Introduction
Uric acid, the end product of purine metabolism, is
a well known danger associated molecular pattern (DAMP) molecule as
encountered in its crystallized form in the extracellular space.
Urate functions as a DAMP through NOD-like receptor family, pyrin
domain containing 3 (NLRP3) inflammasome-dependent caspase-1
activation and interleukin (IL)-1β production has been confirmed in
experimental models such as the bleomycin-induced lung injury model
(1), the acetaminophen-induced liver
injury model (2), and importantly, in
gout (3), the most well characterized
urate-related clinical condition. Although the exact mechanisms
involved in NALP3 activation remain to be elucidated, phagocytosis
of urate crystals, fusion with lysosomes and subsequent rapture of
the formed phagolysosomes and release of cathepsins or crystals,
and reactive oxygen species production have been recognized as
prerequisite steps (4).
By activating antigen presenting cells (APCs), urate
crystals boost adaptive immunity as well. The last has been
demonstrated in studies that have demonstrated that urate crystals
acting as an adjuvant enhance antibody response following
vaccination against hepatitis B surface antigen (5), tuberculosis (6) and tumors (7). It has been also proposed that aluminum
hydroxide, the most widely adjuvant used in human vaccines, exerts
its effect by activating dendritic cells through local cell injury
and urate release (8).
Despite the fact that the effect of urate in
boosting adaptive immunity indirectly by activating APCs is well
documented, there are studies demonstrating that urate crystals are
able to activate isolated T-cells in the absence of APCs (9–11), an
unexpected event since T-cells are unable to phagocytose
crystallized particles. However, there is evidence that urate
crystals can be effective in activating intracellular events
without previous internalization. More precisely, in dendritic
cells urate crystals sequester lipid rafts and consequently
aggregate their bound receptors. This non-specific clustering of
receptors that contain immunoreceptor tyrosine-based activation
motifs (ITAM) ultimately triggers an intracellular signal
transduction cascade (12). In case of
direct T-cell activation by urate crystals, the aforementioned
model applied in dendritic cells becomes quite attractive. If urate
crystals are able to aggregate lipid rafts in general, then they
could cloister many TCR-complexes and co-receptors together,
considering the fluidity of the membrane and the abundance of
TCR-complexes and co-receptors in T-cells' lipid rafts (13,14). Such a
sequestration of receptors would eventually lead to T-cell
activation.
In the current study, the ability of urate crystals
to activate directly TCR-complexes was assessed by evaluating the
phosphorylation state of ζ chain. A ζ-chain homodimer contains six
ITAMs and is part of the TCR-complex (15,16).
Phosphorylation of ζ-chain ITAMs is a crucial and very proximal
event in signal transduction that normally starts immediately
following the binding of the TCR on the surface of T-cell to the
processed and presented with major histocompatibility complex
antigen on the surface of APC (17,18). In
addition, the later outcome of TCR-complex activation, T-cell
proliferation was assessed.
Materials and methods
Isolation and culture of T-cells
Blood samples were collected from 10 healthy
volunteers (5 women/5 men, 25–46 years old). Informed consent was
obtained from each individual enrolled into the study and the
Ethics Committee of the Faculty of Medicine, University of Thessaly
(Larissa, Greece) approved the study protocol.
Peripheral blood mononuclear cells (PBMCs) were
isolated from blood by Ficoll-Hypaque density gradient
centrifugation (Histopaque 1077; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany). T-cells were isolated from the PBMCs by
negative selection. Non T-cells were indirectly magnetically
labeled with a cocktail of biotin-conjugated monoclonal antibodies
and were depleted using the Pan T-cell Isolation kit (Miltenyi
Biotec GmbH, Bergisch Gladbach, Germany). The purity of the
isolated T-cells was tested and confirmed by means of flow
cytometry. Isolated T-cells were counted on a Neubauer chamber with
an optical microscope, and cell viability was assessed using the
trypan blue exclusion assay (Sigma-Aldrich; Merck KGaA).
T-cells were resuspended in RPMI-1640 medium with
L-glutamine and 10 mM HEPES (Sigma-Aldrich; Merck KGaA) and
supplemented with 10% fetal bovine serum (Sigma-Aldrich; Merck
KGaA) and antibiotic-antimycotic solution (Sigma-Aldrich; Merck
KGaA). T-cells were cultured for 48 h at a density of
1×106 cells/well in 12 well-plates or 1×105
cells/well in 96 well-plates. Cells were cultured with or without
urate at a concentration of 10 mg/dl (Sigma-Aldrich; Merck KGaA).
