Introduction
ADAM metalloproteinase with thrombospondin type-1
motif, member 13 (ADAMTS13) is a zinc-containing enzyme
specifically cleaving the multimeric von Willebrand factor (VWF)
between Tyr1605 and Met1606 within the VWF A2 domain (1). ADAMTS13 is produced exclusively in
hepatic stellate cells adjacent to endothelial cells (2). VWF is synthesized in vascular endothelial
cells and released into plasma as ‘unusually large’ VWF multimers
(UL-VWFM) (3). ADAMTS13 deficiency,
caused by either ADAMTS13 gene mutations (4) or inhibitory autoantibodies against
ADAMTS13 (5) increases UL-VWFM plasma
levels, causing platelet clumping and thrombi under high shear
stress, resulting in microcirculatory disturbances (3,5). ADAMTS13
deficiency is linked to thrombotic thrombocytopenic purpura (TTP)
occurrence (5) characterized by
thrombocytopenia, renal dysfunction, fluctuating neurological
symptoms, microangiopathic hemolytic anemia and fever (6).
The role of ADAMTS13 role in alcoholic hepatitis
(7), liver cirrhosis (8) and acute liver failure (ALF) (9) progression is reported by studies,
suggesting that ADAMTS13 enzyme-VWF substrate imbalances are
related to liver disorders and multiple organ failure (MOF)
progression.
Endotoxemia, due to Kupffer cell dysfunction and
increased intestinal permeability, triggers pro-inflammatory
cytokine production, possibly causing a systemic inflammatory
response syndrome and microcirculatory disturbances, leading to MOF
(10,11)
Recent in vitro studies demonstrated associations of
inflammatory cytokines with decreased ADAMTS13 activity
(ADAMTS13:AC) (12) and increased
UL-VWFM release from endothelial cells (13). Inflammation-associated ADAMTS13
deficiency promotes UL-VWFM formation in patients with sepsis
(14), indicating a close linkage
between cytokinemia, endotoxemia and ADAMTS13:AC in ALF.
The authors explored the potential mechanisms
underlying reduced plasma ADAMTS13:AC in ALF patients by
determining plasma cytokine and endotoxin concentrations and
evaluating ADAMTS13 inhibitor activity.
Materials and methods
Ethical approval
All procedures performed in studies involving human
participants were in accordance with the ethical standards of the
Institutional, National Research Committee and with the 1964
Helsinki Declaration. All subjects gave informed consent for
participation. The study was approved by the Ethics Committee of
Nara Medical University (Kashihara, Japan).
Patients
A total of 27 acute hepatitis (AH) patients (14 men,
13 women; mean age, 59.8 years) and 11 ALF patients (6 men, 5
women; mean age, 47.9 years; Table I)
were enrolled and admitted to Nara Medical University between
December 2001 and September 2009. None had a history of
coagulopathies, sepsis or platelet disorders. ALF was diagnosed
based on physical findings and laboratory tests according to the
criteria of Intractable Hepato-Biliary Diseases Study Group of
Japan (15). ALF was defined as
prothrombin time values 40% or less of standardized values or
international normalized ratios 1.5 or more caused by severe liver
damage within 8 weeks of symptom onset. For the control group, 10
healthy volunteers aged 20–40 years were also analyzed.
 | Table I.Clinical data of patients with acute
hepatitis and acute liver failure. |
Table I.
Clinical data of patients with acute
hepatitis and acute liver failure.
| Variable | Acute hepatitis
(n=27) | Acute liver failure
(n=11) | P-value |
|---|
| Age (years) | 47.9±19.1 | 59.8±18.0 | NS |
| Sex
(male/female) | 14/13 | 6/5 | NS |
| Serum total bilirubin
(mg/dl) | 6.3±7.0 | 11.0±7.1 | P<0.05 |
| Aspartate
aminotransferase (IU/l) | 515.8±520.8 | 2,253.1±2,572.4 | P<0.05 |
| Alanine
aminotransferase (IU/l) | 717.1±856.4 | 1,642.6±1,515.7 | P<0.05 |
| Lactate dehydrogenase
(IU/l) | 824.7±1,784.9 | 3,490.7±5,212.4 | P<0.05 |
| alkaline phosphatase
(IU/l) | 499.6±630.0 | 1,093.3±1,693.5 | NS |
| γ-glutamyl
transpeptidase (IU/l) | 213.7±261.1 | 106.9±82.8 | NS |
| Cholinesterase
(IU/l) | 234.1±80.1 | 149.0±55.8 | NS |
| Serum albumin
(g/dl) | 3.7±0.7 | 3.4±0.9 | P<0.05 |
| Blood urea nitrogen
(mg/dl) | 13.2±12.1 | 43.0±38.9 | P<0.05 |
| Serum creatinine
(mg/dl) | 0.8±0.5 | 1.7±1.2 | P<0.05 |
| Prothrombin time
(%) | 86.6±24.8 | 35.5±21.9 | P<0.05 |
| Fibrin/fibrinogen
degradation products (µg/ml) | 29.1±23.5 | 3.5±2.5 | P<0.05 |
| White blood cell
count (/mm3) |
6,018.3±2,575.4 |
11,444.4±5,789.0 | P<0.05 |
| Hemoglobin
(g/dl) | 12.7±2.2 | 11.2±1.9 | NS |
| Platelet count
(×104/mm3) | 22.0±9.0 | 10.9±11.4 | P<0.05 |
| Hepatic
encephalopathy (grade II–III) | 0 | 11 | P<0.05 |
| Renal failure/heart
failure/ascites/sepsis/DIC | 0/0/0/0/1 | 4/3/4/4/6 |
|
| UL-VWFM
positive | 0 | 4 | P<0.05 |
| Outcome
(alive/dead) | 27/0 | 2/9 | P<0.05 |
ADAMTS13:AC, VWF antigen (VWF:Ag),
UL-VWF, and ADAMTS13 inhibitor assays
Blood samples were collected from patients at
admission into plastic tubes containing 1/10th volume of 3.8%
sodium citrate (anticoagulant). Platelet-poor plasma was prepared
by centrifuging plasma samples at 3,000 × g for 15 min at 4°C;
aliquots were stored at −80°C. Plasma ADAMTS13:AC was determined
using a chromogenic ELISA (ADAMTS13-act-ELISA kit, cat. no. 019491;
Kainos Laboratories Inc., Tokyo, Japan). The detection limit of the
activity was 0.5%; normal value was 99±22% (mean ± standard
deviation). Plasma UL-VWFM was analyzed by 0.9% SDS-agarose gel
electrophoresis using 1 µl sample aliquots (16). High-molecular-weight bands undetected
in normal plasma were defined as UL-VWFM (17). Plasma VWF:Ag was measured by sandwich
ELISA using a rabbit polyclonal anti-human VWF antibody (1:1,000;
cat. no. A0082; Dako; Agilent Technologies, Inc., Santa Clara, CA,
USA). ADAMTS13 inhibitor activity was measured using
heat-inactivated plasma (at 56°C for 30 min). One Bethesda's unit
(BU) of inhibitor was defined as the amount required to reduce
ADAMTS13:AC to 50% of control values (18); its titer was estimated to be
significant at >0.5 BU/ml.
