Introduction
Somatic cell nuclear transfer (SCNT) is an effective
method of reprogramming differentiated nuclei and has many
potential applications (1). Owing to
incomplete or faulty reprogramming, however, the cloning efficiency
of SCNT embryos remains low (2,3). Aberrant
reprogramming leads to changes in the expression levels of certain
genes important for embryonic development. Studies on the
expression of development-associated genes may be valuable for
improving the developmental competence of SCNT embryos.
Wnt signaling is a critical regulator of many
cellular and physiological processes. Wnt signaling regulates cell
proliferation and differentiation, controls migration and
patterning during embryonic development, and maintains tissue
homeostasis in adults (4,5). Depending on the involvement of β-catenin,
these signaling pathways have been classified as canonical
(β-catenin-dependent) or non-canonical (β-catenin-independent)
(6). In the canonical Wnt signaling
pathway, which has been better examined (7), β-catenin accumulates in the cytoplasm and
eventually translocates to the nucleus where it acts as a
transcriptional co-activator of transcription factors belonging to
the T-cell factor/lymphoid enhancer factor-1(TCF/LEF) family.
β-catenin that accumulates in the cytoplasm is degraded, however,
by a protein complex that includes axin, glycogen synthase kinase-3
(GSK-3), protein phosphatase 2A (PP2A), casein kinase 1 (CK1) and
adenomatous polyposis coli (APC) (6,8). This
degradation complex becomes disrupted as soon as the Wnt protein
binds to receptors of frizzled (Fz) and low-density lipoprotein
receptor-related protein (LRP5/6), which activates multiple
signaling cascades.
Dysfunctions in Wnt signaling result in severe
complications of embryonic development. For example, abnormalities
were observed in the size and shape of mouse embryos lacking intact
maternal/zygotic β-catenin during pre- and post-implantation
development (5). By contrast,
transient expression of Wnt2 enhances cellular reprogramming
efficiency by elevating β-catenin nuclear accumulation (9).
SCNT cloning was associated with altered expression
levels of specific Wnt-associated genes in extra-embryonic tissue
in a time- and tissue-specific manner in cattle (10). Impaired expression of E-cadherin and
β-catenin proteins, along with defective β-catenin signaling,
contributes to insufficient placentation in bovine SCNT-derived
fetuses (11). IWP2 is a potential
inhibitor of Wnt processing and secretion; however, studies
evaluating the effect of IWP2 on embryonic development are lacking.
Therefore, the aim of the present study was to determine whether
the low cloning efficiency of SCNT embryos was correlated with
irregular coordination between Wnt signaling and embryonic
development by applying IWP2 treatment.
Materials and methods
Somatic cell nuclear transfer
Oocyte collection, in vitro maturation and
SCNT were performed in accordance with a previously described
protocol (12) with slight
modifications. Porcine ovaries were obtained from a local
slaughterhouse (HuaZheng Agriculture Development Co. Ltd.) and
transported to our laboratory (Jilin Provincial Key Laboratory of
Animal Embryo Engineering) within 3 h in sterile saline solution
containing penicillin (12 mg/l) and streptomycin (20 mg/l) at
30–35°C. Cumulus-oocyte complexes (COCs) were harvested by
aspirating ovarian follicles (diameter, 3–6 mm) with an 18-gauge
needle attached to a 10-ml disposable syringe, and COCs with at
least three uniform layers of cumulus cells were selected. The
selected COCs were rinsed using PVA-TL HEPES stock solution and
cultured in TCM-199 (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
supplemented with 0.1% polyvinyl alcohol, 3.05 mM D-glucose, 0.91
mM sodium pyruvate, 10 ng/ml epidermal growth factor
(Sigma-Aldrich; Merck KGaA), 0.57 mM cysteine, 0.5 µg/ml
follicle-stimulating hormone (Sigma-Aldrich; Merck KGaA), 0.5 µg/ml
luteinizing hormone (Sigma-Aldrich; Merck KGaA), 75 µg/ml
penicillin and 50 µg/ml streptomycin for 42–44 h at 38.5°C in
humidified air containing 5% CO2.