This concentration is above the concentration of 6.7 mg/dl at which
uric acid is crystallized, yet still within the levels observed in
hyperuricemic patients (19). As
expected, at the used concentration urate was precipitated forming
monosodium urate crystals. Cultures were incubated at 37°C in an
atmosphere of 95% relative humidity and 5% CO2.
Assessment of ζ-chain, its
phosphorylation state and c-Myc levels
Cells were lysed using the T-PER tissue protein
extraction reagent (Thermo Fisher Scientific, Inc., Waltham, MA,
USA) supplemented with protease and phosphatase inhibitors
(Sigma-Aldrich; Merck KGaA and Roche Diagnostics, Basel,
Switzerland, respectively). Protein was quantified via Bradford
assay (Sigma-Aldrich; Merck KGaA) and 10 µg from each sample were
used for western blotting. Blots were incubated with the primary
antibody for 16 h, followed by the secondary antibody incubation
for 30 min. In case of re-probing polyvinylidene difluoride blots,
the previous primary and secondary antibody were removed via the
use of the Restore Western Blot Stripping Buffer (Thermo Fisher
Scientific Inc.) according to the manufacturer's protocol. Blots
were analyzed using the Image J software (version 1.49; National
Institutes of Health, Bethesda, MD, USA).
The primary antibodies used in western blotting were
specific for ζ-chain (rabbit polyclonal antibody; dilution 1:1,000;
cat. no. sc-20919; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA), phosphorylated ζ-chain (mouse monoclonal antibody; dilution
1:1,000; cat. no. sc-9975; Santa Cruz Biotechnology, Inc.), c-Myc
(rabbit monoclonal antibody; dilution 1:1,000; cat. no. 9402; Cell
Signaling Technology, Inc., Danvers, MA, USA) and β-actin (dilution
1:5,000; cat. no. 4967; Cell Signaling Technology, Inc.). The
secondary antibodies were anti-mouse IgG, horseradish-peroxidase
(HRP)-linked antibody (cat. no. A9044; dilution 1:1,000;
Sigma-Aldrich; Merck KGaA) and anti-rabbit IgG, HRP-linked antibody
(cat. no. 7074; Cell Signaling Technology, Inc.).
Assessment of T-cell
proliferation
T-cell proliferation was assessed by Cell
Proliferation ELISA (cat. no. 11669915001; Roche Diagnostics) using
bromodeoxyuridine (BrdU) labelling and immunoenzymatic detection
according to the manufacturer's protocol. Proliferation index was
calculated by the equation Proliferation index (%) = (optical
density (OD) derived from cultures with urate/OD derived from
cultures without urate) × 100. All these experiments were performed
in triplicate and the results refer to the mean of the three
measurements.
Statistical analysis
Normality of the evaluated variables was assessed
and confirmed by one-sample Kolmogorov-Smirnov test. For comparison
of means paired t-test was used. Results were expressed as mean ±
standard deviation and a P<0.05 was considered to indicate
statistically significant difference.
The P-values were calculated by comparing the means
of OD derived either form BrdU ELISA or from western blot analysis.
Statistical analysis following normalization to the controls' OD
values was avoided in order to prevent violation of the
prerequisite for normal distribution of the compared variables when
applying parametric statistical tests. However, for reader's
convenience, the results were expressed and depicted following
normalization of means for the control group according to the
equation: Relative OD=OD from each culture ÷ OD from the respective
cultures without urate.
