Introduction
Pain is a feared and common complication in patients
with cancer (1,2), which increases in prevalence and
intensity with disease progression (3,4), and
influences multiple aspects of quality of life (5–8).
Pharmacogenetic, pharmacokinetic and pharmacodynamic variations
among individuals result in a wide variety of responses to pain
sensation and analgesics; therefore, investigations of biomarkers
for opioid treatment have been performed to improve the efficacy of
morphine treatment (9). According to a
review published in 2015 (10),
numerous studies have proposed that the
catechol-O-methyltransferase (COMT) 472G→A (rs4680,
p.Val158Met) genotype may be a predictive biomarker for
the response to morphine treatment. In these studies, patients with
the G/G genotype received the highest dose of morphine (11,12), while
those with the A/A genotype received the lowest morphine dose
(13). However, the majority of these
studies had a small sample size, were retrospective, or were
targeting non-cancer pain (14). A
large sample study has indicated that none of the 112 single
nucleotide polymorphisms (SNPs) in 25 candidate genes demonstrated
significant associations with opioid dose (15); however, the quantity of concomitant
analgesics [non-steroidal anti-inflammatory drugs (NSAIDs) and
acetaminophen] and the method for dose determination of opioids
remain unclear. Furthermore, the mean Brief Pain Index (16) following treatment was relatively high
(15).
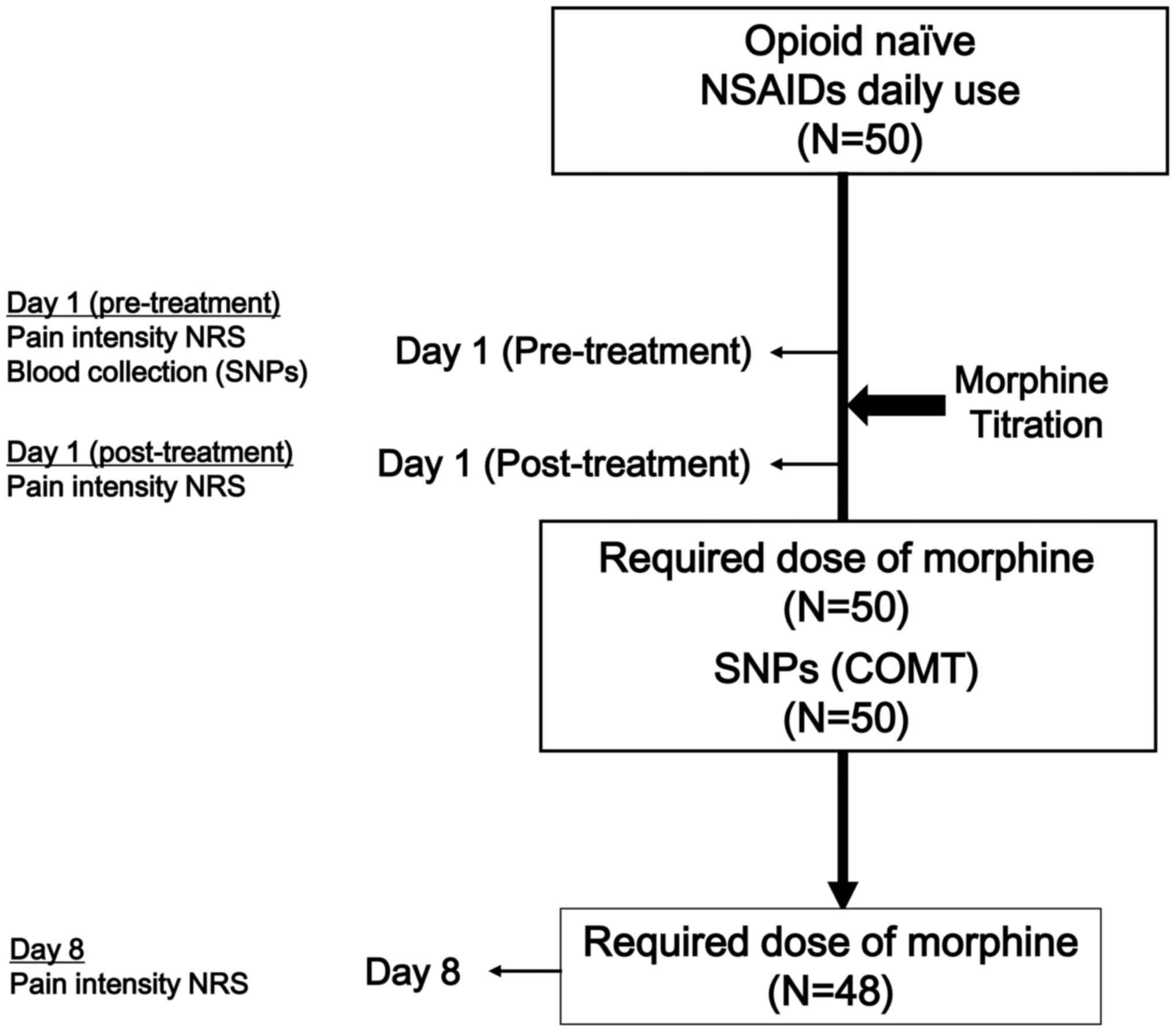
An exploratory study was planned, which included 100
patients with opioid-treatment-naïve cancer, in whom the use of
NSAIDs and acetaminophen, as well as the method of dose
determination of morphine were kept constant. The investigation was
divided into a pilot study of 50 cases, and a replication study
(the current study) of 50 more cases. Expression levels of
functional genetic variants as predictive biomarkers of the
response to morphine were examined. The pilot study indicated that
COMT 472G→A may be a predictive biomarker (17). The aim of the current prospective study
was to replicate these findings in an independent cohort of 50
opioid-treatment-naïve patients exhibiting various types of
cancer.
Materials and methods
Patients and samples
This prospective study was conducted from April 2011
to March 2012 at the Kindai University Faculty of Medicine (Osaka,
Japan). A total of 50 patients with opioid-treatment naïve and
histologically confirmed malignant neoplasms who were scheduled to
undergo opioid treatment were evaluated in the study. These
patients were recruited and selected by non-probabilistic
convenience sampling by their physician from the outpatients and
inpatients service of the Department of Medical Oncology (Kindai
University Faculty of Medicine). All patients met the following