The cumulus cells of the COCs were
denuded by repeated blowing and suction in 0.1% hyaluronidase with
a 100-µl pipette
Denuded oocytes with an extruded first polar body,
round shape and intact cytoplasm were selected as SCNT recipients.
Oocytes were enucleated with a beveled glass pipette by aspirating
the first polar body and part of the surrounding cytoplasm. A
single donor cell was injected into the perivitelline space of the
enucleated oocyte cytoplast with the same glass pipette. SCNT was
conducted in manipulation medium (Sigma-Aldrich; Merck KGaA)
supplemented with 7.5 µg/ml cytochalasin B (Sigma-Aldrich; Merck
KGaA). The oocyte-cell complexes were fused and activated in medium
consisting of 0.3 M mannitol, 1.0 mM
MgCl2·6H2O, 1.0 mM
CaCl2·2H2O, and 0.5 mM HEPES using a BTX
Electro Cell Manipulator 2001 (BTX, San Diego, CA, USA).
Reconstructed embryos were then cultured in PZM-3 (12) at 38.5°C in a humidified atmosphere
containing 5% CO2. Embryos at the 2-cell, 4-cell,
morula, early blastocyst, and hatching blastocyst stages were
collected at 24, 36, 96, 132 and 156 h after activation,
respectively.
Collection of in vivo embryos
All animal experiments were conducted according to
the guidelines on animal care and use established by the Animal
Care and Welfare Committee of Jilin University. All experimental
protocols were approved by the Ethics Committee of Jilin
University. Gilts for embryo collection were artificially
inseminated at estrus and kept in Changchun HuiChang Livestock Co.
Ltd. (Changchun, China). Subsequently, 2-cell, 4-cell, morula, and
blastocyst embryos were collected by flushing the oviduct or uterus
with 10% fetal bovine serum-phosphate-buffered saline (v/v; Wuhan
Boster Biological Technology, Ltd., Wuhan, China) ~24, 36, 96, or
132 h post coitum, respectively. Embryo collection surgery was
performed under anesthesia.
Synthesis of single-embryo cDNAs and
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR)
Briefly, RNA was extracted from 4–5 SCNT or in
vivo embryos at the 2-cell, 4-cell, morula, and blastocyst
stages. The zona pellucida of each embryo was removed by treatment
with Acidic Tyrode's Solution (Sigma-Aldrich; Merck KGaA) and
rinsed in bovine serum albumin (Sigma-Aldrich; Merck
KGaA)-Dulbecco's phosphate-buffered saline (BSA-DPBS; Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA). Each zona-free embryo
was immediately selected and transferred into lysis buffer by mouth
pipetting. The samples were incubated at 70°C for 90 sec, followed
by 1 min at 4°C. Following release of mRNA, SuperScript III reverse
transcriptase (Invitrogen; Thermo Fisher Scientfic, Inc.) was used
for RT. Exonuclease I (New England BioLabs, Inc., Ipswich, MA, USA)
was then used to remove the free primers. A poly (A) tail was added
to the 3′-end of each first-strand cDNA using terminal
deoxynucleotidyl transferase (Invitrogen; Thermo Fisher Scientific,
Inc.). cDNA amplification was performed with 20 cycles of PCR using
TaKaRa Ex Taq™ HS DNA Polymerase (Takara Bio, Shiga, Japan).
Purification of PCR products was conducted with a
NucleoSpin® Extract II kit (Macherey-Nagel Co., Düren,
Germany).
qPCR was conducted using the iQ5™ real-time PCR
detection system (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Quantification was performed in triplicate using a BioEasy
SYBR-Green I Real Time PCR kit (BIOER, Hangzhou, China) according
to the following procedure: Denaturation at 94°C for 2 min,
followed by 40 cycles of 10 sec at 94°C, 30 sec at the annealing
temperature [with primers as previously described (13)], and 30 sec at 72°C for extension. The
fold change in gene expression was calculated from three replicates
with the formula 2−∆∆Cq (14) followoing normalization against β-actin
expression.
Inactivation of Wnt signaling
The small-molecule inhibitor IWP2 (Stemgent, Inc.,
Cambridge, MA, USA) was used to inactivate Wnt signaling in the
SCNT embryos. Following electrical activation, embryos were
transferred into PZM-3 with 5.0 µM IWP2. Control SCNT embryos were
maintained in PZM-3 without any treatment.
