Introduction
Behçet's disease (BD) is a systemic
auto-inflammatory disorder principally characterized by recurrent
oral aphthous ulcers, genital ulcers and ocular inflammation. BD
also affects numerous other organs involved in the vascular,
articular, gastrointestinal, pulmonary, and central nervous systems
(1). As the prevalence of BD is high
in certain regions of the world, including the Mediterranean,
Middle East, Turkey and East Asia (2),
previous studies have attempted to identify the etiopathogenic
mechanisms underlying BD (1,2); however, the causes and pathogenesis
remain unclear.
B lymphocytes serve various functions, by acting as
antigen-presenting cells to activate cluster of differentiation
(CD)4+ T cells, by providing costimulatory molecules and
cytokines, and by producing antibodies within the humoral immune
system (3). B cells are also
considered to serve a critical role in the pathogenesis of BD
(4–6).
Patients with active BD have been reported to exhibit elevated
numbers of immunoglobulin (Ig)-secreting B cells, which are
representative of fully differentiated B cells in vivo
(4). Increased levels of activated and
memory B cell subsets also suggests that alterations in B cell
function may be involved in the development of BD (5). The role of B cell activating factor in
signaling in B cells may contribute to B cell abnormalities and the
development of skin lesions in patients with BD (6). Although studies have also evaluated the
roles of T cells in BD (7–9), numerous other reports have continued to
emerge regarding the contributions of abnormalities in B
cell-associated factors, including CD43 (10–13),
activation-induced cytidine deaminase (AID) (14–19), and
interleukin (IL)-10 (20–26), to the progression of autoimmune
disease.
CD43, also known as leukosialin or sialophorin, is a
cell surface glycoprotein that is considered to be involved in the
modulation of apoptosis, cell differentiation, immune homeostasis,
cell adhesion, anti-adhesion and signal transduction (10). CD43 antigen is expressed on the
majority of leukocytes, and in particular, is expressed on
activated B and plasma cells, though not on resting (naïve) B
cells. Abnormal expression of CD43 has been reported in a number of
autoimmune pathologies, including systemic lupus erythematosus
(SLE), Wiskott-Aldrich syndrome and human immunodeficiency virus
infection (11–13).
From the perspective of humoral immunity, AID is
proposed to be an important mechanistic factor that influences B
cell function (14). AID deaminates
target cytidines (C) to uracil's (U) in the Ig-encoding region and
triggers U-G mismatches; through this mechanism, AID initiates Ig
somatic hypermutation (SHM) and class switch recombination (CSR)
(14,15), resulting in the affinity maturation of
antibodies and production of different Ig classes against
pathogenic antigens (15). Thus,
changes in AID expression have been associated with the severity of
autoimmune diseases, including lupus nephritis and rheumatoid
arthritis in mouse models (16–19).
Among the various subsets of B cells, some specific
types negatively regulate the cellular immune response and
inflammation (20). In particular,
IL-10-producing subsets of regulatory B cells (BREGS), known as B10
cells, are now considered to serve major functions in the
downregulation of autoimmunity, inflammation, and innate and
adaptive immune responses, and are amongst the most intensively
studied BREG subsets (21–23). IL-10 is an anti-inflammatory cytokine
that is involved in the development and maintenance of immune
tolerance and homeostasis (24), and
suppresses proinflammatory cytokine production and antigen
presentation (25). B10 cells not only
limit inflammation and immune responses through the production of
IL-10, but also facilitate the clearance of antigens by producing
antigen-specific antibodies during the humoral immune response
(26).
Accordingly, in the present study, the role of B
cells in the pathogenesis of BD was investigated. In particular,
the phenotypic proportions of B cells were assessed to determine
their effects of the autoimmune system, and the expression of AID
in B cells from patients with BD was evaluated for the first time
in vivo. Additionally, the expression and plasma
concentration of IL-10 were measured to provide insight into the
effects of IL-10 and the roles of B10 cells in BD.
Materials and methods
Patients and healthy controls
(HCs)
A total of 16 Korean patients with BD (11 women and
5 men; mean age, 50.06±9.43) and 16 age- and sex-matched HCs were
recruited from Konyang University Hospital (Daejeon, Republic of
Korea). All participants provided informed consent for
participation in the study. All patients with BD met the
International Study Group Classification Criteria for BD (27) and received outpatient treatment. The
clinical manifestations of the patients are presented in Table I. HCs were identified as having no
history of autoimmune disease or other health problems following
blood sample collection. The present study was approved by the
Institutional Review Board of Konyang University Hospital (approval
no.: KYUH 2016-12-015-002).
 | Table I.Clinical manifestations of patients
with Behçet's disease. |
Table I.