inclusion criteria: Aged ≥18 years, a verified cancer diagnosis,
cancer pain suitable for morphine treatment (excluding patients
with neuropathic pain, predominant incidental pain, glomerular
filtration rate <30 ml/min, and history of opioid/drug abuse or
alcoholism from a self-reported questionnaire), daily use of NSAIDs
and able to provide written informed consent. The required dose of
morphine (day 1) was evaluated in 50 patients, and was assessed in
48 patients, (2 were excluded who could not continuously receive
morphine due to adverse effects or mortality; Fig. 1).
Clinical features, including age, sex, Eastern
Cooperative Oncology Group performance status (PS) (18), and types of primary malignant neoplasm
were recorded. Morphine was administered using the standard method,
including titration [NCCN Guidelines (19), Adult Cancer Pain], performed by
specialized palliative care doctors. Immediate release (IR)
morphine (5 mg) was administered orally, and efficacy and adverse
effects were reassessed at 60 min. When the pain score was not
decreased, the same dose of IR morphine (5 mg) was re-administered.
Dose titration was performed to decrease pain by ≥33% on the
numerical rating scale (NRS) (20)
pain scale at day 1 (pre-treatment), as well as to reduce the NRS
to a score of ≤3.
Criteria for discontinuation of dose titration were
appearance of adverse effects graded ≥3 (Common Terminology
Criteria for Adverse Events, v4.0) (21), or an attending physician determining
titration to be difficult to continue, even if the adverse effect
was graded ≤2. Controlled release (CR) morphine, six times the dose
of IR morphine, was administered orally once per day. If pain was
insufficiently controlled by 10 mg IR morphine in total, 60 mg CR
morphine was administered at night on day 1. The attending
physician could reduce the dose of CR morphine based on the general
condition of the patient. Morphine administration was discontinued
following a side effect of grade ≥3. Standardized information on
potential benefits and adverse effects was provided to the
patients. The study was approved by the Regional Committee for
Medical Research Ethics, Kindai University Faculty of Medicine.
Informed consent was obtained from all participants in the study.
All procedures performed in studies involving human participants
were in accordance with the ethical standards of the institutional
research committee and the 1964 Helsinki declaration and its later
amendments or comparable ethical standards.
Pain score
The pain score was assessed using a 0–10 NRS (0, no
pain to 10, worst imaginable pain) on day 1 (pre-treatment), day 1
(post-treatment) and day 8 (one week after treatment) by asking
patients the following question: “How intense was your average pain
for the past 24 h?”
