the best of the authors' knowledge, this is the
first study to investigate the diagnostic performance of R-HE4 in
the diagnosis of OC. In the present study, R-HE4 demonstrated
significant differences between the OC and CKD patients, and
healthy controls (HC >OC> CKD; P<0.01). In addition, ROC
analysis indicated that R-HE4 was useful for differentiating OC
from CKD and HC, with the optimal cut-off values of 36.85 and
96.15, respectively, with sensitivity of 82.6% and specificity of
85.4% in differentiating OC patients from non-cancer objects
(including CKD and HC group).
OC is a heterogeneous group of diseases that
exhibits various pathological characteristics and clinical
manifestations (1). Early diagnosis is
critical for the management and prognosis of OC. An increasing
number of studies have demonstrated the important role of HE4 as a
tumor marker in the diagnosis of OC (5). For example, a large study demonstrated
that S-HE4 has a higher sensitivity and specificity in the
diagnosis of OC when compared with serum CA-125 (23). However, the evaluation of S-HE4 levels
may be problematic when patients suffer from additional conditions,
such as CKD (17), heart failure
(4), and breast (24) and lung (25) cancer. Thus, the accuracy of S-HE4 in OC
diagnosis remains a challenge. Research has shown that S-HE4 levels
demonstrate a high false-positive rate in the differential
diagnosis of OC, with the main factor being the presence of CKD
(4). Therefore, the differential
diagnoses of CKD should be considered for patients with elevated
S-HE4 levels.
HE4, like various other tumor biomarkers, is
detected in the urine and used as a potentially non-invasive
diagnostic tool for OC diagnosis (10,18).
However, previous studies have not investigated the combination
detection efficacy of S-and U-HE4 levels. In the present study, S-
and U-HE4 levels and R-HE4 were analyzed in OC and CKD patients,
and HCs. The results indicated that the S-HE4 level in the OC and
CKD groups was significantly higher than that in the HC group
(P<0.001), and that no significant difference regarding S-HE4
levels was identified between the OC and CKD groups. These
observations were consistent with those of the study by Lv et
al (17), which demonstrated that
S-HE4 levels in OC and CKD patients significantly increased in
comparison to the levels detected in the HC group, and the study
indicated that the S-HE4 level in the CKD group was higher than
that in the OC group. The present study and that of Lv et al
(17) indicate that the diagnosis of
OC on the basis of S-HE4 levels may be problematic in patients who
suffer from CKD.
Hellstrom et al (19) described high levels of U-HE4 in
patients with OC. Macuks et al (18) reported that OC patients had higher
urinary concentrations of HE4 than patients with benign ovarian
tumors, and U-HE4 had comparable accuracy with S-HE4 in
differentiating malignant ovarian tumors from benign disease
(18). Similarly, the present study
demonstrated that the U-HE4 level in the OC group was significantly
higher than that in the CKD and HC groups (P<0.001), and there
was no significant difference in U-HE4 levels between the CKD
patients and the HC group. These results indicate that U-HE4 level
presented superior diagnostic efficacy in differentiating OC from
CKD when compared with that of S-HE4 level. In the analysis of the
R-HE4 diagnostic performance, there were significant differences
regarding the R-HE4 among the OC and CKD groups, and the HC group
(HC>OC>CKD; P<0.01). This result implies that R-HE4 maybe
a candidate diagnostic marker in differentiating OC from CKD and
HC.
Macuks et al compared the diagnostic
performances of S- and U-HE4 levels (18). The study concluded that urine sample
was an acceptable alternative for HE4 measurement, but S-HE4
measurement (AUC=0.868) was more accurate than U-HE4 measurement
(AUC=0.856) for the discrimination of patients with benign and
malignant diseases. In the study by Hellstrom et al
(19), the ratio of urinary HE4 to
urinary creatinine presented a very high diagnostic accuracy for
diagnosis of OC (AUC=0.969). The present study demonstrated that
the AUCs of R-HE4 reached 0.935 and 0.815 in differentiating OC
from CKD and HC, respectively. Furthermore, the AUC of R-HE4 was as
high as 0.972 when distinguishing CKD from HC. While ROC analysis
demonstrated that S-HE4 could not differentiate OC from CKD, U-HE4
could not distinguish CKD from HC. All of the data from this study
indicated that R-HE4 demonstrated good diagnostic performance in
differentiating OC from CKD and in differentiating CKD from HC.