Cytokine concentrations
Interleukin (IL)-6 and IL-8 plasma concentrations
were determined using immunoassay kits (cat. no. of IL-6, KHC0061;
cat. no. of IL-8, KHC0081; BioSource International; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). The detection limits of IL-6
and IL-8 plasma levels are below 7.8 pg/ml and 15.6 pg/ml,
respectively. AH or ALF patients were classified into three groups
according to IL-6 plasma level (8): i)
<7.8 pg/ml; ii) 7.8–100 pg/ml; and iii) >100 pg/ml. Patients
were also classified into two groups according to IL-8 plasma
level: i) <15.6 pg/ml and ii) ≥15.6 pg/ml (7).
Endotoxin determination
Blood specimens from 10 healthy controls and AH or
ALF patients were obtained under aseptic conditions by peripheral
venipuncture using pyrogen-free syringes and needles and were mixed
in pyrogen-free tubes containing 1/10th volume of 3.8% sodium
citrate (anticoagulant). Plasma was immediately separated in a
refrigerated centrifuge at 3,000 × g for 15 min at 4°C and stored
at −80°C for further analysis. Endotoxin activity was measured
using a chromogenic substrate assay (Toxicolor LS-M Set; Seikagaku
Kogyo Co., Ltd., Tokyo, Japan) with kinetic analysis (19). Briefly, 50 µl plasma sample was mixed
with 450 µl 0.02% Triton X-100, and heated at 70°C for 10 min to
inactivate any inhibitor reacting with endotoxin; serial standard
solutions were prepared to final exogenous endotoxin concentrations
(180, 90, 45, 22.5, 11.3 and 5.6 pg/ml). Absorbance was measured at
37°C every 15 sec for 30 min by a microprocessor-controlled reader
(Wellreader, SK603; Seikagaku Kogyo Co., Ltd.). The linear part of
the kinetics curve was read, and endogenous plasma endotoxin
concentrations were calculated from the obtained standard curve.
Determinations were performed in duplicate and mean values were
utilized.
Statistical analysis
Differences between paired and unpaired groups were
analyzed using the Mann-Whitney U-test and the Steel-Dwass test
after the hypothesis in analysis of variance was rejected.
Correlations were calculated using the Spearman rank test.
Categorical data were analyzed using the Fisher's exact test.
Analyses were performed using EZR (version 1.35; Saitama Medical
Center, Jichi Medical University, Saitama, Japan), a graphical user
interface for R (version 2.13.0; The R Foundation for Statistical
Computing, Vienna, Austria) (20).
Data are expressed as means ± standard deviation. A two-tailed
P<0.05 was considered to indicate a statistically significant
difference.
Results
Clinical characteristics and
laboratory values
Clinical data from AH and ALF patients are presented
in Table I. Serum total bilirubin,