Genotyping
SNPs of COMT 472G→A (rs4680; p.Val158Met)
modulate the genetic response to opioid medications (22). In an exploratory study of 50 cases, a
functional genotype analysis of opioid receptor µ 1 118A→G
(rs1799971; p.Asn40Asp) and COMT 472G→A (rs4680;
p.Val158Met) was performed to establish the predictive
biomarkers for the treatment outcome of morphine, based on the
plasma level of morphine and the required dose according to
genotype on days 1, 2 and 8. On day 1, blood was sampled for
genotyping, and the pain score was recorded on days 1 (before and
after treatment), 2 and 8. The required dose of morphine on day 1
was significantly higher in patients with the COMT G/G
genotype, compared with those with A/A and A/G genotypes in the
previous pilot study (P=0.03) (16).
Therefore, in the current prospective study of 50 independent
cases, COMT alone was the main focus.
Genomic DNA was isolated from blood samples using a
QIAamp(r) DNA Mini kit (cat. no. 51304; Qiagen GmbH, Hilden,
Germany) and was subjected to a genotype analysis using Taqman® SNP
Genotyping Assay for COMT rs4680 (cat. no.4362691; Thermo Fisher
Scientific Inc., Waltham, MA, USA) and a StepOnePlus™ Real-Time PCR
Systems (cat no. 376600; Thermo Fisher Scientific Inc.).
Statistical analysis
All data are expressed as means ± standard
deviation. Differences in the effects of morphine between gene
polymorphism groups were evaluated using a Mann-Whitney U test.
Two-sided P<0.05 was considered to indicate a statistically
significant difference. All analyses were performed using SPSS
software (v19.0; IBM SPSS, Armonk, NY, USA).
Results
Patient characteristics
The characteristics of the 50 patients (25 men and
25 women) are presented in Table I.
The median age was 63 years (age, 36–80 years; 62±9.17). The
primary tumors were as follows: Lung cancer, n=19 (38%); breast
cancer, n=9 (18%); colorectal cancer, n=5 (10%); head and neck
cancer, n=4 (8%); gastric cancer, n=3 (6%); unknown primary cause,
n=3 (6%); pancreatic cancer, n=2 (4%); gallbladder cancer, n=2
(4%); and others, n=3 (6%). More than 80% of the subjects exhibited
metastasis and a PS of 0 to 2. The median required doses of
morphine were 31.2 mg (20–60 mg) on day 1 and 32.6 mg (20–60 mg) on
day 8. The pain NRS before and after treatment did not differ
between the G/G and non-G/G groups (P=0.177). A dose of 60 mg
morphine was required in 4 cases of lung cancer and 1 case of
unknown primary cancer. The causes of pain were lymph node
metastases and bone metastases in 4 cases each, and pleural seeding
in 1 case. Features of 5 high-dose morphine-requiring cases,
defined as requiring 2 or more cycles of 5 mg IR morphine, are
presented in Table II.
 | Table I.Clinical characteristics of the
patients (n=50). |
Table I.
Clinical characteristics of the
patients (n=50).
| Characteristics | Patients (n) |
|---|
| Age, years |
|
|
<65 | 27 |
| ≥65 | 23 |
| Sex |
|
| Male | 25 |
|
Female | 25 |
| Performance
status |
|
| 0–2 | 40 |
| 3–4 | 10 |
| Genotype: COMT
472G→A (rs4680; p.Val158Met) |
|
| G/G | 29 |
| A/G | 19 |
| A/A | 2 |
| Tumor type |
|
| Lung | 19 |
|
Breast | 9 |
|
Colorectal | 5 |
| Head and
neck | 4 |
|
Gastric | 3 |
| Unknown
primary | 3 |
|
Gallbladder | 2 |
|
Pancreas | 2 |
|
Others | 3 |
| Required dose of
morphine on day 1 (mg) |
|
| 20 | 2 |
| 30 | 43 |
| 60 | 5 |
| Required dose of
morphine on day 8 (mg) |
|
| As
required | 8 |
| 20 | 3 |
| 30 | 30 |
| 60 | 6 |
| 90 | 1 |
|
N.E. | 2 |
| Day 1
(pre-treatment) pain NRS, mean (SD) |
|
| All
patients | 6.88 (2.43) |
|
G/G | 7.07 (2.25) |
| Non-G/G
(A/A and A/G) | 6.62 (2.69) |
| Day 1
(post-treatment) pain NRS, mean (SD) |
|
| All
patients | 2.46 (1.46) |
|
G/G | 2.38 (1.45) |
| Non-G/G
(A/A and A/G) | 2.57 (1.50) |
| Day 8 pain NRS,
mean (SD) |
|
| All
patients | 3.60 (2.73) |
|
G/G | 3.17 (2.63) |
| Non-G/G
(A/A and A/G) | 4.19 (2.80) |
 | Table II.Characteristics of patients with
high-dose morphine-requiring cases. |
Table II.