Results
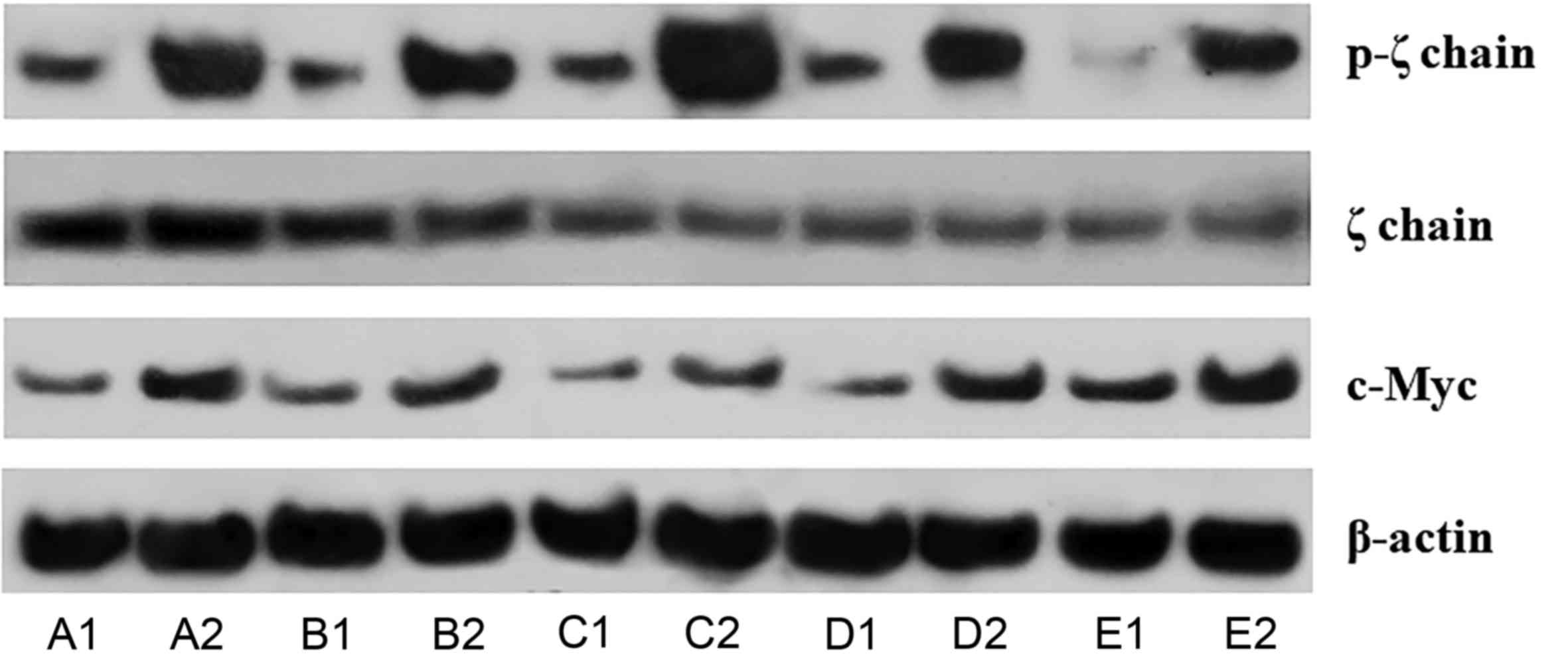
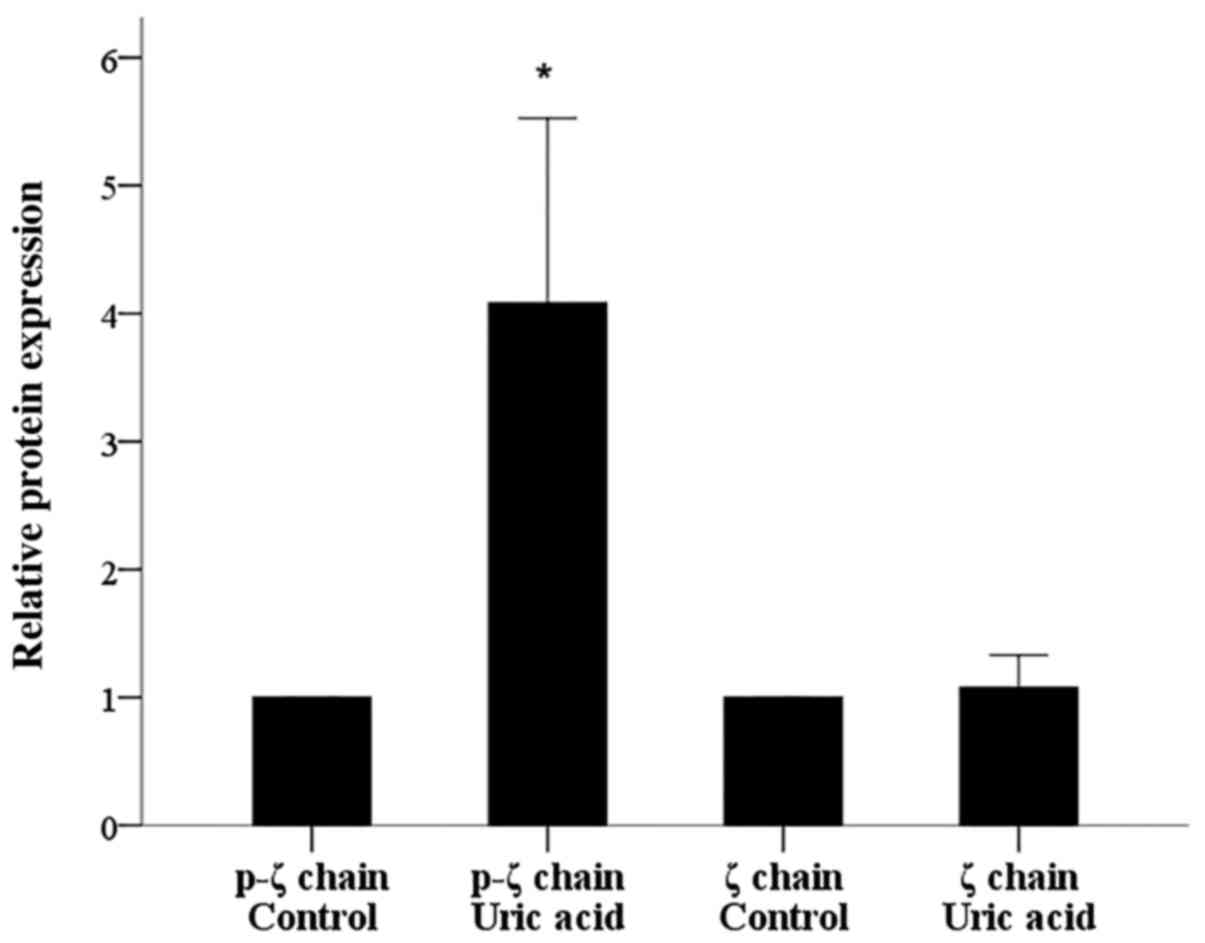
Urate did not affect ζ-chain
expression but it increased its phosphorylation significantly
Urate crystals did not affect ζ-chain expression in
isolated T-cells since its expression altered only by a factor of
1.08±0.26 (P=0.447; Figs. 1 and
2). On the contrary, urate crystals
induced ζ-chain phosphorylation significantly. In isolated T-cells
cultured with urate at a concentration of 10 mg/dl, the level of
phosphorylated ζ-chain (p-ζ) increased by a factor of 4.08±1.44
(P<0.001; Figs. 1 and 2).
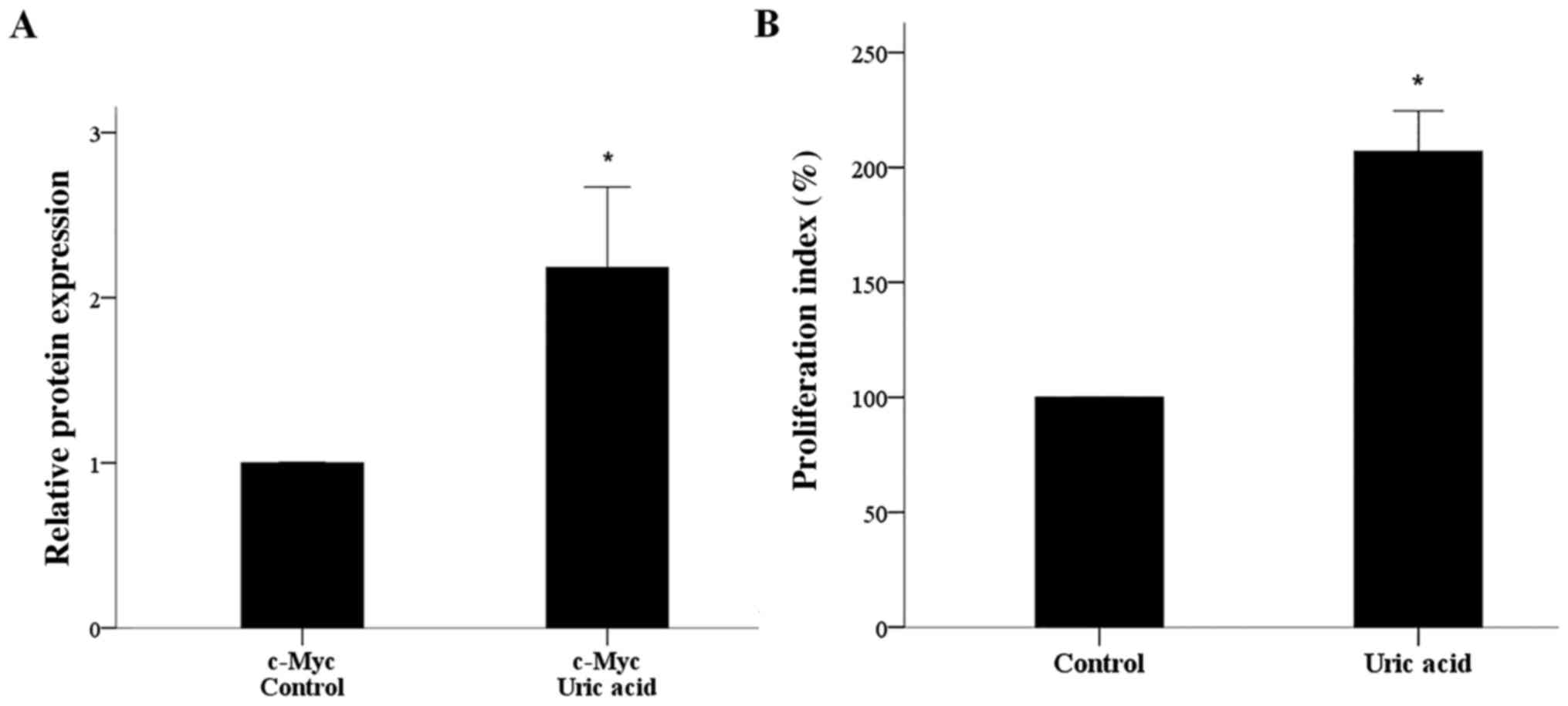
Urate increased the expression of
c-Myc significantly
Culture of isolated T-cells in the presence of urate
at a concentration of 10 mg/dl increased the expression of the