aspartate aminotransferase, alanine aminotransferase, lactate
dehydrogenase, blood urea nitrogen, creatinine and white blood cell
counts were higher in ALF patients than in AH patients, whereas
serum albumin, prothrombin time, and platelet count were lower in
ALF patients than in AH patients. All AH patients survived, while 9
of 11 ALF patients died of hepatic failure within 12–132 days of
admission. In ALF patients, various causes were implicated,
including the hepatitis B virus (2 patients), autoimmune hepatitis
(1 patient), drugs (2 patients) and systemic circulatory
disturbances (2 patients); in 4 patients, the cause was cryptogenic
or unknown. ALF type was considered to be acute in 6 patients,
subacute in 4, and late-onset hepatic failure in 1. The 9
non-survivors with ALF showed grade II–III hepatic encephalopathy,
while 3 had ascites, 3 had heart failure and 4 had sepsis,
indicating MOF occurrence. Only 5 of these patients had
disseminated intravascular coagulation (DIC). Of the remaining 2
survivors with ALF, one was complicated by hepatic encephalopathy,
ascites and DIC but not by heart failure or sepsis, while the other
had moderate ascites and required treatment with a liver
transplant. A total of 9 ALF patients was treated with plasma
exchange and standard therapy. Two patients did not receive plasma
exchange because of systemic circulatory disturbances and were
treated with liver transplantation.
Plasma ADAMTS13:AC, VWF:Ag, and
UL-VWFM
Plasma ADAMTS13:AC on admission was significantly
lower in AH (63.9±14.5%; P<0.05) and ALF (14.4±8.6%; P<0.05)
patients than in healthy subjects (126.6±15.0%). Activity further
decreased in ALF patients compared with AH patients (P<0.05;
Fig. 1A). Values of plasma VWF:Ag were
higher in AH (231.4±90.6%; P<0.05) and ALF (535.2±238.9%;
P<0.05) patients than in healthy subjects (108.8±76.6%); VWF:Ag
values were higher in ALF patients than in AH patients (P<0.05;
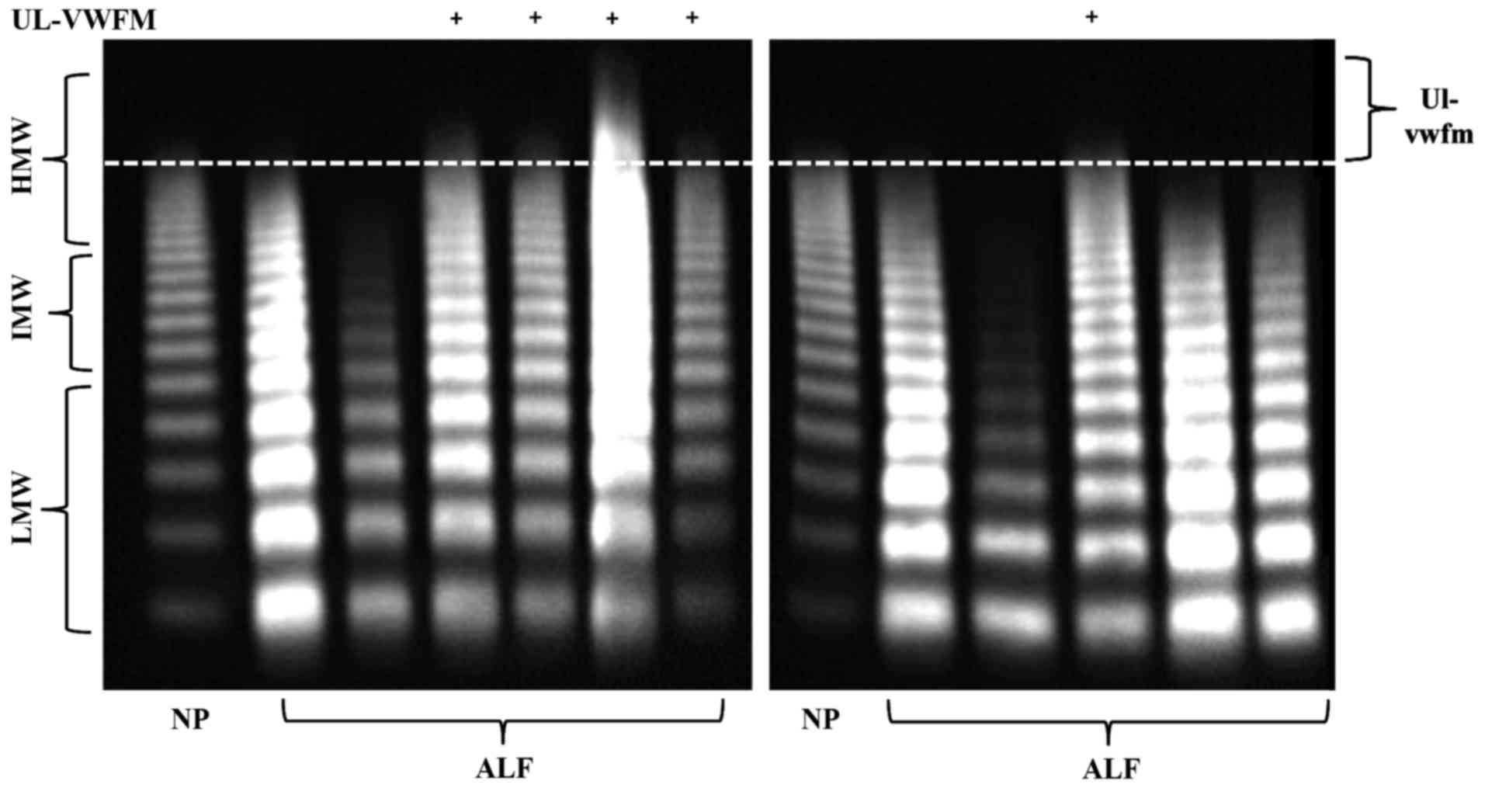
Fig. 1B). Plasma UL-VWFM (Fig. 2) was detected in 5 (13.2%) of 38 AH and
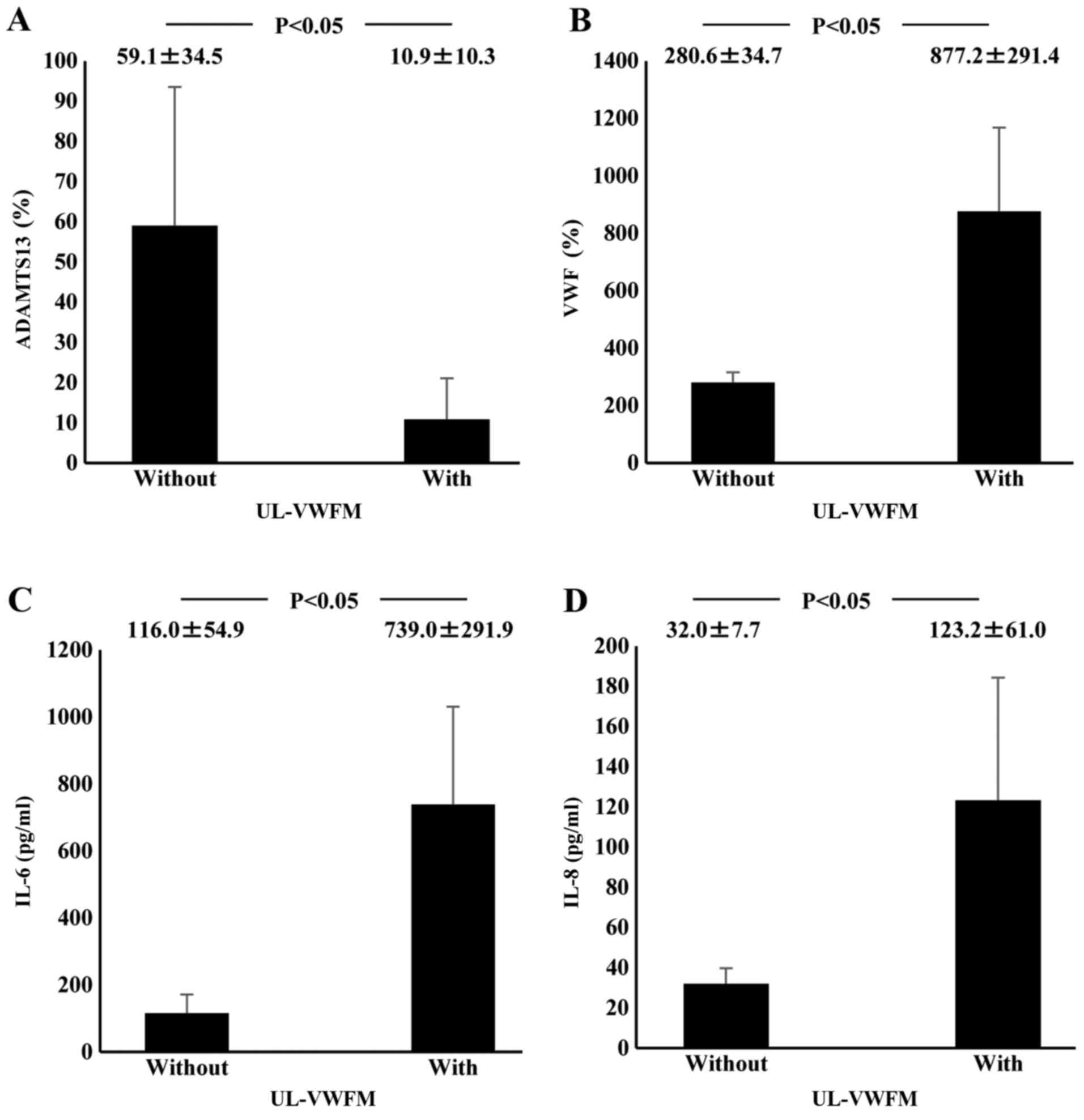
ALF patients. Plasma ADAMTS13:AC values of patients with UL-VWFM
(10.9±10.3%) were significantly lower than values of those without
UL-VWFM (59.1±34.5%; P<0.05; Fig.
3A). Plasma VWF:Ag values of patients with UL-VWFM
(877.2±291.4%) were significantly higher than values of those
without (280.6±34.7%; P<0.05; Fig.
3B). Plasma ADAMTS13:AC values on admission (53.9±34.6% vs.
24.1±16.8%; P<0.05) were higher in survivors than in
non-survivors, respectively. Plasma VWF:Ag values on admission
(252.8±127.4% vs. 419.6±99.5%; P<0.05) were lower in survivors
than in non-survivors, respectively (Table II).