Indirect immunofluorescence
staining
The zona pellucida of each embryo was removed by
treatment with Acidic Tyrode's Solution and rinsed in BSA-DPBS.
Zona-free embryos were fixed in 4% paraformaldehyde (v/v) for 30
min at room temperature and permeabilized with 0.2% Triton X-100
(Sigma-Aldrich; Merck KGaA) for 30 min. Embryos were blocked in 5%
goat serum (Wuhan Boster Biological Technology, Ltd.) in PBS for 30
min and incubated overnight with primary antibodies at 4°C. Primary
antibodies used in the analysis were anti-β-catenin mouse
monoclonal antibody (1:200; sc-65480; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) and anti-active-β-catenin mouse monoclonal
antibody (1:500; 05–655; Upstate Biotechnology, Inc., Lake Placid,
NY, USA). Subsequent to removing the primary antibodies, embryos
were rinsed three times with 0.2% Tween-20 in PBS. Embryos were
incubated with Alexa Fluor 594 goat anti mouse IgG (1:2,000;
R37121; Invitrogen; Thermo Fisher Scientific, Inc.) at 4°C
overnight. Embryos were stained with Hoechst 33342 and observed
under an epifluorescent microscope (Nikon Corporation, Tokyo,
Japan).
Results
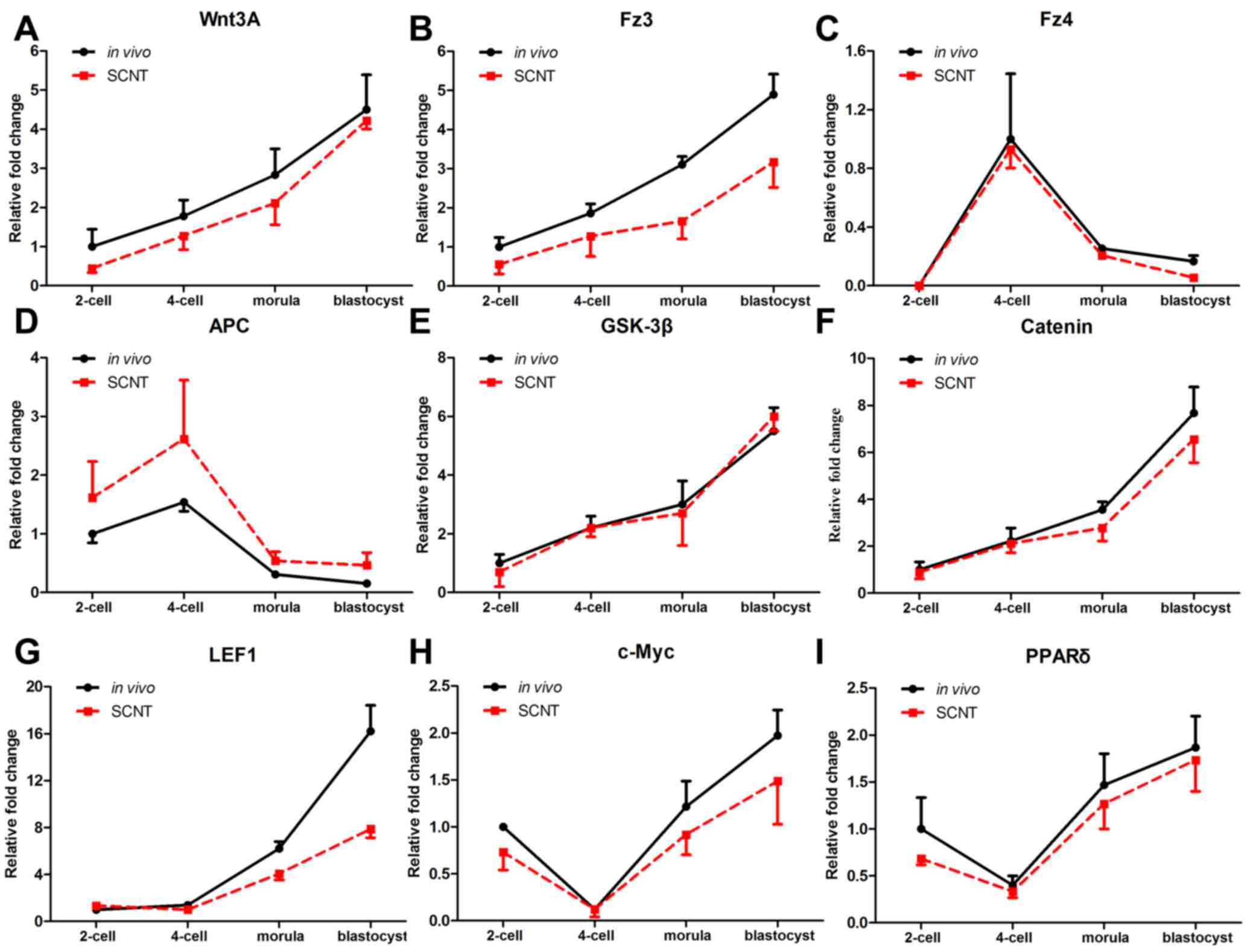
Temporal expression patterns of Wnt
signaling genes in porcine pre-implantation embryos
To determine the roles of canonical Wnt signaling
pathway genes in porcine SCNT embryos, their temporal expression
patterns were examined. The expression of various canonical Wnt
signaling genes, including Wnt3A, frizzled receptor transcripts
(Fz3 and Fz4), adenomatous polyposis coli (APC), glycogen synthase
kinase (GSK)3β, β-catenin, lymphoid enhancer binding factor 1
(LEF1), c-Myc and peroxisome proliferator activated receptor
(PPAR)δ, was analyzed in individual porcine embryos by RT-qPCR. As
shown in Fig. 1, changes in the