Clinical manifestations of patients
with Behçet's disease.
| Case | Sex/age
(years) | Oral ulcer | Genital ulcers | Skin lesions | Eye lesions | Thrombosis | Arthritis | Vasculitis | ESR (mm/h) | CRP (mg/l) |
|---|
| 1 | F/57 | AU/DU/Mul/PL | Y | EN/PF | Uveitis | Y | Y | Y | 11 | 0.2 |
| 2a | F/55 | AU/DU/Mul/PL | Y | EN | Uveitis,
conjunctivitis | N | Y | Y | 68 | 3.0 |
| 3a | M/52 | AU | Y | EN | Conjunctivitis | N | Y | Y | 15 | 0.1 |
| 4a | M/25 | AU/Mul | Y | EN | Uveitis | N | N | Y | 16 | 0.2 |
| 5 | F/50 | AU/Mul | N | EN | – | N | N | Y | 16 | 0.1 |
| 6 | F/47 | AU/Mul | Y | EN | Conjunctivitis | N | Y | N | 29 | 0.1 |
| 7a | M/43 | DU/S | N | EN | Uveitis | N | N | N | 5 | 0.1 |
| 8 | F/61 | AU/DU/Mul | Y | EN | – | N | N | Y | 15 | 0.3 |
| 9 | M/48 | DU/ | Y | EN | – | N | N | N | 7 | 0.1 |
| 10 | M/53 | AU/DU/Mul | Y | EN/PF | Uveitis | N | N | N | 44 | 3.1 |
| 11 | F/50 | AU | Y | – | – | N | N | N | 12 | 0.1 |
| 12 | F/38 | AU/Mul/PL | Y | EN | Uveitis | N | Y | N | 45 | 4.0 |
| 13 | F/49 | DU | Y | EN/PF | Uveitis | N | N | N | 9 | 0.9 |
| 14 | F/59 | AU/DU | Y | EN | – | N | N | N | 24 | 0.1 |
| 15 | F/55 | AU/DU/Mul | Y | EN | – | N | N | N | 39 | 1.4 |
| 16a | F/38 | AU/DU/Mul | Y | EN | Conjunctivitis,
optic neuritis | N | N | N | 10 | 2.7 |
Plasma and cell separation
Peripheral blood (~8 ml) was obtained from each
patient and HC by venipuncture using plastic blood collection tubes
containing lithium heparin (BD Biosciences, San Jose, CA, USA). The
samples were treated to separate B cells within 1 h at room
temperature. An equivalent volume of phosphate-buffered saline
(PBS) was added to the fresh blood samples, the samples were
centrifuged for 15 min at 500 × g at room temperature, and the
plasma supernatant was collected. The heparinized-plasma was then
stored at −70°C until analysis by enzyme-linked immunosorbent assay
(ELISA). After removing the remaining supernatant, corpuscles below
the plasma were used to create a layer of peripheral blood
mononuclear cells (PBMCs) by Histopaque density-gradient
centrifugation (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) as
previously described (28). The
samples were then washed twice with Hank's balanced salt solution
(Welgene, Inc., Daegu, Korea), and the acquired PBMCs were
incubated for 30 min with CD19 microbeads (20 µl per
1×107 cells; Miltenyi Biotec GmbH., Bergisch Gladbach,
Germany) on ice. As CD19 is only expressed on B cells (20), magnetic-activated cell sorting (MACS)
allowed us to positively select B cells by flow cytometric analysis
using a FACS Calibur flow cytometer (BD Biosciences, San Jose, CA,
USA) (29).
The lymphocyte and B cell numbers in the blood
samples were evaluated at two time points: Immediately after
layering of the PBMCs, and following the isolation of B cells by
MACS sorting. Two independent researchers counted the number of
cells twice using an inverted fluorescence microscope (CKX41;
Olympus Corp., Tokyo, Japan).
ELISA
The frozen plasma samples were used to measure the
plasma levels of IL-10 and IgA. ELISA was performed with commercial
ELISA kits [IgA human simplestep ELISA kit (cat. no. ab196263;
Abcam, Cambridge, UK) and high sensitivity human IL-10 ELISA kit
(cat. no. D1000B, R&D Systems Inc., Minneapolis, MN, USA)]
according to the manufacturer's instructions.
Flow cytometry analysis
Suspended B cells sorted using CD19 microbeads were
washed with MACS buffer (Miltenyi Biotec GmbH, Bergisch Gladbach,
Germany) and then incubated with anti-human CD19-phycoerythrin
(25–0199) and anti-human CD43-fluorescein isothiocyanate (FITC;
11-0439) antibodies (each 0.25 µg/5 µl; Affeymetrix; Thermo Fisher
Scientific Inc., Waltham, MA, USA) for 30 min on ice. After washing
twice with PBS, the cells were fixed with 1% paraformaldehyde at
4°C until use. The cells were analyzed with the FACSCalibur
cytometer using CellQuest software, and the data were confirmed
with FlowJo V10 (Tree Star, Inc., Ashland, OR, USA).