Characteristics of patients with
high-dose morphine-requiring cases.
| Patient | Age (y) | Sex | PS | COMT genotype | Tumor type | Pain NRS (before
administration) |
|---|
| 1 | 63 | M | 1 | G/G | Lung | 8 |
| 2 | 59 | M | 1 | G/G | Lung | 7 |
| 3 | 70 | M | 2 | G/G | Lung | 10 |
| 4 | 64 | F | 1 | G/G | Unknown
primary | 8 |
| 5 | 60 | F | 2 | G/G | Lung | 8 |
Pain titration
Morphine at the required dose was administered to
patients as a controlled-release agent. On day 1, dose titration
was performed to reach NRS ≤3 and pain control ≥33%. Titration was
successful in 76% of all cases, but unsuccessful in 60% of G/G
genotype cases.
Genotypes of COMT and treatment
outcomes of morphine
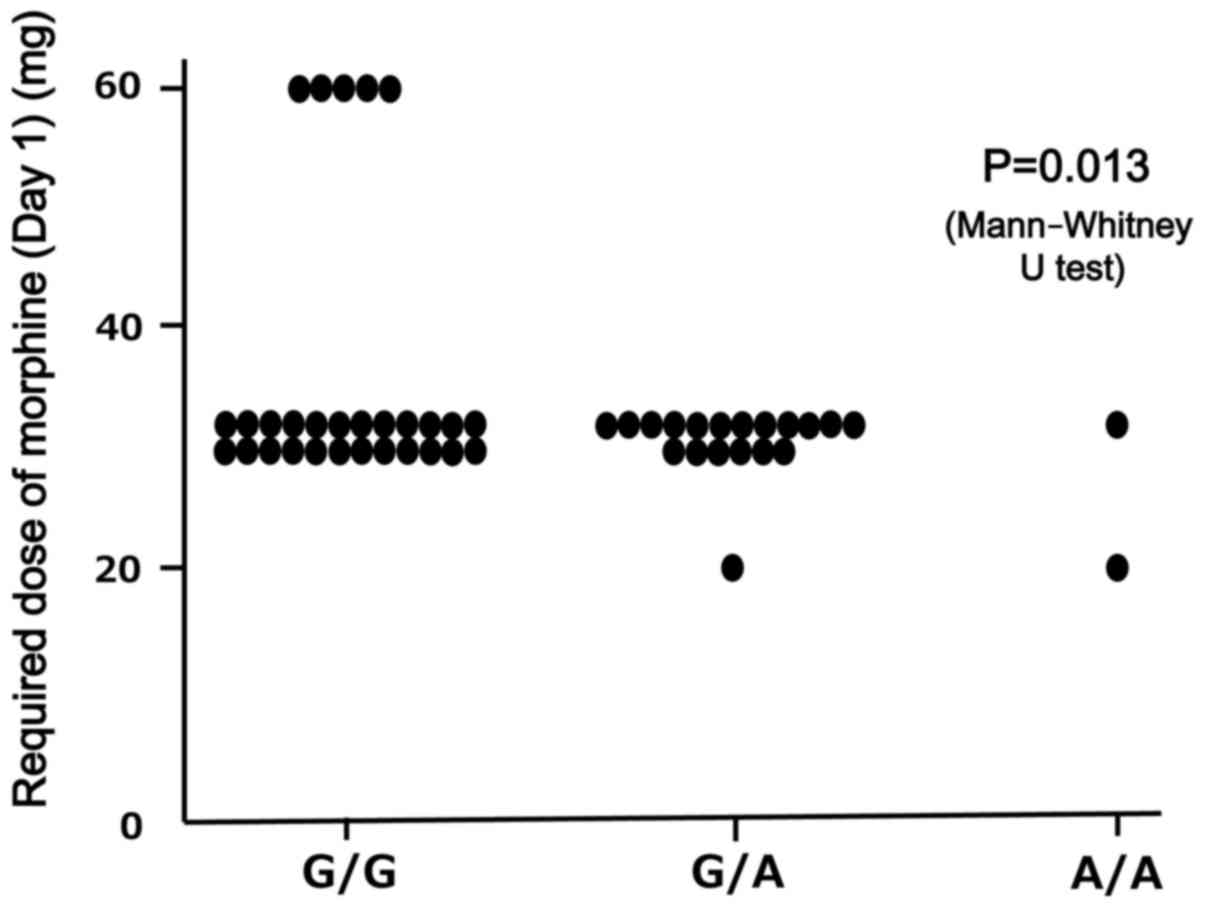
A functional genotype analysis of COMT 472G→A
(rs4680; p.Val158Met) was performed to establish the
predictive biomarkers for the outcome of morphine treatment
(Fig. 2). The outcome based on the
required morphine dose on days 1 and 8 was examined according to
the genotype. The required dose of morphine (mg) on day 1 in GG,
AG, and AA were 35.2±11.5, 29.5±2.3, and 25.0±7.1, respectively,
and was significantly higher for patients with the COMT G/G
genotype, compared with those with non-G/G (A/A and A/G) genotypes
(P=0.013).
Discussion
To the best of our knowledge, the present study is
the first to demonstrate the reproducibility of differences between
required doses of morphine among individuals, due to genetic
polymorphisms, in an independent cohort of opioid-treatment-naïve
patients exhibiting various types of cancer. The most important
finding is that patients with the COMT G/G genotype require
a higher dose of morphine (day 1), compared with patients with A/A
and A/G genotypes.
The morphine effect varies due to genetic
differences; therefore, genetic polymorphisms are considered to be
predictive markers. Various clinical studies have demonstrated that
the required dose of morphine is higher in subjects with a
COMT G/G genotype, compared with other genotypes, in cancer
and non-cancer pain (11–14). The current findings are consistent with