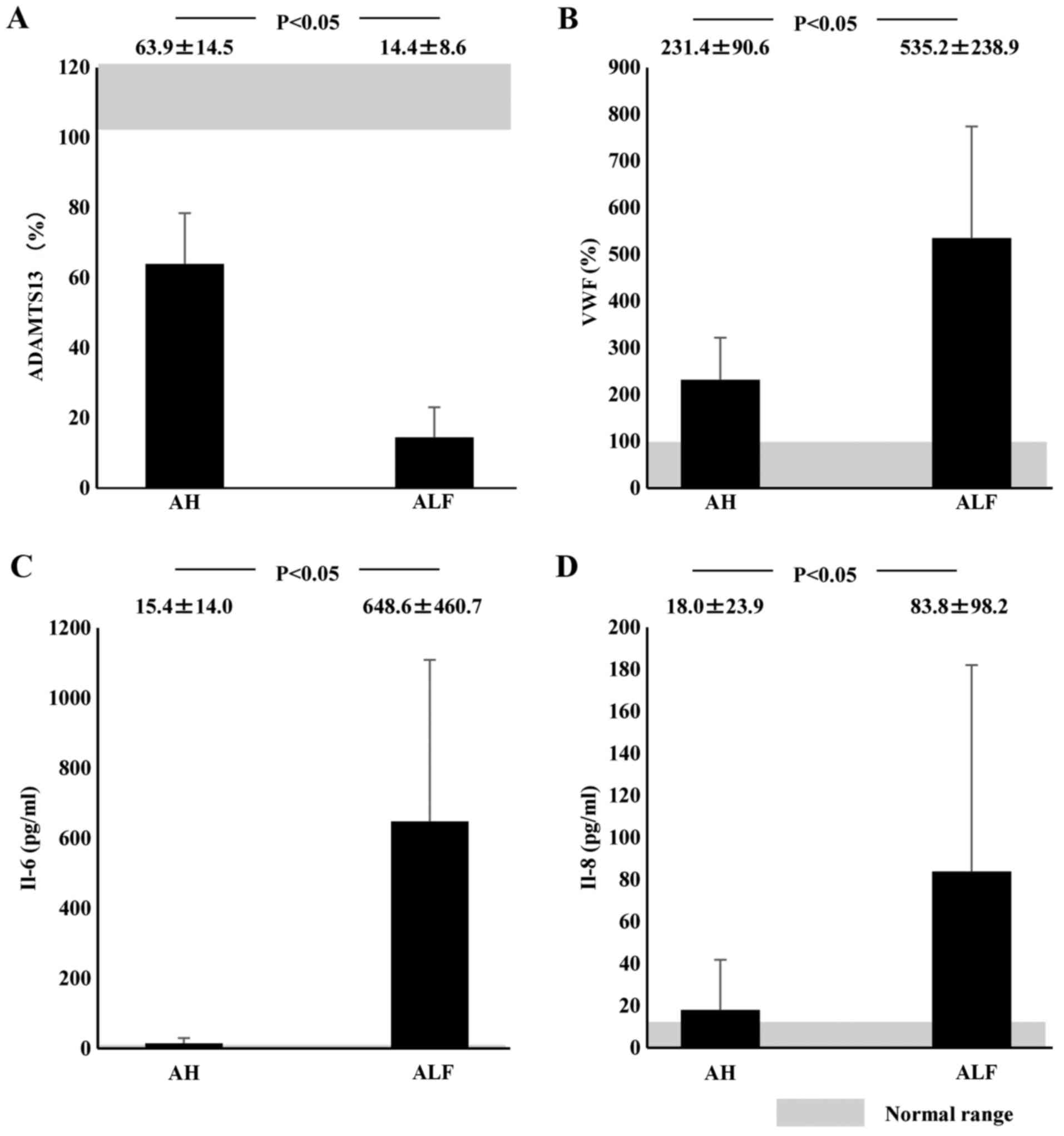 | Figure 1.Plasma ADAMTS13:AC, VWF:Ag, and
cytokine levels in AH or ALF patients on admission. Shaded area
shows the normal range. (A) Plasma ADAMTS13:AC levels were
significantly lower in ALF patients than in AH patients. (B) Plasma
VWF:Ag levels were significantly higher in ALF patients than in AH
patients. (C) IL-6 and (D) IL-8 concentrations were significantly
higher in ALF patients than in AH patients. Data are expressed as
means ± standard deviation. P<0.05 as indicated. ADAMTS13, ADAM
metalloproteinase with thrombospondin type-1 motif, member 13; AC,
activity; VWF, von Willebrand factor; Ag, antigen; AH, acute
hepatitis; ALF, acute liver failure; IL, interleukin. |
 | Table II.Changes in plasma ADAMTS13:AC and
VWF:Ag in survivors and non-survivors with acute hepatitis and
acute liver failure on admission. |
Table II.
Changes in plasma ADAMTS13:AC and
VWF:Ag in survivors and non-survivors with acute hepatitis and
acute liver failure on admission.
| Variables | Survivors
(n=29) | Non-survivors
(n=9) | P-value |
|---|
| ADAMTS13:AC
(%) | 53.9±34.6 | 24.1±16.8 | P<0.05 |
| VWF:Ag (%) | 252.8±127.4 | 419.6±99.5 | P<0.05 |
Plasma cytokine levels and their
relationship to ADAMTS13:AC, VWF:Ag, and UL-VWFM
Plasma IL-6 concentrations at admission in ALF
patients (648.6±460.7 pg/ml) were significantly higher than those
in healthy controls (<7.8 pg/ml; P<0.05) or those in AH
patients (15.4±14.0 pg/ml; P<0.05). However, plasma IL-6 levels
did not differ between AH patients and healthy subjects (Fig. 1C). Plasma IL-8 concentrations were
significantly higher in ALF patients (83.8±98.2 pg/ml) than in AH
patients (18.0±23.9 pg/ml; P<0.05). However, IL-8 concentrations
did not differ between AH patients and healthy subjects (Fig. 1D). ADAMTS13:AC on admission
concomitantly decreased along with the elevation of plasma IL-6
levels (P<0.05; Fig. 4A). Plasma
ADAMTS13:AC levels in patients with IL-8 concentrations ≥15.6 pg/ml
were significantly lower than those in patients with IL-8
concentrations <15.6 pg/ml (P<0.05; Fig. 4B). VWF:Ag on admission increased with
increasing IL-6 levels (P<0.05; Fig.
4C). In patients with IL-8 levels ≥15.6 pg/ml, VWF:Ag was
significantly higher than in those with IL-8 levels <15.6 pg/ml
(Fig. 4D). Plasma IL-6 levels of
patients with UL-VWFM (739.0±291.9 pg/ml) were significantly higher
than levels of those without (116.0±54.9 pg/ml; P<0.05;
(Fig. 3C). Plasma IL-8 levels of
patients with UL-VWFM (123.2±61.0 pg/ml) were significantly higher
than levels of those without (32.0±7.7 pg/ml; P<0.05; Fig. 3D).