expression patterns of Wnt signaling genes were similar between in
vivo and SCNT embryos from the 2-cell to the blastocyst stage. The
expression level of Wnt3A, Fz3, GSK3β, β-catenin and LEF1 steadily
increased from the 2-cell to the blastocyst stage (Fig. 1A,B,E-G). For Fz4 and APC, there was a
peak in expression at the 4-cell stage, and expression then
gradually declined to the blastocyst stage (Fig. 1C and D). By contrast, the expression
levels of Wnt target genes, c-Myc and PPARδ decreased from the
2-cell to the 4-cell stage, and subsequently increased, reaching a
peak at the blastocyst stage (Fig. 1H and
I). Thus, the temporal expression patterns of canonical Wnt
signaling genes in SCNT embryos were similar with those of in
vivo embryos.
Development of SCNT embryos was
compromised with Wnt signaling inactivation
The results revealed that various genes were
expressed abnormally in pre-implantation embryos, although the
actual contribution of the Wnt signaling pathway to the development
competence of SCNT embryos requires further elucidation. Our
previous study revealed that parthenogenetic blastocyst hatching
may be affected by activation or inactivation of Wnt signaling.
Therefore, the effects of Wnt signaling blockade were accessed in
SCNT embryos. As presented in Table I,
no significant differences were identified between control and
IWP2-treated embryos in blastocyst-formation rates or cell numbers
in the early blastocyst. However, the hatching rate of embryos at
156 h was significantly reduced following IWP2 treatment.
 | Table I.Effects of IWP2 on the in vitro
blastocyst development of somatic cell nuclear transfer
embryos. |
Table I.
Effects of IWP2 on the in vitro
blastocyst development of somatic cell nuclear transfer
embryos.