these results. A large sample study has indicated that none of 112
SNPs in the 25 candidate genes showed significant associations with
opioid dose (15); however, this study
lacked clarity regarding the doses of concomitant analgesics, the
dose determination of opioids, and the high average total dose of
morphine of 90 mg/day. Despite this dose, residual pain was
relatively high (pain NRS in the past 24 h, 3.20±2.10 subsequent to
analgesic use and no data for pain NRS prior to analgesic use)
(15). This suggests a high proportion
of refractory pain. By contrast, in the current study, the dose of
concomitant analgesics was controlled (all patients received daily
regular doses of NSAIDs and acetaminophen), the method of dose
titration was consistent, and pain was relatively well-controlled
(pain NRS following treatment, 2.46±1.46). Patients who report a
higher NRS for baseline pain generally exhibit a larger change in
raw pain intensity (23). The patients
in the present study had relatively strong pain (pain NRS in the
past 24 h, 6.88±2.43) that was relatively well-controlled, and this
may have made it easier to identify a difference due to genetic
polymorphism.
Notably, the required dose of morphine on day 1 was
significantly higher in patients with the COMT G/G genotype.
The enzyme activity of COMT is defined as high in G/G, intermediate
in G/A and low in A/A. Impairment of the COMT enzyme suppresses the
production of enkephalin, which causes subsequent opioid receptor
expression upregulation (10). In the
A/A genotype, the function of the µ receptor is weak and patients
tend to be sensitive to pain. However, the density of µ receptors
is proposed to be increased in this genotype, which may be the
reason for the low quantity of morphine required (24,25).
The nature of the pain NRS questionnaire may also be
relevant. As patients with the COMT A/A genotype are more
sensitive to pain, the reported NRS may be higher than that for the
quantity of morphine required (26). A
meta-analysis by Lee et al (27) revealed an association between
fibromyalgia and the COMT A/A genotype.