master regulator of T-cell proliferation transcription factor c-Myc
2.18±0.49 times (P<0.001; Figs. 1
and 3A).
Urate induced T-cell
proliferation
In isolated T-cells treated with 10 mg/dl urate, the
increase in c-Myc expression was accompanied by increased cell
proliferation. Compared to untreated isolated T-cells, isolated
T-cells cultured with urate exhibited a proliferation index
increased by 206.98±8.79% (Fig.
3B).
Discussion
Triggered by studies that have indicated that urate
crystals are able to activate isolated T-cells (9–11), which are
unable to phagocytose crystal particles, the authors evaluated
whether urate is able to activate directly the TCR-complex. The
rationale behind this attempt was based on the fact that the
TCR-complex and other co-receptors lie within lipid rafts in cell
membrane (13,14), and, at least in dendritic cells, urate
crystals by sequestering lipid rafts they bring together ITAM
containing receptors resulting in their activation and the
initiation of intracellular signal transduction events (12). This potential of urate crystals to
activate cells through non-specific clustering of cell membrane
receptors due to lipid raft sequestration has been confirmed in
macrophages as well, in which they induce NALP3 activation
(20). Interestingly, other crystals
share this property of urate crystals. Alum, the widest used
adjuvant in human vaccines, activates dendritic cells by inducing
lipid rafts sequestration (21). This
property of alum crystals has been also confirmed in a biophysical
study of lipid domain clustering in a simple model of phospholipid
monolayers containing dipalmitoyl-phosphatidylcholine and
dioleoyl-phosphatidylcholine (22).