RNA amplification and reverse
transcription-quantitative polymerase chain reaction(RT-qPCR)
Total RNA was extracted from B cells using an
RNAqueous-micro total RNA isolation kit (Invitrogen; Thermo Fisher
Scientific, Inc.). The concentration and quality of the total RNA
were assessed using an Optizen 3220 UV spectrophotometer (Rose
Scientific Ltd., Edmonton, Canada). However, the quantities of
isolated total RNA were inadequate, and thus mRNA was amplified
after synthesizing cDNA from RNA samples with qualities of >1.6
(absorbance 260/280) using a QuantiTect whole transcriptome kit
(Qiagen, Inc., Valencia, CA, USA) to obtain a concentration of 100
ng/µl complementary DNA according to the manufacturer's
instructions. For subsequent use, the amplified cDNA was diluted to
2 ng/µl in diethylpyrocarbonate (DEPC)-treated distilled
H2O (Sigma-Aldrich; Merck KGaA). qPCR was performed in a
final volume of 20 µl containing 2 µl diluted cDNA, 0.5 µl of 100
pmol/µl of each forward and reverse primer, 10 µl buffer (iQ
SYBR-Green Supermix; Bio-Rad Laboratories, Inc., Hercules, CA, USA)
and 7 µl DEPC-treated distilled H2O using a CFX96 touch
real-time PCR detection 29 system (Bio-Rad Laboratories, Inc.). The
primers used in the study were as follows: IL-10, forward,
5′-ACCTGGGTTGCCAAGCCTT-3′ and reverse, 5′-ATCGATGACAGCGCCGTAG-3′;
AID, forward, 5′-CCTCTTGATGAACCGGAGGAA-3′ and reverse,
5′-AGCACTGTCACGCCTCTTCACT-3′; and glyceraldehyde 3-phosphate
dehydrogenase (GAPDH), forward, 5′-ACAGTCAGCCGCATCTTCTT-3′ and
reverse, 5′-ACGACCAAATCCGTTGACTC-3′. The qPCR conditions to amplify
AID, IL-10 and GAPDH were as follows: Initial denaturation at 95°C
for 3 min followed by 50 cycles of denaturation at 95°C for 10 sec,
annealing for 10 sec at an appropriate temperature (56°C for IL-10
and GAPDH, 60°C for AID), and extension at 72°C for 10 sec. The
targeted mRNA expression levels were quantified following
normalization to the expression of GAPDH using the comparative
threshold cycle (∆∆Cq) method (30).
Statistical analysis
The data are presented as mean ± standard error of
the mean unless otherwise stated. The significance of differences
was determined by two-tailed paired Student's t tests, and
differences with P<0.05 were considered to indicate a
statistically significant difference. All statistical analyses were
performed with Excel 2013 (Microsoft Corp., Redmond, WA, USA).
Results
Plasma concentrations of IL-10 and
IgA
IL-10 is an anti-inflammatory cytokine that inhibits
the production of proinflammatory cytokines and serves a role in
the development and maintenance of immune tolerance and homeostasis
(24,25). IgA is present at high levels at mucosal
sites and is responsible for mucosal immunity (31); however, a number of patients with BD
have been documented to have IgA nephropathy (32). Therefore, the present study measured
the plasma levels of IL-10 and IgA by ELISA. The results indicated
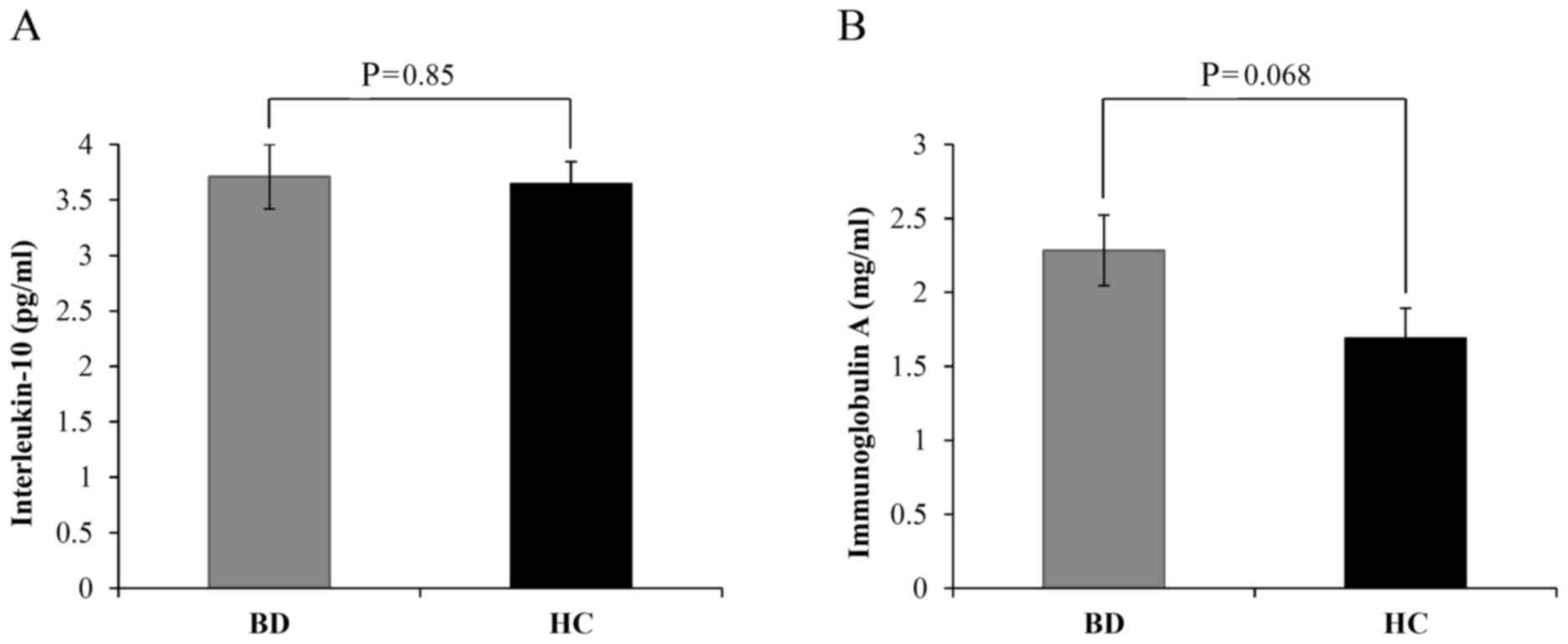
that the levels of IL-10 between patients with BD and HCs did not
markedly differ (3.71±0.29 vs. 3.64±0.02, respectively; P=0.85;
Fig. 1A). Meanwhile, IgA levels were
notably increased in patients with BD compared with HCs; however,
the difference was not significant (2.28±0.24 vs. 1.69±0.20,
respectively; P=0.068; Fig. 1B).