Thus, the clinical diagnosis of OC should be considered if the
R-HE4 is between 36.85 and 96.15 (providing a sensitivity of 82.6%
and a specificity of 85.4%). Furthermore, the clinical diagnosis of
CKD should be considered if R-HE4 is <36.85. Otherwise, the
individual should be considered healthy.
There were various limitations of the present study.
As a result of the difficulty in recruiting patients who
simultaneously suffer from OC and CKD, the diagnostic efficacy of
R-HE4 for these patients was not investigated. Numerous studies
have confirmed that patients with OC have high levels of S-HE4 and
patients with CKD have low levels of U-HE4. Therefore, it is
reasonably speculated that the lowest R-HE4 exists in patients who
simultaneously suffer from OC and CKD, as compared with OC and CKD
patients, and HC subjects. In addition, the sample size of the
current study was small, consisting of only 31 OC patients and 38
CKD patients.
In conclusion, S- and U-HE4 levels and R-HE4 were
analyzed in OC and CKD patients, and HC subjects. Results
demonstrated that OC patients had higher R-HE4 than patients with
CKD and lower R-HE4 than the HC subjects. Thus, R-HE4 serves as an
effective diagnostic marker for differentiating OC from CKD and HC.
When R-HE4 is between 36.85 and 96.15, a clinical diagnosis of OC
should be considered. The combined determination of S-and U-HE4
levels facilitates the diagnosis of OC.
Acknowledgements
The authors would like to thank the Department of
Gynecology for providing the data from medical records. The authors
would also like to thank the study subjects for their participation
in the present study.
References
|
1
|
Jayson GC, Kohn EC, Kitchener HC and
Ledermann JA: Ovarian cancer. Lancet. 384:1376–1388. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bristow RE, Chang J, Ziogas A, Randall LM
and Anton-Culver H: High-volume ovarian cancer care: Survival
impact and disparities in access for advanced-stage disease.
Gynecol Oncol. 132:403–410. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hamed EO, Ahmed H, Sedeek OB, Mohammed AM,
Abd-Alla AA and Ghaffar HM Abdel: Significance of HE4 estimation in
comparison with CA125 in diagnosis of ovarian cancer and assessment
of treatment response. Diagn Pathol. 8:112013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kappelmayer J, Antal-Szalmás P and Nagy B
Jr: Human epididymis protein 4 (HE4) in laboratory medicine and an
algorithm in renal disorders. Clin Chim Acta. 438:35–42. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Speeckaert MM, Speeckaert R and Delanghe
JR: Human epididymis protein 4 in cancer diagnostics: A promising
and reliable tumor marker. Adv Clin Chem. 59:1–21. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Terlikowska KM, Dobrzycka B, Witkowska AM,
Mackowiak-Matejczyk B, Sledziewski TK, Kinalski M and Terlikowski
SJ: Preoperative HE4, CA125 and ROMA in the differential diagnosis
of benign and malignant adnexal masses. J Ovarian Res. 9:432016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wei SU, Li H and Zhang B: The diagnostic
value of serum HE4 and CA-125 and ROMA index in ovarian cancer.
Biomed Rep. 5:41–44. 2016.PubMed/NCBI
|
|
8
|
Xu Y, Zhong R, He J, Ding R, Lin H, Deng
Y, Zhou L, Li X, Jiang J, Bao Y, et al: Modification of cut-off
values for HE4, CA125 and the ROMA algorithm for early-stage
epithelial ovarian cancer detection: Results from 1021 cases in
South China. Clin Biochem. 49:32–40. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang P, Wang C, Cheng L, Zhang P, Guo L,
Liu W, Zhang Z, Huang Y, Ou Q, Wen X, et al: Development of a
multi-marker model combining HE4, CA125, progesterone, and
estradiol for distinguishing benign from malignant pelvic masses in
postmenopausal women. Tumour Biol. 37:2183–2191. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liao JB, Yip YY, Swisher EM, Agnew K,
Hellstrom KE and Hellstrom I: Detection of the HE4 protein in urine
as a biomarker for ovarian neoplasms: Clinical correlates. Gynecol
Oncol. 137:430–435. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wan J, Wang Y, Cai G, Liang J, Yue C, Wang
F, Song J, Wang J, Liu M, Luo J, et al: Elevated serum
concentrations of HE4 as a novel biomarker of disease severity and
renal fibrosis in kidney disease. Oncotarget. 7:67748–67759.