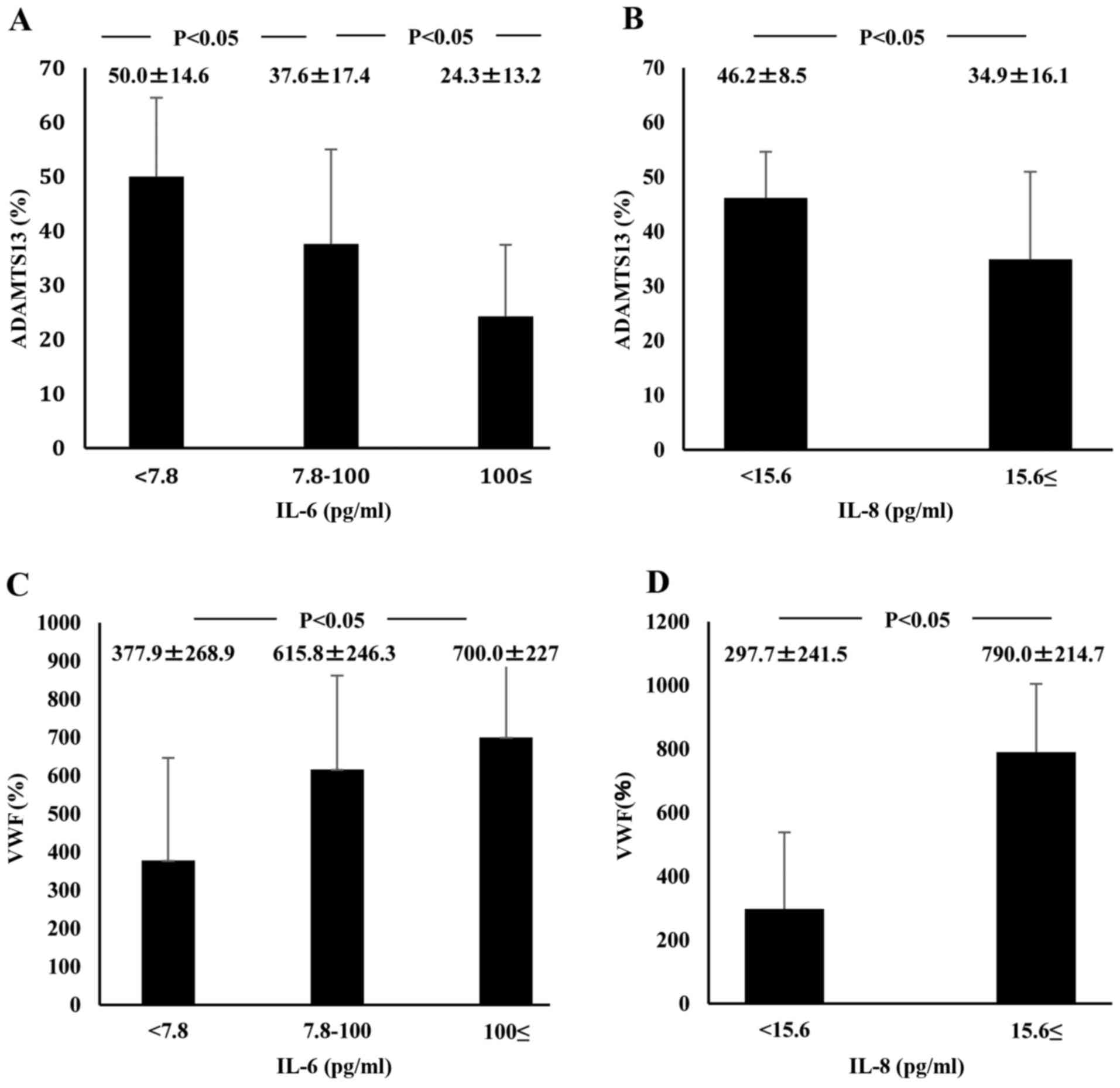 | Figure 4.Relationship between plasma cytokine
levels, ADAMTS13 activity, and VWF:Ag in AH and ALF patients on
admission. IL-6 plasma level detection limit is below 7.8 pg/ml and
that of IL-8 plasma level is below 15.6 pg/ml. (A) ADAMTS13
activity concomitantly decreased with increasing IL-6 plasma
levels. (B) Activity was lower in patients with IL-8 levels ≥15.6
pg/ml than in those with IL-8 <15.6 pg/ml levels. (C) VWF:Ag
concomitantly increased with increasing plasma levels of IL-6. (D)
In addition, the antigen increased in patients with IL-8 levels
≥15.6 pg/ml compared with those with IL-8 levels <15.6 pg/ml.
Data are expressed as means ± standard deviation. P<0.05 as
indicated. ADAMTS13, ADAM metalloproteinase with thrombospondin
type-1 motif, member 13; VWF, von Willebrand factor; Ag, antigen;
AH, acute hepatitis; ALF, acute liver failure; IL, interleukin. |
Plasma endotoxin levels and their
relationships to ADAMTS13:AC and VWF:Ag
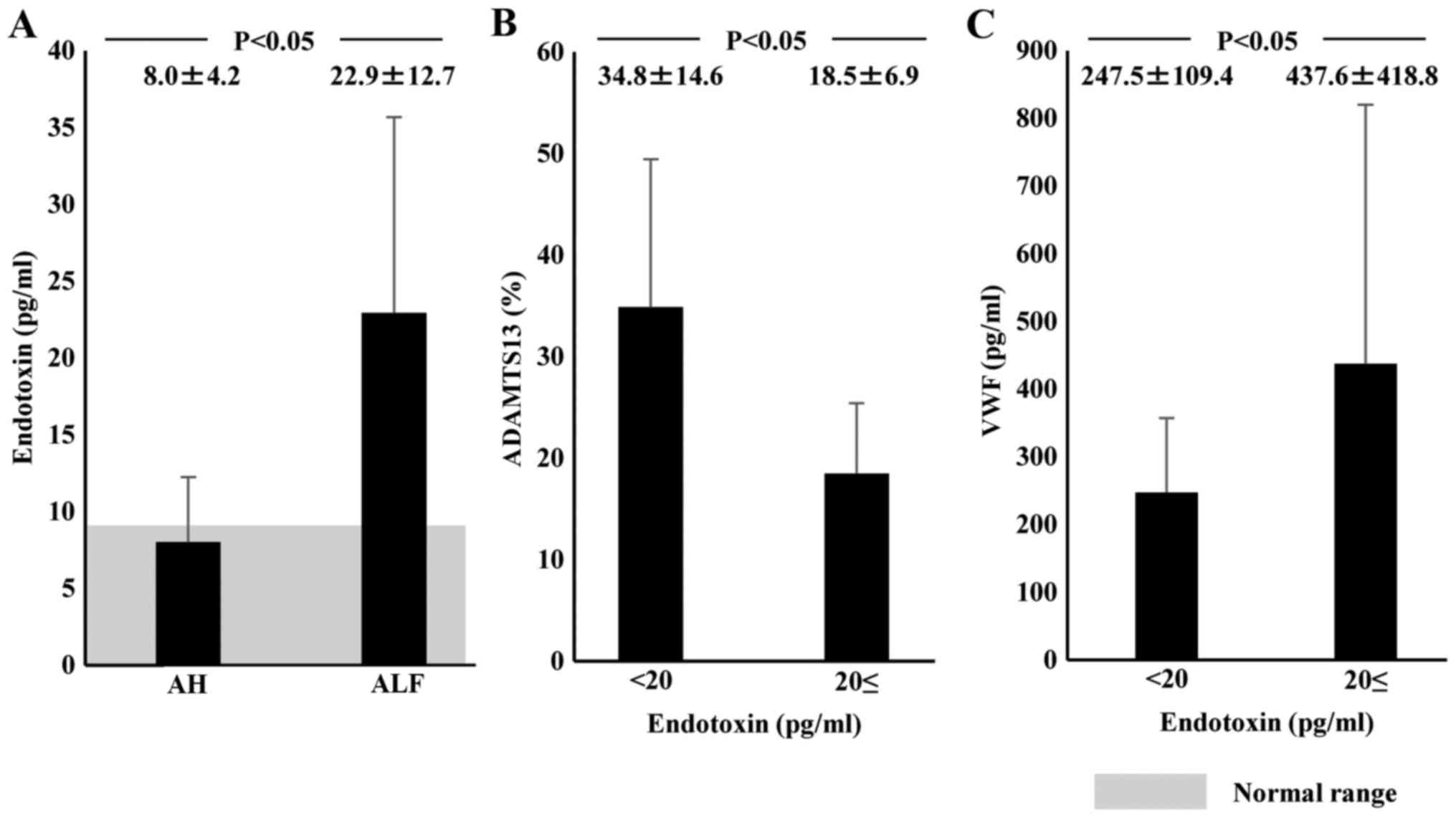
Plasma endotoxin levels in normal subjects were
<10 pg/ml (average, 7.9±1.7 pg/ml). Plasma endotoxin
concentration on admission was significantly higher in ALF patients
(22.9±12.7 pg/ml) than in AH patients (8.0±4.2 pg/ml; P<0.05;
Fig. 5A). Plasma ADAMTS13:AC levels
were significantly lower in patients with endotoxin levels ≥20
pg/ml (18.5±6.9%) than in those with lower endotoxin levels
(34.8±14.6%; P<0.05; Fig. 5B).