CD43+CD19+ B
cell count is indistinguishable between patients with BD and
HCs
Lymphocyte and B cell numbers were slightly reduced
in the PBMC populations of patients with BD compared with those in
HCs, and the percentage of B cells to lymphocytes was markedly
lower in patients with BD; however, the differences were not
significant (Table II).
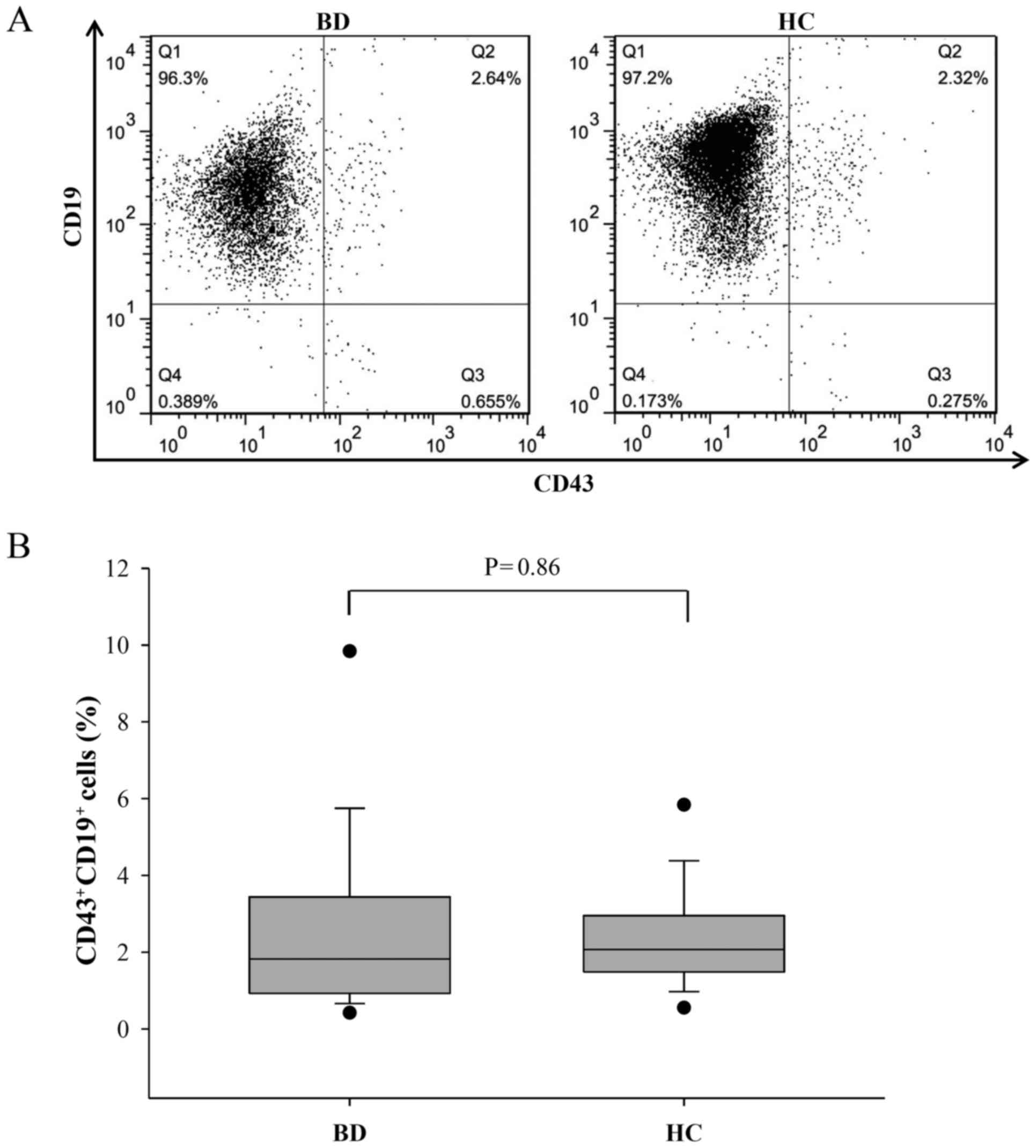
CD19+ B cells were isolated to ~97% purity (Fig. 2A) from the human PBMCs and anti-human
CD43-FITC antibodies were used to determine changes in the
activated (CD43+CD19+) (33) B cell population by flow cytometry
analysis. Despite the reduction in the B cell percentage, the
number of CD43+CD19+ B cells in patients with
BD did not differ significantly compared with that in HCs
(2.41±0.57 vs. 2.29±0.31, respectively; P=0.86; Fig. 2B). This result corresponds to a
previous report that observed no marked alteration in the
CD43+ B cell component of patients with SLE compared
with healthy donors (34).