The present study has various limitations. First, it
is not possible to rule out that differences in patients’
background between the COMT G/G genotype and COMT
non-G/G genotype groups may be responsible for the higher required
dose of morphine in the G/G group. Although there was no
significant difference in age between the G/G genotype and non-G/G
genotype groups (P=0.658), the PS tended to be poor in the G/G
genotype group (P=0.053). However, there were no patients with poor
PS (3 and 4) in the high-dose morphine group. The COMT G/G
genotype group has a marginally higher NRS score (prior to
treatment) than the non-G/G genotype group. Although there was no
significant difference in the NRS score before treatment between
these groups (P=0.177), NRS scores tended to be higher in high-dose
morphine-requiring cases compared with other cases. Thus, patient
backgrounds require evaluation in future studies. In addition, the
patients were included in this study based on the decision of
attending physicians in a single hospital; consequently, the
results may not be generalizable to other centers. Furthermore,
disease duration, comorbidity, use of drugs other than opioids,
NSAIDs, acetaminophen, and psychosocial factors, including
education level and occupation, were not examined. Finally, the
sample size is not considered to be large enough for a genetic
study, which increases the rate of false positives. Therefore, the
current results should be treated with caution until the present
study is repeated using a larger population sample.
In conclusion, within these limitations, these
results indicate that the COMT genotype influences the
outcome of morphine treatment; therefore, it may be useful as a
predictive biomarker for morphine treatment. The frequency of the
GG allele has been found to be around 20% in previous studies
(11,12,25), but
close to 50% in a study targeting Japanese patients (17). This demonstrates ethnic differences in
allele frequencies. This observation supports that it is clinically
meaningful not to choose morphine as the first opioid in G/G
patients with cancer pain, as higher doses may be required.
However, these findings are from a prospective observational study,
and an interventional study is required for further evaluation.
Acknowledgements
The present study was supported by the Third-Term
Comprehensive 10-Year Strategy for Cancer Control and a
Grant-in-Aid for Cancer Research from the Ministry of Health,
Labour and Welfare (grant no. H22-037). The authors would like to
thank Mrs. Tomoko Kitayama, Mrs. Erina Hatashita, Mrs. Kiyoko
Kuwata, Mrs. Haruka Yamaguchi, Mr. Hiromasa Wadaguri and Mrs. Akiko
Mizumoto for their technical assistance.
References
|
1
|
Cleeland CS: Undertreatment of cancer pain
in elderly patients. JAMA. 279:1914–1915. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Patrick DL, Ferketich SL, Frame PS, Harris
JJ, Hendricks CB, Levin B, Link MP, Lustig C, McLaughlin J, Reid
LD, et al: National Institutes of Health State-of-the-Science
Panel: National Institutes of Health State-of-the-Science
Conference Statement: Symptom management in cancer: pain,
depression, and fatigue. July;15–17. 2002.J Natl Cancer Inst
Monogr. 32:9–16, 2004.
|
|
3
|
Potter J and Higginson IJ: Pain
experienced by lung cancer patients: A review of prevalence, causes
and pathophysiology. Lung Cancer. 43:247–257. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Butler LD, Koopman C, Cordova MJ, Garlan
RW, DiMiceli S and Spiegel D: Psychological distress and pain
significantly increase before death in metastatic breast cancer
patients. Psychosom Med. 65:416–426. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bischoff K, Weinberg V and Rabow MW:
Palliative and oncologic co-management: Symptom management for
outpatients with cancer. Support Care Cancer. 21:3031–3037. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chase DM, Huang HQ, Wenzel L, Cella D,
McQuellon R, Long HJ, Moore DH and Monk BJ: Quality of life and
survival in advanced cervical cancer: A Gynecologic Oncology Group
study. Gynecol Oncol. 125:315–319. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kelsen DP, Portenoy RK, Thaler HT,
Niedzwiecki D, Passik SD, Tao Y, Banks W, Brennan MF and Foley KM:
Pain and depression in patients with newly diagnosed pancreas
cancer. J Clin Oncol. 13:748–755. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Turk DC, Sist TC, Okifuji A, Miner MF,
Florio G, Harrison P, Massey J, Lema ML and Zevon MA: Adaptation to
metastatic cancer pain, regional/local cancer pain and non-cancer
pain: Role of psychological and behavioral factors. Pain.
74:247–256. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lötsch J, Geisslinger G and Tegeder I:
Genetic modulation of the pharmacological treatment of pain.