Plasma levels of VWF:Ag were significantly higher in patients with
endotoxin levels ≥20 pg/ml (437.6±418.8%) than in those with lower
endotoxin levels (247.5.6±109.4%; P<0.05; Fig. 5C).
Plasma ADAMTS13 inhibitor and its
relationship to ADAMTS13:AC, VWF:Ag and clinical features
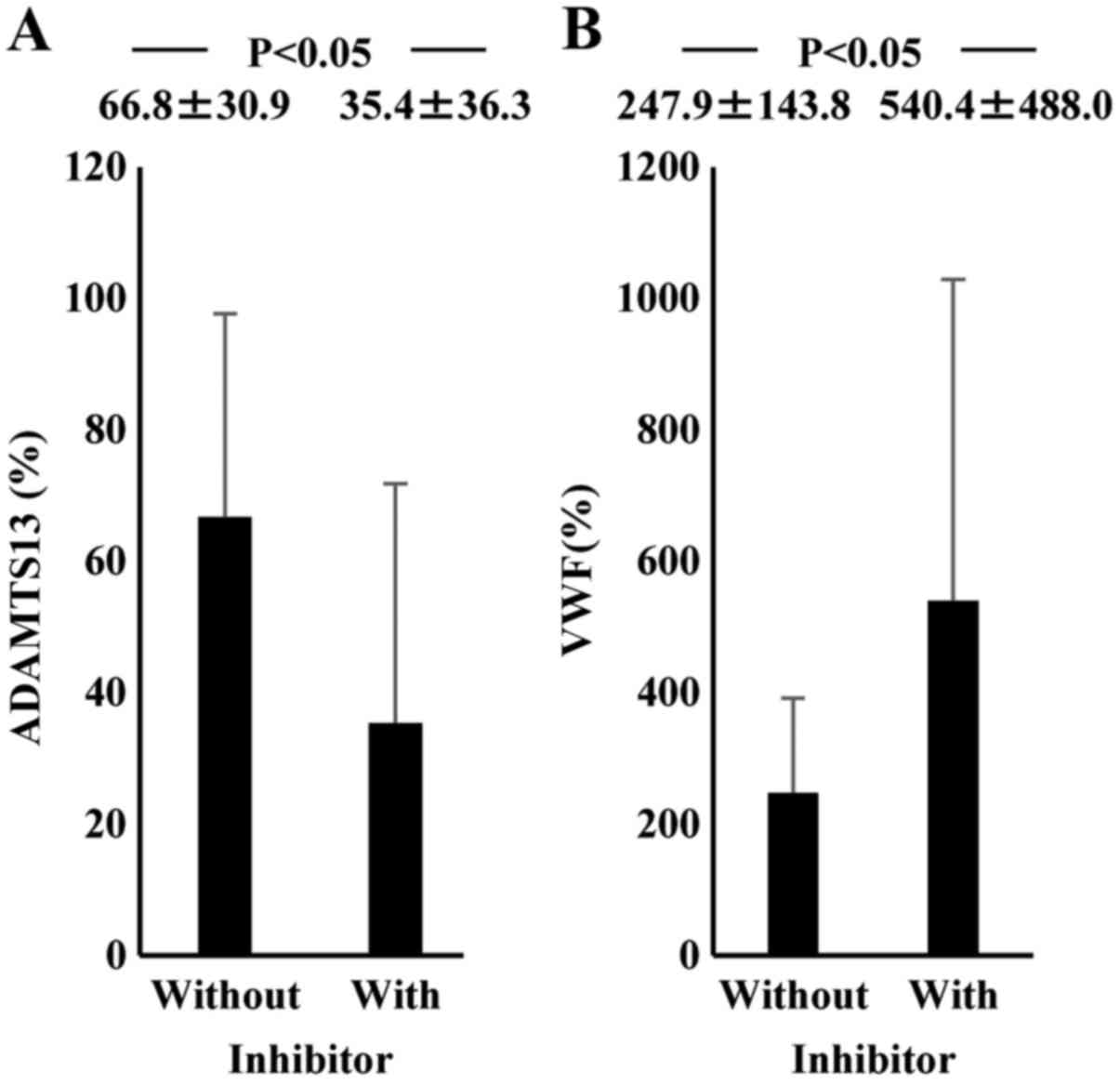
The plasma ADAMTS13 inhibitor was detected on
admission in 8 ALF (66.7%) and 6 AH patients (22.2%). The average
inhibitory titer was 1.2 BU/ml (range, 0.6–2.4 BU/ml) in ALF
patients and 0.7 BU/ml (range, 0.6–0.8 BU/ml) in AH patients.
Patients with the inhibitor showed lower ADAMTS13:AC and higher
VWF:Ag than those without the inhibitor (Fig. 6). AH and ALF patients who had the
inhibitor presented lower levels of serum albumin (3.2±0.6 g/dl vs.
3.8±0.6 g/dl; P<0.05) and higher levels of blood urea nitrogen
(35.8±36.2 g/dl vs. 13.4±13.3 mg/dl; P<0.05), creatinine
(1.5±1.1 mg/dl vs. 0.8±0.5 mg/dl; P<0.05) and white blood cell
count (9,839.2±5,813.4/µl vs. 6,160.5±2,629.4/µl; P<0.05).
Discussion
The authors reported that low ADAMTS13:AC and high
VWF:Ag were closely related to plasma endotoxin, pro-inflammatory
cytokines and poor prognosis in AH and ALF patients. Hugenholtz
et al (9) reported low
ADAMTS13:AC and high VWF:Ag in AH and ALF patients. However, they
stated that low ADAMTS13:AC, but not high VWF:Ag, was associated
with poor outcome. The reported lower ADAMTS13:AC and higher
VWF:Ag, in ALF patients than in AH patients. A total of 9 of our 11
ALF patients died from hepatic failure within 12–132 days of
admission. An analysis of the relationship of these parameters in
all our subjects (AH and ALF) in relation to clinical course showed
that ADAMTS13:AC and VWF:Ag changes were related to poor outcome
and developing various complications. UL-VWFM was identified in 5
of 38 AH and ALF patients who also had marked ADAMTS13:AC
deficiency and marked high VWF values. These observations provide
an explanation of the deleterious UL-VWFM effects on severe liver
injury progression. Conversely, Hugenholtz et al (9) reported that UL-VWFM proportion was
reduced in spite of markedly low ADAMTS13:AC in AH and ALF
patients. They explained this discrepancy from the action of other
proteases on VWF and/or the effect of treatment. They collected
blood sample from the patients after starting the treatment. Thus,
they did not analyze UL-VWFM on admission correctly but agreed to
UL-VWFM effects on platelet thrombi formation in hepatic
microvasculature and resultant tissue ischemia.