 | Table II.Comparisons of B cell and lymphocyte
counts between patients with BD and HCs. |
Table II.
Comparisons of B cell and lymphocyte
counts between patients with BD and HCs.
| Cell type | BD | HC |
P-valuea |
|---|
| Lymphocytes
(×105/ml) | 7.02±0.85 | 7.76±0.72 | 0.51 |
| B cells
(×104/ml) | 7.24±1.52 | 9.60±1.07 | 0.21 |
| B cells/lymphocytes
(%) | 9.43±1.34 | 12.61±1.15 | 0.08 |
Expression of IL-10 and AID mRNA in B
cells
To further investigate abnormalities that may affect
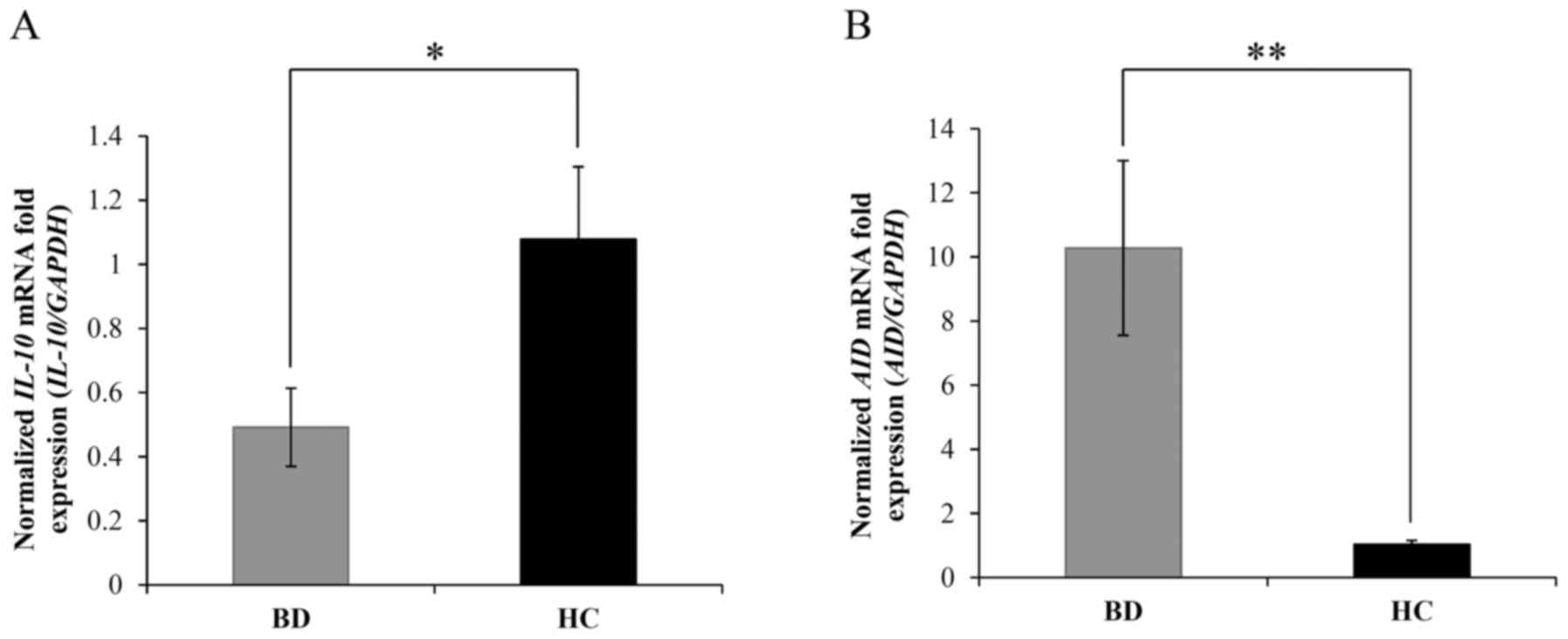
the etiopathology of BD, the expression of IL-10 and AID mRNA was
evaluated. The results indicated that the mRNA levels of IL-10 were
significantly downregulated in patients with BD compared with those
in HCs (0.49±0.12 vs. 1.08±0.26, respectively; P=0.03; Fig. 3A). Meanwhile, the levels of AID mRNA
were significantly increased in patients with BD compared with
those in HCs (10.27±2.72 vs. 1.04±0.12, respectively; P=0.003;
Fig. 3B). These results were
consistent with previous studies suggesting that high expression of
AID triggers autoimmune disease in lupus-prone mice (18) and that abnormal AID expression
contributes to autoimmune diseases (35).