2016.PubMed/NCBI
|
|
12
|
Mckinnon B, Mueller MD, Nirgianakis K and
Bersinger NA: Comparison of ovarian cancer markers in endometriosis
favours HE4 over CA125. Mol Med Rep. 12:5179–5184. 2015.PubMed/NCBI
|
|
13
|
Anton C, Carvalho FM, Oliveira EI, Maciel
GA, Baracat EC and Carvalho JP: A comparison of CA125, HE4, risk
ovarian malignancy algorithm (ROMA), and risk malignancy index
(RMI) for the classification of ovarian masses. Clinics (Sao
Paulo). 67:437–441. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nagy B Jr, Krasznai ZT, Balla H, Csobán M,
Antal-Szalmás P, Hernádi Z and Kappelmayer J: Elevated human
epididymis protein 4 concentrations in chronic kidney disease. Ann
Clin Biochem. 49:377–380. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Piek A, Meijers WC, Schroten NF,
Gansevoort RT, de Boer RA and Sillje HH: HE4 Serum levels are
associated with heart failure severity in patients with chronic
heart failure. J Card Fail. 23:12–19. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang Z, Zhang Z, Qin B, Wu P, Zhong R,
Zhou L and Liang Y: Human Epididymis Protein 4: A novel biomarker
for Lupus nephritis and chronic kidney disease in systemic Lupus
erythematosus. J Clin Lab Anal. 30:897–904. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lv YW, Yang L, Zhang M, Jiang LH, Niu JH,
Hou J and Cui XH: Increased human epididymis protein 4 in benign
gynecological diseases complicated with chronic renal insufficiency
patients. Genet Mol Res. 14:2156–2161. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Macuks R, Baidekalna I and Donina S:
Urinary concentrations of human epidydimis secretory protein 4
(He4) in the diagnosis of ovarian cancer: A case - control study.
Asian Pac J Cancer Prev. 13:4695–4698. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hellstrom I, Heagerty PJ, Swisher EM, Liu
P, Jaffar J, Agnew K and Hellstrom KE: Detection of the HE4 protein
in urine as a biomarker for ovarian neoplasms. Cancer Lett.
296:43–48. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
FIGO Committee on Gynecologic Oncology, .
FIGO staging for carcinoma of the vulva, cervix, and corpus uteri.
Int J Gynaecol Obstet. 125:97–98. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kidney Disease Improving Global Outcomes
(KDIGO) CKD Work Group, . KDIGO 2012 clinical practice guideline
for the evaluation and management of chronic kidney disease. Kidney
inter. 3:1–150. 2013.
|
|
22
|
Levey AS, Stevens LA, Schmid CH, Zhang YL,
Castro AF III, Feldman HI, Kusek JW, Eggers P, van Lente F, Greene
T and Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology
Collaboration), : A new equation to estimate glomerular filtration
rate. Ann Intern Med. 150:604–612. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ferraro S, Braga F, Lanzoni M, Boracchi P,
Biganzoli EM and Panteghini M: Serum human epididymis protein 4 vs
carbohydrate antigen 125 for ovarian cancer diagnosis: A systematic
review. J Clin Pathol. 66:273–281. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gündüz UR, Gunaldi M, Isiksacan N, Gündüz
S, Okuturlar Y and Kocoglu H: A new marker for breast cancer
diagnosis, human epididymis protein 4: A preliminary study. Mol
Clin Oncol. 5:355–360. 2016.PubMed/NCBI
|
|
25
|
Lamy PJ, Plassot C and Pujol JL: Serum
HE4: An independent prognostic factor in non-small cell lung
cancer. PLoS One. 10:e01288362015. View Article : Google Scholar : PubMed/NCBI
|