The dysfunctional ADAMTS13:AC and VWF:Ag mechanisms
in hepatic failure remain unknown. Here, ADAMTS13:AC gradually
decreased and VWF:Ag progressively increased with concomitant
increases in IL-6 and IL-8 concentrations. Plasma IL-6 and IL-8
levels were significantly higher in patients with UL-VWFM than in
those without it. It has been demonstrated in vitro with
human umbilical vein endothelial cells that IL-6 inhibits the
ADAMTS13 actions under flow conditions, while IL-8 stimulates
UL-VWFM release (12).
ALF is characterized by massive necroinflammation of
liver tissue and is associated with high mortality (21,22). High
serum proinflammatory cytokines levels have been reported (21,22) and are
related to the development of multiorgan dysfunction (23). Pro-inflammatory cytokines are also
involved in the systemic inflammatory response syndrome
pathophysiology (24).
Pro-inflammatory cytokine removal is important during continuous
hemodiafiltration for acute hepatic failure (25). Marked increases in pro-inflammatory
cytokines (IL-6 and IL-8) may have decreased ADAMTS13:AC and
increased VWF:Ag, along with UL-VWFM, possibly leading to MOF
development in ALF with through microcirculatory disturbances.
However, endotoxemia serves an important role in the
initiation and aggravation of acute liver injury through the
enhancement of proinflammatory cytokines, including IL-6 and IL-8
(10,26). Here, pro-inflammatory cytokine (IL-6
and IL-8) concentrations and plasma endotoxin levels were
significantly increased in ALF patients. The chromogenic endotoxin
assay with kinetic analysis following plasma pretreatment with
detergent (Triton X-100) and heating at 70°C for 10 min was based
on the authors' previous basic experiments (19,27).
Compared with endotoxin levels <20 pg/ml, those ≥20 pg/ml were
related to lower ADAMTS13:AC and higher VWF:Ag. These results
indicated that enhanced endotoxemia may be closely related to
decreased ADAMTS13:AC and increased VWF:Ag through enhanced
cytokinemia.
To the best of the authors' knowledge, the present
study is the first report to demonstrate a potential linkage
between endotoxemia, enhanced inflammatory cytokines and the
imbalance between decreased ADAMTS13:AC and increase in its
substrate VWF:Ag, leading to systemic microcirculatory disturbances
in ALF patients. A previous study (13) demonstrated that deficiency of
ADAMTS13-associated inflammation promotes UL-VWFM the formation and
severe, secondary ADAMTS13 deficiency can be associated with
sepsis-induced DIC and may contribute to renal failure development
(28), supporting the authors' data
and hypothesis.
Likewise, another mechanism reducing ADAMTS13:AC is
the presence of the plasma inhibitor against ADAMTS13. Here, the
inhibitor was detected in 66.7% of ALF patients and in 22.2% of AH
patients; inhibitory activity averaged 1.2 BU/ml in ALF and 0.7
BU/ml in AH. Patients with the inhibitor reported lower ADAMTS13:AC
and higher VWF:Ag than those without the inhibitor. AH and ALF
patients with the inhibitor presented higher blood urea nitrogen,
serum creatinine and white blood cell counts, and lower serum
albumin than those without the inhibitor, suggesting that decreased
ADAMTS13:AC is caused by the presence of its inhibitor, closely
related to lower functional liver capacity, marked inflammation and
enhanced endotoxemia in ALF patients.
Clarifying what types of inhibitors may be involved
in the association with inflammatory cytokines and endotoxin is
necessary. The authors recently encountered two patients who
developed TTP; one occurring during hepatitis C virus (HCV)-related
advanced liver cirrhosis course (29)
and the other occurring a month following pegylated-interferon α-2a
therapy in a HCV-related chronic hepatitis patient (30). In both, plasma ADAMTS13:AC levels were
extremely low; the inhibitor against ADAMTS13 was detected in each
patient's heated plasma (2.0 and 1.6 BU/ml, respectively) and
purified IgG (0.19 and 0.4 BU/ml IgG, respectively). The authors of
a previous study detected IgG-inhibitor using western blotting in
four patients with advanced liver cirrhosis and extremely low
ADAMTS13:AC (<3% controls) without clinical TTP features
(8). Some end-stage cirrhotic patients
have extremely low ADAMTS13:AC with the IgG-inhibitor against
ADAMTS13, similar to TTP patients. However, rapid IgG-inhibitor
development following ALF onset is unknown. Intravenous endotoxin
infusion in healthy volunteers decreased plasma ADAMTS13:AC and
increased VWF:Ag and UL-VWFM during acute systemic inflammation
(31). These results and those of
others indicated that endotoxemia may reduce plasma ADAMTS13:AC, or
together with inflammatory cytokines, in patients with ALF. Further
studies are warranted to clarify the kind of inhibitors, other than
the IgG-inhibitor, involved in AHF patients with lower
ADAMTS13:AC.
Therefore, enhanced cytokinemia and ADAMTS13:AC
inhibitor, closely related to enhanced endotoxemia in ALF patients,
decreased ADAMTS13:AC and increased VWF:Ag. Cytokinemia and
inhibitor presence cause an enzyme-substrate imbalance, resulting
in MOF, particularly in ALF patients. Regarding mechanism of
ADAMTS13 depletion, further experimental studies are required. The
presented results raise the possibility of novel supportive
therapies for ALF patients, including ADAMTS13 supplementation or
anti-inflammatory cytokine agents.
Acknowledgements
The present study was completed with the great help
of the late Professor Masahito Uemura. The authors would like to
thank Ayami Isonishi for her great help in the assay of ADAMTS13:AC
and VWF:Ag. The study was supported in part by research grants
(grant no. 20590794) from the Ministry of Education, Culture,
Sports, Science and Technology of Japan.
References
|
1
|
Zheng X, Chung D, Takayama TK, Majerus EM,
Sadler JE and Fujikawa K: Structure of von Willebrand
factor-cleaving protease (ADAMTS13), a metalloprotease involved in
thrombotic thrombocytopenic purpura. J Biol Chem. 276:41059–41063.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Uemura M, Tatsumi K, Matsumoto M, Fujimoto
M, Matsuyama T, Ishikawa M, Iwamoto TA, Mori T, Wanaka A, Fukui H
and Fujimura Y: Localization of ADAMTS13 to the stellate cells of
human liver. Blood. 106:922–924. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Moake JL, Turner NA, Stathopoulos NA,
Nolasco LH and Hellums JD: Involvement of large plasma von
Willebrand factor (vWF) multimers and unusually large vWF forms
derived from endothelial cells in shear stress-induced platelet
aggregation. J Clin Invest. 78:1456–1461. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Levy GG, Nichols WC, Lian EC, Foroud T,
McClintick JN, McGee BM, Yang AY, Siemieniak DR, Stark KR, Gruppo
R, et al: Mutations in a member of the ADAMTS gene family cause
thrombotic thrombocytopenic purpura. Nature. 413:488–494. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Furlan M, Robles R, Galbusera M, Remuzzi
G, Kyrle PA, Brenner B, Krause M, Scharrer I, Aumann V, Mittler U,
et al: von Willebrand factor-cleaving protease in thrombotic
thrombocytopenic purpura and the hemolytic-uremic syndrome. N Engl
J Med. 339:1578–1584. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Griffin D, Al-Nouri ZL, Muthurajah D, Ross
JR, Ballard RB, Terrell DR, Vesely SK, George JN and Marques MB:
First symptoms in patients with thrombotic thrombocytopenic
purpura: What are they and when do they occur? Transfusion.