Discussion
The objective of the present study was to
investigate changes in B cells in patients with BD. Specifically,
phenotypic analysis was conducted by comparing CD43+
populations among patients with BD and HCs. Although the number of
CD43+CD19+ B cells did not differ between
patients with BD and HCs, it was observed, to the best of our
knowledge for the first time, that the expression of IL-10 mRNA in
B cells was significantly decreased in patients with BD compared
with HCs. Furthermore, AID mRNA levels in B cells were increased by
~10-fold in patients with BD compared with those in HCs.
To date, studies of AID, as a factor involved in
immunity, have demonstrated that this protein can induce
autoantibody production (16–19). A study by Hsu et al (16) reported that BXD2 mice, presenting with
age-related development and progression of arthritis,
glomerulonephritis and high immune complex titers, exhibited
significant alterations in autoantibody production and AID
expression in the germinal center when compared with wild-type
mice. Murphy roths large (MRL) mice, which present SLE-like
symptoms, also exhibit increased AID expression, and hyperactivity
of SHM and CSR when focusing on heavy mutations in the Ig locus
(18). Additionally, in AID-knockdown
and AID-knockout MRL mice, lupus nephritis, as a main condition
triggered by autoantibodies, was alleviated compared with AID
wild-type MRL mice (17,19). Furthermore, AID may account for the
antibody-independent role of B cells in T cell activation and
autoimmunity (36). In the present
study, it was observed that AID mRNA expression was markedly
increased in patients with BD patient compared with HCs. Although
the majority of previous studies have been performed in mice, the
indicated effects of autoantibodies may also be relevant in the
pathogenesis of BD in humans. However, the specific underlying
mechanisms remain unclear, and additional studies are required to
determine the exact relationship between AID and BD. Specifically,
to elucidate the effect of aberrant AID expression on Ig CSR and
SHM in B cells in BD, the expression patterns and functional roles
of AID splicing variants should be examined in BD B cells, as AID
splicing variants have been indicated to serve various functions in
Ig CSR and SHM (37).
BREGS are involved in immunomodulation and
suppression of the immune response through a range of mechanisms.
The roles of BREGS have been studied in mouse models of autoimmune
diseases including SLE (38) and
experimental autoimmune encephalomyelitis (21). However, there is no clear understanding
of the characteristics of BREGS (39).
Three mature B cell subsets have been identified in mice [B1 cells,
follicular B (FOB) cells, marginal zone B (MZB) cells]; these
subsets have different characteristics regarding their phenotypes
and functional activities (40). In
particular, FOB cells, also known as adaptive B cells, and MZB
cells belong to a subset of innate-like B (ILB) cells termed B2
cells. These B2 cells contribute to the adaptive immune response
and mediate humoral immunity; they may also produce high-affinity
antibodies and generate immunological memory (41). Human B1 cells are typically considered
as CD43+ B cells, while B2 cells may be regarded as
CD43− B cells (34).
Furthermore, CD43− IL-10-producing ILB cells have been
identified to have BREG activity in immune responses during
chlamydia infection (42). In the
present study, IL-10 mRNA expression was reduced in the B cells of
patients with BD, suggesting that the percentage of CD43-
IL-10-producing B cells may be reduced in BD, as these cells have
more potential than CD43+ IL-10-producing B cells to
affect the immune system. However, only the whole CD43−
B cell was distinguished from the whole CD43+ B cell
proportion; therefore, further studies are necessary to clarify the
involvement of IL-10-producing subtypes in BD. Meanwhile, in
contrast to IL-10 mRNA levels, plasma IL-10 levels were not
altered. This result may be explained by the observation that T
cells and neutrophils also produce IL-10 (43,44). In
addition, the change in IL-10 level may not be reflected in the
plasma as the proportion of total B cells in the peripheral blood
is relatively low (~10%) (45).
There were a number of limitations to the present
study. Firstly, the patients were not grouped according to the
status of BD (active vs. inactive). Due to the experimental methods
used in the study, fresh blood samples were required from patients,
and blood was drawn from patients immediately following their visit
to the hospital. However, it was not possible to request that
patients visit the hospital specifically when they had symptoms.
Thus, the majority of the patients were evaluated during the
inactive disease period, and only three patients presented with
active BD during evaluation. Secondly, all the patients with BD
were treated with medication at the time of blood sampling. This
may have affected the results of serum cytokine analysis and
RT-qPCR. Therefore, further studies are required with improved
control of patient treatment and blood sample collection in order
to obtain results of higher accuracy.