Calcium phosphate crystals (23), as
well as poly methyl-methacrylate microspheres of 153 µm diameter
(24), impossible to be phagocytosed,
are also able to activate macrophages by inducing lipid rafts
clustering. A similar mechanism may be responsible for innate
immune cells activation by cholesterol or heme crystals, since in
both cases activation can take place in the absence of phagocytosis
(25,26). It is remarkable that urate crystals are
also able to affect non immune system cells, and more precisely to
induce apoptosis in osteoblasts, independently of phagocytosis
(27). Thus, it is likely that many
crystal structures are able to affect cell function by sequestering
cell membrane lipid rafts.
In the current study, the ability of urate crystals
to act in a similar way in T-cells and consequently activate the
TCR-complex in a non specific and antigen independent manner was
evaluated by assessing the phosphorylation status ζ-chain. This 16
kD protein is an indispensable component of the TCR-complex and its
phosphorylation is the first event that occurs after TCR activation
(15–18). Indeed, western blotting revealed that
urate crystals did not affect ζ-chain expression following 48 h of
isolated T-cells culture, but they increased its phosphorylation
significantly. Thus, urate crystals activate the TCR-complex
directly likely by inducing lipid raft clustering.
Next, the authors evaluated if urate crystals are
able to induce later effects of TCR-complex activation, and more
precisely T-cell proliferation. Indeed, urate crystals increased
the expression of the transcription factor c-Myc. This
transcription factor serves a pivotal role in T-cell proliferation
and controls metabolic reprogramming upon T-cell activation
(28,29). Expression of c-Myc decreased in case of
decreased ζ-chain expression (30), as
well as of reduced phosphorylation of the TCR-complex ITAM
(31). In parallel with increase of
c-Myc expression, urate crystals increased T-cell
proliferation.
Since activation of the TCR-complex without
co-stimulation by activation of co-receptors, such as CD28, results
in T-cell anergy (32,33), it is possible that urate crystals
sequesters lipid rafts containing both TCR-complexes and
co-receptors in order to achieve full T-cell activation and
proliferation. Another possibility may reside in the ability of
urate crystals to induce active IL-1β production in a
NALP3-dependent manner. The fact that, in isolated T-cells cultured
with urate crystals, inhibition of NALP3 abrogates both IL-1β
production and T-cell proliferation (9), favors such a possibility. The first
transcription factors that are activated upon full T-cell
activation are the nuclear factor of activated T cells (NFAT),
nuclear factor (NF)-κB cells and activator protein 1 (AP1).
Especially for activation of AP1 co-stimulation is required;
otherwise T-cell falls in a state of anergy (32,33). The
cytokine IL-1β activates AP1 and NF-κΒ (34). Consequently, IL-1β may provide the
additional to NFAT (32,33), which is activated by the TCR-complex,
transcription factor AP1 leading to full T-cell activation even in
the absence of co-stimulation.
According to the results of the present study, one
could assume that hyperuricemia may result in T-cell activation.
However, this aspect requires further investigation. Certainly, an
acute raise in extracellular urate levels due to any tissue damage
may enhance T-cell immune response both indirectly by activating
APCs (1–3,8), and
according to this and other studies directly (9–11).
Nevertheless, in the chronic hyperuricemic state, the opposite may
occur since chronic inflammation (30,35–37), or TCR-complex activation (17,18),
resulting in ζ-chain downregulation.
In conclusion, the results of the present study
support that urate crystals are able to activate the TCR-complex
directly and induce T-cell proliferation, expanding the known
immune cell targets of urate and supporting its
phagocytosis-independent way of action.
References
|
1
|
Gasse P, Riteau N, Charron S, Girre S,
Fick L, Pétrilli V, Tschopp J, Lagente V, Quesniaux VF, Ryffel B,
et al: Uric acid is a danger signal activating NALP3 inflammasome
in lung injury inflammation and fibrosis. Am J Respir Crit Care
Med. 179:903–913. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kono H, Chen CJ, Ontiveros F and Rock KL:
Uric acid promotes an acute inflammatory response to sterile cell
death in mice. J Clin Invest. 120:1939–1949. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Busso N and So A: Microcrystals as DAMPs
and their role in joint inflammation. Rheumatology (Oxford).
51:1154–1160. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rock KL, Kataoka H and Lai JJ: Uric acid
as a danger signal in gout and its comorbidities. Nat Rev
Rheumatol. 9:13–23. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ma XJ, Tian DY, Xu D, Yang DF, Zhu HF,
Liang ZH and Zhang ZG: Uric acid enhances T cell immune responses
to hepatitis B surface antigen-pulsed-dendritic cells in mice.