| Treatment | Total oocytes | Early blastocysts (%
± SD) | Hatching blastocysts
[% ± SD) | Cell numbers in early
blastocysts (mean ± SD) |
|---|
| Control | 948 | 248
(28.3±9.1)a | 153
(64.3±11.1)a | 46±10.2a |
| IWP2 | 575 | 160
(27.4±8.8)a | 25
(15.4±2.2)b | 47±8.1a |
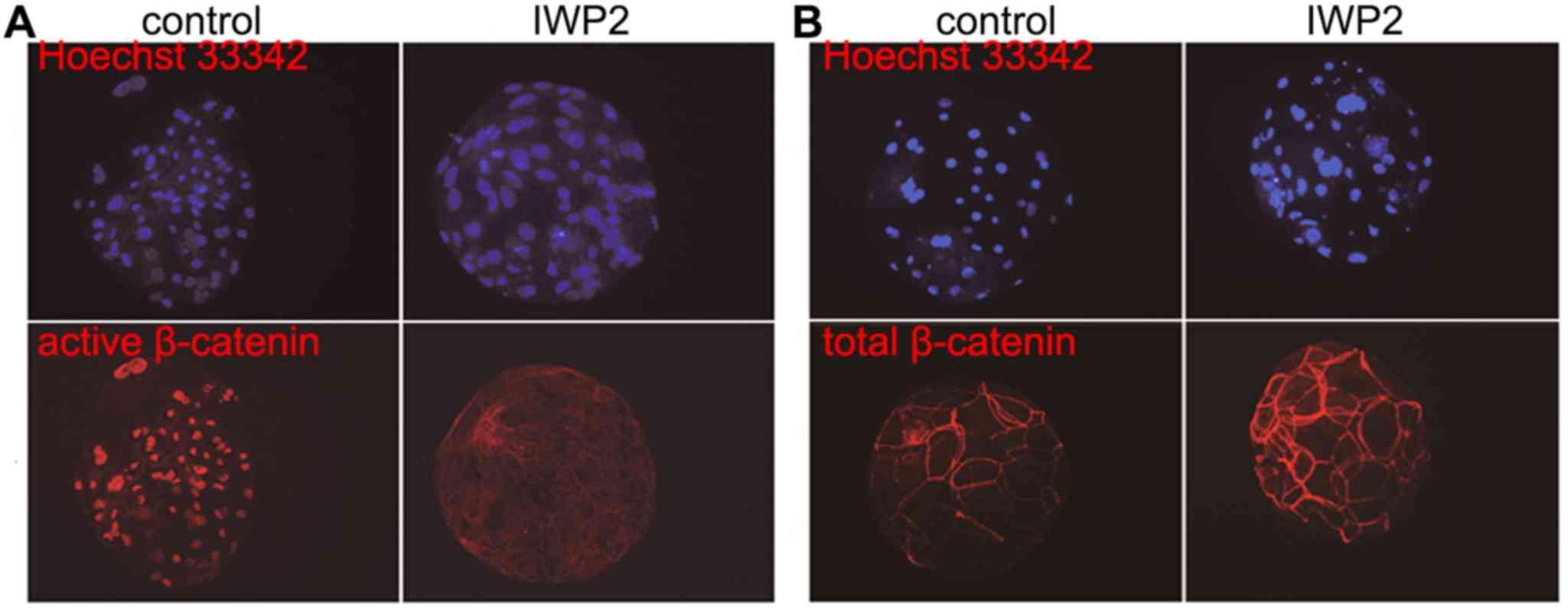
To confirm the inhibition of Wnt signaling following
IWP2 exposure, β-catenin immunofluorescence was observed in SCNT
blastocysts as cytosolic β-catenin is transported into the nucleus
when Wnt signaling is active. As shown in Fig. 2, no significant differences in total
β-catenin abundance between control and IWP2-treated blastocysts
were observed, although expression of active β-catenin was nearly
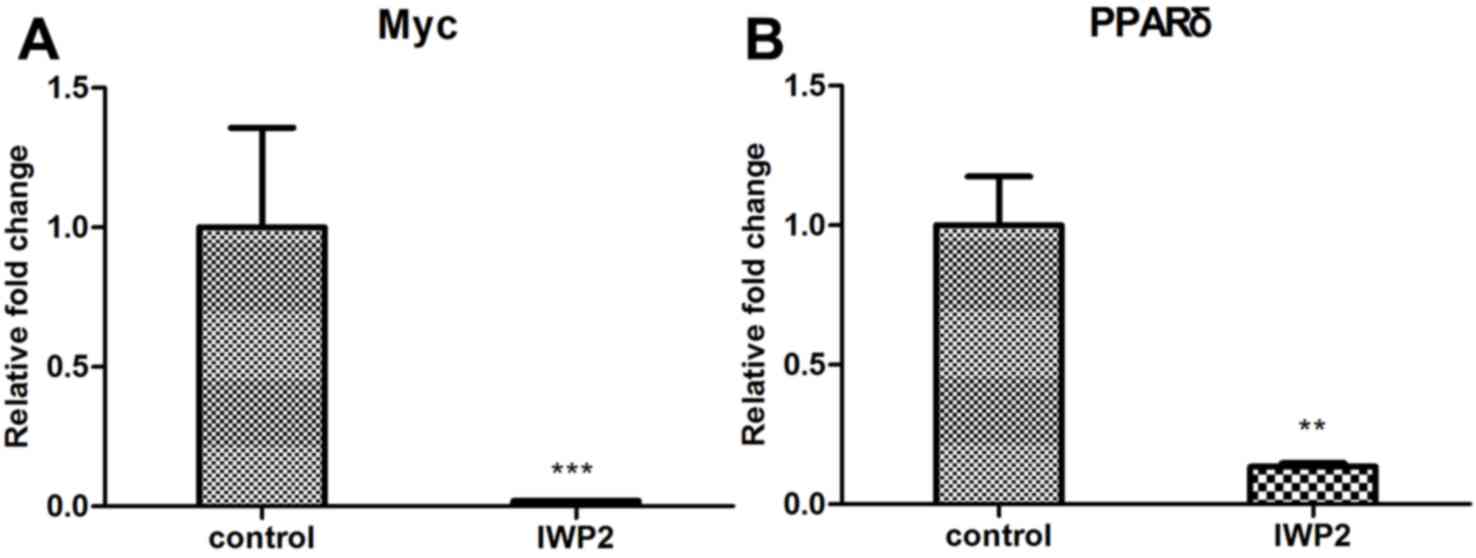
undetectable following IWP2 treatment. In addition, expression
levels of the Wnt target genes, c-Myc and PPARδ were analyzed. As
presented in Fig. 3A and B, expression
levels of c-Myc and PPARδ were downregulated in SCNT blastocyst
following IWP2 treatment. These results confirm that the Wnt
signaling pathway is inactivated by IWP2 treatment.
Discussion
The Wnt signaling pathway is important for embryonic
development, tissue regeneration and homeostasis (15,16). In the
present study, certain Wnt signaling genes were observed to be
aberrantly expressed in SCNT embryo, such as the expression of
Wnt3A, FZ3, LEF1 and APC. In our previous study, it was primarily
Wnt genes that exhibited different expression patterns in
parthenogenetic and in vivo embryos (13). This may be one of the reasons for
differences in developmental competence between parthenogenetic and
SCNT embryos. While parthenogenetic embryos do not develop to term,
a small number of SCNT embryos go through gestation and form cloned
piglets.
Furthermore, the present study identified that