In conclusion, the present study described the
concentrations of IL-10 and IgA in plasma, the number of
CD43+/CD43− B cells and the mRNA levels of
IL-10 and AID in B cells from fresh peripheral blood samples of
patients with BD and matched HCs. The present findings indicated,
to the best of our knowledge for the first time, that AID mRNA was
upregulated in patients with BD. These data may be a starting point
for examining the mechanisms and influence of AID in BD in further
studies. Correlations between disease severity and AID expression
should also be evaluated, which may implicate the applications of
AID in the diagnosis or treatment of BD. Furthermore, based on the
present results that IL-10 mRNA was downregulated in B cells in
vivo, future studies should be performed to analyze B10 cells,
which downregulate immune responses, to elucidate the immunological
mechanisms involved in BD.
Acknowledgements
The current study was supported by the National
Research Foundation of Korea funded by the Korean Government (grant
nos. NRF-2015R1D1A3A01019948 and NRF-2017R1C1B2008199).
Glossary
Abbreviations
Abbreviations:
|
AID
|
activation-induced cytidine
deaminase
|
|
BD
|
Behçet's disease
|
|
Bregs
|
regulatory B cells
|
|
CD
|
cluster of differentiation
|
|
CSR
|
class switch recombination
|
|
DEPC
|
diethylpyrocarbonate
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
FITC
|
fluorescein isothiocyanate
|
|
FOB
|
follicular B cells
|
|
HCs
|
healthy controls
|
|
Ig
|
immunoglobulin
|
|
IL
|
interleukin
|
|
ILB
|
innate-like B
|
|
MACS
|
magnetic-activated cell sorting
|
|
MZB
|
marginal zone B
|
|
MRS
|
Murphy Roths Large
|
|
PBMCs
|
peripheral blood mononuclear cells
|
|
PBS
|
phosphate-buffered saline
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
SHM
|
somatic hypermutation
|
|
SLE
|
systemic lupus erythematosus
|
References
|
1
|
Kural-Seyahi E, Fresko I, Seyahi N,
Ozyazgan Y, Mat C, Hamuryudan V, Yurdakul S and Yazici H: The
long-term mortality and morbidity of Behçet syndrome: A 2-decade
outcome survey of 387 patients followed at a dedicated center.
Medicine (Baltimore). 82:60–76. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Khairallah M, Accorinti M, Muccioli C,
Kahloun R and Kempen JH: Epidemiology of Behçet disease. Ocul
Immunol Inflamm. 20:324–335. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
LeBien TW and Tedder TF: B lymphocytes:
How they develop and function. Blood. 112:1570–1580. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Suzuki N, Sakane T, Ueda Y and Tsunematsu
T: Abnormal B cell function in patients with Behçet's disease.
Arthritis Rheum. 29:212–219. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ekşioglu-Demiralp E, Kibaroglu A,
Direskeneli H, Yavuz S, Karsli F, Yurdakul S, Yazici H and Akoglu
T: Phenotypic characteristics of B cells in Behçet's disease:
Increased activity in B cell subsets. J Rheumatol. 26:826–832.
1999.PubMed/NCBI
|
|
6
|
Hamzaoui K, Houman H, Ben Dhifallah I,
Kamoun M and Hamzaoui A: Serum BAFF levels and skin mRNA expression
in patients with Behçet's disease. Clin Exp Rheumatol. 26:64–71.
2008.
|
|
7
|
Imamura Y, Kurokawa MS, Yoshikawa H, Nara
K, Takada E, Masuda C, Tsukikawa S, Ozaki S, Matsuda T and Suzuki
N: Involvement of Th1 cells and heat shock protein 60 in the
pathogenesis of intestinal Behcet's disease. Clin Exp Immunol.
139:371–378. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Geri G, Terrier B, Rosenzwajg M, Wechsler
B, Touzot M, Seilhean D, Tran TA, Bodaghi B, Musset L, Soumelis V,
et al: Critical role of IL-21 in modulating TH17 and regulatory T
cells in Behçet disease. J Allergy Clin Immunol. 128:655–664. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hughes T, Ture-Ozdemir F, Alibaz-Oner F,
Coit P, Direskeneli H and Sawalha AH: Epigenome-wide scan
identifies a treatment-responsive pattern of altered DNA
methylation among cytoskeletal remodeling genes in monocytes and
CD4+ T cells from patients with Behçet's disease. Arthritis
Rheumatol. 66:1648–1658. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pedraza-Alva G and Rosenstein Y: CD43- One
molecule, many tales to recount. Signal Transduct. 7:372–385. 2007.
View Article : Google Scholar
|
|
11
|
Liang ZB, Zhang SF and Xu J: CD43
preliminary study of expression of CD43 antigen on lymphocytes of
peripheral blood in patients with SLE. Zhonghua Pifuke Zazhi.