World J Gastroenterol. 13:1060–1066. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Taus F, Santucci MB, Greco E, Morandi M,
Palucci I, Mariotti S, Poerio N, Nisini R, Delogu G and Fraziano M:
Monosodium Urate Crystals Promote Innate Anti-Mycobacterial
Immunity and Improve BCG Efficacy as a Vaccine against
Tuberculosis. PLoS One. 10:e01272792015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang Y, Ma X, Su C, Peng B, Du J, Jia H,
Luo M, Fang C and Wei Y: Uric acid enhances the antitumor immunity
of dendritic cell-based vaccine. Sci Rep. 5:164272015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kool M, Soullié T, van Nimwegen M, Willart
MA, Muskens F, Jung S, Hoogsteden HC, Hammad H and Lambrecht BN:
Alum adjuvant boosts adaptive immunity by inducing uric acid and
activating inflammatory dendritic cells. J Exp Med. 205:869–882.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Eleftheriadis T, Pissas G, Antoniadi G,
Makri P, Liakopoulos V and Stefanidis I: Urate crystals induce
NLRP3 inflammasome-dependent IL-1β secretion and proliferation in
isolated primary human T-cells. Hippokratia. 19:41–46.
2015.PubMed/NCBI
|
|
10
|
Eleftheriadis T, Pissas G, Karioti A,
Antoniadi G, Golfinopoulos S, Liakopoulos V, Mamara A, Speletas M,
Koukoulis G and Stefanidis I: Uric acid induces caspase-1
activation, IL-1β secretion and P2X7 receptor dependent
proliferation in primary human lymphocytes. Hippokratia.
17:141–145. 2013.PubMed/NCBI
|
|
11
|
Webb R, Jeffries M and Sawalha AH: Uric
acid directly promotes human T-cell activation. Am J Med Sci.
337:23–27. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ng G, Sharma K, Ward SM, Desrosiers MD,
Stephens LA, Schoel WM, Li T, Lowell CA, Ling CC, Amrein MW, et al:
Receptor-independent, direct membrane binding leads to cell-surface
lipid sorting and Syk kinase activation in dendritic cells.
Immunity. 29:807–818. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dinic J, Riehl A, Adler J and Parmryd I:
The T cell receptor resides in ordered plasma membrane nanodomains
that aggregate upon patching of the receptor. Sci Rep. 5:100822015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Varshney P, Yadav V and Saini N: Lipid
rafts in immune signalling: Current progress and future
perspective. Immunology. 149:13–24. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Samelson LE, Harford JB and Klausner RD:
Identification of the components of the murine T cell antigen
receptor complex. Cell. 43:223–231. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Reth M: Antigen receptor tail clue.
Nature. 338:383–384. 1989. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chan AC, Desai DM and Weiss A: The role of
protein tyrosine kinases and protein tyrosine phosphatases in T
cell antigen receptor signal transduction. Annu Rev Immunol.
12:555–592. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Eleftheriadis T, Antoniadi G, Liakopoulos
V and Kortsaris A: T-Cell Zeta Chain Expression, Phosphorylation
and Degradation and their Role in T-Cell Signal Transduction and
Immune Response Regulation in Health And Disease. Curr Signal
Transduct Ther. 1:191–208. 2006. View Article : Google Scholar
|
|
19
|
Richette P, Doherty M, Pascual E, Barskova
V, Becce F, Castañeda-Sanabria J, Coyfish M, Guillo S, Jansen TL,
Janssens H, et al: 2016 updated EULAR evidence-based
recommendations for the management of gout. Ann Rheum Dis.
76:29–42. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hari A, Zhang Y, Tu Z, Detampel P, Stenner
M, Ganguly A and Shi Y: Activation of NLRP3 inflammasome by
crystalline structures via cell surface contact. Sci Rep.