Pharmacol Ther. 124:168–184. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bell GC, Donovan KA and McLeod HL:
Clinical implications of opioid pharmacogenomics in patients with
cancer. Cancer Control. 22:426–432. 2015.PubMed/NCBI
|
|
11
|
Rakvåg TT, Klepstad P, Baar C, Kvam TM,
Dale O, Kaasa S, Krokan HE and Skorpen F: The Val158Met
polymorphism of the human catechol-O-methyltransferase
(COMT) gene may influence morphine requirements in cancer
pain patients. Pain. 116:73–78. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rakvåg TT, Ross JR, Sato H, Skorpen F,
Kaasa S and Klepstad P: Genetic variation in the
catechol-O-methyltransferase (COMT) gene and morphine
requirements in cancer patients with pain. Mol Pain. 4:642008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Reyes-Gibby CC, Shete S, Rakvåg T, Bhat
SV, Skorpen F, Bruera E, Kaasa S and Klepstad P: Exploring joint
effects of genes and the clinical efficacy of morphine for cancer
pain: OPRM1 and COMT gene. Pain. 130:25–30. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Candiotti KA, Yang Z, Buric D, Arheart K,
Zhang Y, Rodriguez Y, Gitlin MC, Carvalho E, Jaraba I and Wang L:
Catechol-o-methyltransferase polymorphisms predict opioid
consumption in postoperative pain. Anesth Analg. 119:1194–1200.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Klepstad P, Fladvad T, Skorpen F, Bjordal
K, Caraceni A, Dale O, Davies A, Kloke M, Lundström S, Maltoni M,
et al: European Association for Palliative Care Research Network:
Influence from genetic variability on opioid use for cancer pain: A
European genetic association study of 2294 cancer pain patients.
Pain. 152:1139–1145. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Atkinson TM, Mendoza TR, Sit L, Passik S,
Scher HI, Cleeland C and Basch E: The Brief Pain Inventory and its
‘pain at its worst in the last 24 hours’ item: Clinical trial
endpoint considerations. Pain Med. 11:337–346. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Matsuoka H, Arao T, Makimura C, Takeda M,
Kiyota H, Tsurutani J, Fujita Y, Matsumoto K, Kimura H, Otsuka M,
et al: Expression changes in arrestin β 1 and genetic variation in
catechol-O-methyltransferase are biomarkers for the response to
morphine treatment in cancer patients. Oncol Rep. 27:1393–1399.
2012.PubMed/NCBI
|
|
18
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern Cooperative Oncology Group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Swarm R, Abernethy AP, Anghelescu DL,
Benedetti C, Blinderman CD, Boston B, Cleeland C, Coyle N,
Deleon-Casasola OA, Eilers JG, et al: NCCN Adult Cancer Pain: Adult
cancer pain. J Natl Compr Canc Netw. 8:1046–1086. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Caraceni A, Cherny N, Fainsinger R, Kaasa
S, Poulain P, Radbruch L and De Conno F: Pain measurement tools and
methods in clinical research in palliative care: Recommendations of
an expert working group of the European Association of Palliative
Care. J Pain Symptom Manage. 23:239–255. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
National Cancer Institute: Common
Terminology Criteria for Adverse Events (CTCAE) v4.03, . https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdfJune
14–2010
|
|
22
|
Montagna P: Recent advances in the
pharmacogenomics of pain and headache. Neurol Sci. 28 Suppl
2:S208–S212. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Farrar JT, Polomano RC, Berlin JA and
Strom BL: A comparison of change in the 0–10 numeric rating scale
to a pain relief scale and global medication performance scale in a
short-term clinical trial of breakthrough pain intensity.
Anesthesiology. 112:1464–1472. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tunbridge EM, Harrison PJ and Weinberger
DR: Catechol-o-methyltransferase, cognition, and psychosis:
Val158Met and beyond. Biol Psychiatry. 60:141–151. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zubieta JK, Heitzeg MM, Smith YR, Bueller
JA, Xu K, Xu Y, Koeppe RA, Stohler CS and Goldman D: COMT
val158Met genotype affects mu-opioid neurotransmitter
responses to a pain stressor. Science. 299:1240–1243. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Arnold BS, Alpers GW, Süss H, Friedel E,
Kosmützky G, Geier A and Pauli P: Affective pain modulation in
fibromyalgia, somatoform pain disorder, back pain, and healthy
controls. Eur J Pain. 12:329–338. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee YH, Kim JH and Song GG: Association
between the COMT Val158Met polymorphism and
fibromyalgia susceptibility and fibromyalgia impact questionnaire
score: A meta-analysis. Rheumatol Int. 35:159–166. 2015. View Article : Google Scholar : PubMed/NCBI
|