53:235–237. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ishikawa M, Uemura M, Matsuyama T,
Matsumoto M, Ishizashi H, Kato S, Morioka C, Fujimoto M, Kojima H,
Yoshiji H, et al: Potential role of enhanced cytokinemia and plasma
inhibitor on the decreased activity of plasma ADAMTS13 in patients
with alcoholic hepatitis: relationship to endotoxemia. Alcohol Clin
Exp Res. 34 Suppl 1:25–33. 2010. View Article : Google Scholar
|
|
8
|
Uemura M, Fujimura Y, Matsumoto M,
Ishizashi H, Kato S, Matsuyama T, Isonishi A, Ishikawa M, Yagita M,
Morioka C, et al: Comprehensive analysis of ADAMTS13 in patients
with liver cirrhosis. Thromb Haemost. 99:1019–1029. 2008.PubMed/NCBI
|
|
9
|
Hugenholtz GC, Adelmeijer J, Meijers JC,
Porte RJ, Stravitz RT and Lisman T: An unbalance between von
Willebrand factor and ADAMTS13 in acute liver failure: Implications
for hemostasis and clinical outcome. Hepatology. 58:752–761. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fukui H: Relation of endotoxin, endotoxin
binding proteins and macrophages to severe alcoholic liver injury
and multiple organ failure. Alcohol Clin Exp Res. 29:172S–179S.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nolan JP: The role of intestinal endotoxin
in liver injury: A long and evolving history. Hepatology.
52:1829–1835. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cao WJ, Niiya M, Zheng XW, Shang DZ and
Zheng XL: Inflammatory cytokines inhibit ADAMTS13 synthesis in
hepatic stellate cells and endothelial cells. J Thromb Haemost.
6:1233–1235. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bernardo A, Ball C, Nolasco L, Moake JF
and Dong JF: Effects of inflammatory cytokines on the release and
cleavage of the endothelial cell-derived ultralarge von Willebrand
factor multimers under flow. Blood. 104:100–106. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bockmeyer CL, Claus RA, Budde U, Kentouche
K, Schneppenheim R, Lösche W, Reinhart K and Brunkhorst FM:
Inflammation-associated ADAMTS13 deficiency promotes formation of
ultra-large von Willebrand factor. Haematologica. 93:137–140. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mochida S, Nakayama N, Ido A, Takikawa Y,
Yokosuka O, Sakaida I, Moriwaki H, Genda T and Takikawa H: Revised
criteria for classification of the etiologies of acute liver
failure and late-onset hepatic failure in Japan: A report by the
Intractable Hepato-biliary Diseases Study Group of Japan in 2015.
Hepatol Res. 46:369–371. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Matsumoto M, Kawa K, Uemura M, Kato S,
Ishizashi H, Isonishi A, Yagi H, Park YD, Takeshima Y, Kosaka Y, et
al: Prophylactic fresh frozen plasma may prevent development of
hepatic VOD after stem cell transplantation via ADAMTS13-mediated
restoration of von Willebrand factor plasma levels. Bone Marrow
Transplant. 40:251–259. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Koyama N, Matsumoto M, Tamaki S, Yoshikawa
M, Fujimura Y and Kimura H: Reduced larger von Willebrand factor
multimers at dawn in OSA plasmas reflect severity of apnoeic
episodes. Eur Respir J. 40:657–664. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kasper CK, Aledort L, Aronson D, Counts R,
Edson JR, van Eys J, Fratantoni J, Green D, Hampton J, Hilgartner
M, et al: Proceedings: A more uniform measurement of factor VIII
inhibitors. Thromb Diath Haemorrh. 34:6121975.PubMed/NCBI
|
|
19
|
Fukui H, Brauner B, Bode JC and Bode C:
Plasma endotoxin concentrations in patients with alcoholic and
non-alcoholic liver disease: Reevaluation with an improved
chromogenic assay. J Hepatol. 12:162–169. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shah N, de Oca M Montes, Jover-Cobos M,
Tanamoto K, Muroi M, Sugiyama K, Davies NA, Mookerjee RP, Dhar DK
and Jalan R: Role of toll-like receptor 4 in mediating multiorgan
dysfunction in mice with acetaminophen induced acute liver failure.
Liver Transpl. 19:751–761. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Melgaço JG, Soriani FM, Sucupira PH,
Pinheiro LA, Vieira YR, de Oliveira JM, Lewis-Ximenez LL, Araújo
CC, Pacheco-Moreira LF, Menezes GB, et al: Changes in cellular
proliferation and plasma products are associated with liver
failure. World J Hepatol. 8:1370–1383. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mookerjee RP, Dalton RN, Davies NA, Hodges
SJ, Turner C, Williams R and Jalan R: Inflammation is an important
determinant of levels of the endogenous nitric oxide synthase
inhibitor asymmetric dimethylarginine (ADMA) in acute liver
failure. Liver Transpl. 13:400–405. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rolando N, Wade J, Davalos M, Wendon J,
Philpott-Howard J and Williams R: The systemic inflammatory
response syndrome in acute liver failure. Hepatology. 32:734–739.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shinozaki K, Oda S, Abe R, Tateishi Y,
Yokoi T and Hirasawa H: Blood purification in fulminant hepatic
failure. Contrib Nephrol. 166:64–72. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fujimoto M, Uemura M, Nakatani Y, Tsujita
S, Hoppo K, Tamagawa T, Kitano H, Kikukawa M, Ann T, Ishii Y, et
al: Plasma endotoxin and serum cytokine levels in patients with
alcoholic hepatitis: Relation to severity of liver disturbance.
Alcohol Clin Exp Res. 24 Suppl:48S–54S. 2000.PubMed/NCBI
|
|
27
|
Fukui H, Brauner B, Bode JC and Bode C:
Chromogenic endotoxin assay in plasma. Selection of plasma
pretreatment and production of standard curves. J Clin Chem Clin
Biochem. 27:941–946. 1989.PubMed/NCBI
|
|
28
|
Ono T, Mimuro J, Madoiwa S, Soejima K,
Kashiwakura Y, Ishiwata A, Takano K, Ohmori T and Sakata Y: Severe
secondary deficiency of von Willebrand factor-cleaving protease
(ADAMTS13) in patients with sepsis-induced disseminated
intravascular coagulation: Its correlation with development of
renal failure. Blood. 107:528–534. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yagita M, Uemura M, Nakamura T, Kunitomi
A, Matsumoto M and Fujimura Y: Development of ADAMTS13 inhibitor in
a patient with hepatitis C virus-related liver cirrhosis causes
thrombotic thrombocytopenic purpura. J Hepatol. 42:420–421. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kitano K, Gibo Y, Kamijo A, Furuta K,
Oguchi S, Joshita S, Takahashi Y, Ishida F, Matsumoto M, Uemura M
and Fujimura Y: Thrombotic thrombocytopenic purpura associated with
pegylated-interferon alpha-2a by an ADAMTS13 inhibitor in a patient
with chronic hepatitis C. Haematologica. 91:ECR342006.PubMed/NCBI
|
|
31
|
Reiter RA, Varadi K, Turecek PL, Jilma B
and Knöbl P: Changes in ADAMTS13 (von-Willebrand-factor-cleaving
protease) activity after induced release of von Willebrand factor
during acute systemic inflammation. Thromb Haemost. 93:554–558.
2005.PubMed/NCBI
|