blastocyst hatching was impaired following IWP2 treatment to
inactivate the Wnt signaling pathway. Furthermore, β-catenin has
been shown to be aberrantly expressed in bovine SCNT placentas
(11). These findings indicate that
Wnt signaling is markedly more important for trophectoderm
development, affecting SCNT embryonic development by disturbing
placentation. Notably, one component of Wnt signaling, LEF1, which
was downregulated in SCNT embryos at the morula and blastocyst
stages in the present study, is critical in the development of the
placenta (17). Such aberrant gene
expression patterns may contribute to the failure of embryonic
development or to defects in the SCNT piglets. However, the present
study did not identify the most critical Wnt gene for embryonic
development. Further research should be focused on regulating the
expression of Wnt signaling genes to improve SCNT reprogramming
efficiency and embryonic development.
In summary, the present study demonstrated that only
a small proportion of Wnt signaling genes were abnormally expressed
during pre-implantation development, and this pathway serves
important roles in the development of the trophectoderm in porcine
SCNT blastocysts.
Acknowledgements
The authors would like to thank Mr. Wanhua Xie, Miss
Xianju Chen, Mr. Yang Han, Professor Xiaochun Tang, Professor Daxin
Pang and Professor Zhanjun Li for technical support and helpful
discussion. This work was supported by the China National Key Basic
Research Program (grant no. 2011CB944200), the National Natural
Science Foundation of China (grant no. 81502582), the Fundamental
Research Funds of Northeastern University (grant nos. N152004003
and N141008001-8), and the Postdoctoral Scientific Research Funds
of Northeastern University (grant no. 20150314).