4:72812014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Flach TL, Ng G, Hari A, Desrosiers MD,
Zhang P, Ward SM, Seamone ME, Vilaysane A, Mucsi AD, Fong Y, et al:
Alum interaction with dendritic cell membrane lipids is essential
for its adjuvanticity. Nat Med. 17:479–487. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Antúnez LR, Livingston A, Berkland C and
Dhar P: Physiochemical Properties of Aluminum Adjuvants Elicit
Differing Reorganization of Phospholipid Domains in Model
Membranes. Mol Pharm. 13:1731–1737. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Corr EM, Cunningham CC, Helbert L,
McCarthy GM and Dunne A: Osteoarthritis-associated basic calcium
phosphate crystals activate membrane proximal kinases in human
innate immune cells. Arthritis Res Ther. 19:232017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Malik AF, Hoque R, Ouyang X, Ghani A, Hong
E, Khan K, Moore LB, Ng G, Munro F, Flavell RA, et al: Inflammasome
components Asc and caspase-1 mediate biomaterial-induced
inflammation and foreign body response. Proc Natl Acad Sci USA.
108:20095–20100. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Corr EM, Cunningham CC and Dunne A:
Cholesterol crystals activate Syk and PI3 kinase in human
macrophages and dendritic cells. Atherosclerosis. 251:197–205.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dutra FF, Alves LS, Rodrigues D, Fernandez
PL, de Oliveira RB, Golenbock DT, Zamboni DS and Bozza MT:
Hemolysis-induced lethality involves inflammasome activation by
heme. Proc Natl Acad Sci USA. 111:E4110–E4118. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chhana A, Callon KE, Pool B, Naot D,
Watson M, Gamble GD, McQueen FM, Cornish J and Dalbeth N:
Monosodium urate monohydrate crystals inhibit osteoblast viability
and function: Implications for development of bone erosion in gout.
Ann Rheum Dis. 70:1684–1691. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang R, Dillon CP, Shi LZ, Milasta S,
Carter R, Finkelstein D, McCormick LL, Fitzgerald P, Chi H, Munger
J, et al: The transcription factor Myc controls metabolic
reprogramming upon T lymphocyte activation. Immunity. 35:871–882.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chou C and Egawa T: Myc or no Myc, that is
the question. EMBO J. 34:1990–1991. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Eleftheriadis T, Pissas G, Antoniadi G,
Tsogka K, Sounidaki M, Liakopoulos V and Stefanidis I: Indoleamine
2,3 dioxygenase downregulates T cell receptor complex ζ chain and c
Myc, and reduces proliferation, lactate dehydrogenase levels and
mitochondrial glutaminase in human T cells. Mol Med Rep.
13:925–932. 2016.PubMed/NCBI
|
|
31
|
Guy CS, Vignali KM, Temirov J, Bettini ML,
Overacre AE, Smeltzer M, Zhang H, Huppa JB, Tsai YH, Lobry C, et
al: Distinct TCR signaling pathways drive proliferation and
cytokine production in T cells. Nat Immunol. 14:262–270. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fathman CG and Lineberry NB: Molecular
mechanisms of CD4+ T-cell anergy. Nat Rev Immunol.
7:599–609. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nurieva RI, Liu X and Dong C: Molecular
mechanisms of T-cell tolerance. Immunol Rev. 241:133–144. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Barksby HE, Lea SR, Preshaw PM and Taylor
JJ: The expanding family of interleukin-1 cytokines and their role
in destructive inflammatory disorders. Clin Exp Immunol.
149:217–225. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rodriguez PC, Zea AH, DeSalvo J, Culotta
KS, Zabaleta J, Quiceno DG, Ochoa JB and Ochoa AC: L-arginine
consumption by macrophages modulates the expression of CD3 zeta
chain in T lymphocytes. J Immunol. 171:1232–1239. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bronstein-Sitton N, Cohen-Daniel L, Vaknin
I, Ezernitchi AV, Leshem B, Halabi A, Houri-Hadad Y, Greenbaum E,
Zakay-Rones Z, Shapira L, et al: Sustained exposure to bacterial
antigen induces interferon-gamma-dependent T cell receptor zeta
down-regulation and impaired T cell function. Nat Immunol.
4:957–964. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Eleftheriadis T, Kartsios C, Yiannaki E,
Kazila P, Antoniadi G, Liakopoulos V and Markala D: Chronic
inflammation and T cell zeta-chain downregulation in hemodialysis
patients. Am J Nephrol. 28:152–157. 2008. View Article : Google Scholar : PubMed/NCBI
|