Glossary
Abbreviations
Abbreviations:
|
SCNT
|
somatic cell nuclear transfer
|
|
Wnt3A
|
wingless-type MMTV integration site
family
|
|
Fz3
|
frizzled homolog 3
|
|
Fz4
|
frizzled homolog 4
|
|
APC
|
adenomatous polyposis coli
|
|
Axin
|
axis inhibition protein
|
|
GSK3
|
glycogen synthase kinase 3
|
|
LEF/TCF
|
lymphoid enhancer-binding
factor/T-cell factor
|
References
|
1
|
Iuso D, Czernik M, Zacchini F, Ptak G and
Loi P: A simplified approach for oocyte enucleation in mammalian
cloning. Cell Reprogram. 15:490–494. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Thuan NV, Kishigami S and Wakayama T: How
to improve the success rate of mouse cloning technology. J Reprod
Dev. 56:20–30. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Huang Y, Ouyang H, Yu H, Lai L, Pang D and
Li Z: Efficiency of porcine somatic cell nuclear transfer - a
retrospective study of factors related to embryo recipient and
embryos transferred. Biol Open. 2:1223–1228. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
MacDonald BT, Tamai K and He X:
Wnt/beta-catenin signaling: components, mechanisms, and diseases.
Dev Cell. 17:9–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Messerschmidt D, De Vries WN,
Lorthongpanich C, Balu S, Solter D and Knowles BB:
beta-catenin-mediated adhesion is required for successful
preimplantation mouse embryo development. Development.
143:1993–1999. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bengoa-Vergniory N and Kypta RM: Canonical
and noncanonical Wnt signaling in neural stem/progenitor cells.
Cell Mol Life Sci. 72:4157–4172. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fujimaki S, Wakabayashi T, Takemasa T,
Asashima M and Kuwabara T: The regulation of stem cell aging by Wnt
signaling. Histol Histopathol. 116572015.
|
|
8
|
Minde DP, Anvarian Z, Rudiger SGD and
Maurice MM: Messing up disorder: how do missense mutations in the
tumor suppressor protein APC lead to cancer? Mol Cancer.
10:1012011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kimura M, Nakajima-Koyama M, Lee J and
Nishida E: Transient Expression of WNT2 Promotes Somatic Cell
Reprogramming by Inducing beta-Catenin Nuclear Accumulation. Stem
Cell Reports. 6:834–843. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Biase FH, Rabel C, Guillomot M, et al:
Changes in WNT signaling-related gene expression associated with
development and cloning in bovine extra-embryonic and endometrial
tissues during the peri-implantation period. Mol Reprod Dev.
80:977–987. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kohan-Ghadr HR, Smith LC, Arnold DR,
Murphy BD and Lefebvre RC: Aberrant expression of E-cadherin and
beta-catenin proteins in placenta of bovine embryos derived from
somatic cell nuclear transfer. Reprod Fertil Dev. 24:588–598. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lai L, Kolber-Simonds D, Park KW, et al:
Production of alpha-1,3-galactosyltransferase knockout pigs by
nuclear transfer cloning. Science. 295:1089–1092. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang Y, Ouyang H, Xie W, et al: Moderate
expression of Wnt signaling genes is essential for porcine
parthenogenetic embryo development. Cellular signalling.
25:778–785. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(T)(−Delta Delta C) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Caprioli A, Villasenor A, Wylie LA,
Braitsch C, Marty-Santos L, Barry D, Karner CM, Fu S, Meadows SM,
Carroll TJ and Cleaver O: Wnt4 is essential to normal mammalian
lung development. Dev Biol. 406:222–234. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vlad-Fiegen A, Langerak A, Eberth S and
Muller O: The Wnt pathway destabilizes adherens junctions and
promotes cell migration via beta-catenin and its target gene cyclin
D1. FEBS Open Bio. 2:26–31. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Galceran J, Farinas I, Depew MJ, Clevers H
and Grosschedl R: Wnt3a(−/−)-like phenotype and limb deficiency in
Lef1(−/−)Tcf1(−/−) mice. Genes & Development. 13:709–717. 1999.
View Article : Google Scholar
|