31:14–18. 1998.
|
|
12
|
Parkman R, Remold-O'Donnell E, Kenney DM,
Perrine S and Rosen FS: Surface protein abnormalities in
lymphocytes and platelets from patients with Wiskott-Aldrich
syndrome. Lancet. 2:1387–1389. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gallego MD, Aguado E, Kindelán JM, Peña J,
Santamaría M and Molina IJ: Altered expression of
CD43-hexasaccharide isoform on peripheral T lymphocytes from
HIV-infected individuals. AIDS. 15:477–481. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Park SR: Activation-induced cytidine
deaminase in B cell immunity and cancer. Immune Netw. 12:230–239.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nussenzweig MC and Alt FW: Antibody
diversity: One enzyme to rule them all. Nat Med. 10:1304–1305.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hsu HC, Wu Y, Yang P, Wu Q, Job G, Chen J,
Wang J, Accavitti-Loper MAV, Grizzle WE, Carter RH, et al:
Overexpression of activation-induced cytidine deaminase in B cells
is associated with production of highly pathogenic autoantibodies.
J Immunol. 178:5357–5365. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jiang C, Foley J, Clayton N, Kissling G,
Jokinen M, Herbert R and Diaz M: Abrogation of lupus nephritis in
activation-induced deaminase-deficient MRL/lpr mice. J Immunol.
178:7422–7431. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zan H, Zhang J, Ardeshna S, Xu Z, Park SR
and Casali P: Lupus-prone MRL/faslpr/lpr mice display increased AID
expression and extensive DNA lesions, comprising deletions and
insertions, in the immunoglobulin locus: Concurrent upregulation of
somatic hypermutation and class switch DNA recombination.
Autoimmunity. 42:89–103. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jiang C, Zhao ML and Diaz M:
Activation-induced deaminase heterozygous MRL/lpr mice are delayed
in the production of high-affinity pathogenic antibodies and in the
development of lupus nephritis. Immunology. 126:102–113. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
DiLillo DJ, Matsushita T and Tedder TF:
B10 cells and regulatory B cells balance immune responses during
inflammation, autoimmunity, and cancer. Ann N Y Acad Sci.
1183:38–57. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Matsushita T, Yanaba K, Bouaziz JD,
Fujimoto M and Tedder TF: Regulatory B cells inhibit EAE initiation
in mice while other B cells promote disease progression. J Clin
Invest. 118:3420–3430. 2008.PubMed/NCBI
|
|
22
|
Mauri C and Bosma A: Immune regulatory
function of B cells. Annu Rev Immunol. 30:221–241. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yanaba K, Bouaziz JD, Haas KM, Poe JC,
Fujimoto M and Tedder TF: A regulatory B cell subset with a unique
CD1dhi CD5+ phenotype controls T cell-dependent inflammatory
responses. Immunity. 28:639–650. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Anderson AC, Reddy J, Nazareno R, Sobel
RA, Nicholson LB and Kuchroo VK: IL-10 plays an important role in
the homeostatic regulation of the autoreactive repertoire in naive
mice. J Immunol. 173:828–834. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Moore KW, de Waal Malefyt R, Coffman RL
and O'Garra A: Interleukin-10 and the interleukin-10 receptor. Annu
Rev Immunol. 19:683–765. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Maseda D, Smith SH, DiLillo DJ, Bryant JM,
Candando KM, Weaver CT and Tedder TF: Regulatory B10 cells
differentiate into antibody-secreting cells after transient IL-10
production in vivo. J Immunol. 188:1036–1048. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Weichsler B, Davatchi F, Mizushima Y,
Hamza M, Dilsen N, Kansu E, Yazici H, Barnes CG, Chamberlain MA,
James DG, et al: International Study Group for Behçet's Disease:
Criteria for diagnosis of Behçet's disease. Lancet. 335:1078–1080.
1990.PubMed/NCBI
|
|
28
|
Fuss IJ, Kanof ME, Smith PD and Zola H:
Isolation of whole mononuclear cells from peripheral blood and cord
blood. Curr Protoc Immunol. 85:7.1.1–7.1.8. 2009.https://doi.org/10.1002/0471142735.im0701s85
|
|
29
|
Miltenyi S, Müller W, Weichel W and
Radbruch A: High gradient magnetic cell separation with MACS.
Cytometry. 11:231–238. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cho SB, Ahn KJ, Kim DH, Zheng Z, Cho S,
Kang SW, Lee JH, Park YB, Lee KH and Bang D: Identification of
HnRNP-A2/B1 as a target antigen of anti-endothelial cell IgA
antibody in Behçet's disease. J Invest Dermatol. 132:601–608. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Altay M, Secilmis S, Unverdi S, Ceri M and
Duranay M: Behcet's disease and IgA nephropathy. Rheumatol Int.
32:2227–2229. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Barclay A, Brown M, Alex Law SK, McKnight
A, Tomlinson M and van der Merwe P: The Leukocyte Antigen
Factsbook. Academic press; London: 1997, View Article : Google Scholar
|
|
34
|
Inui M, Hirota S, Hirano K, Fujii H,
Sugahara-Tobinai A, Ishii T, Harigae H and Takai T: Human CD43+ B
cells are closely related not only to memory B cells phenotypically
but also to plasmablasts developmentally in healthy individuals.
Int Immunol. 27:345–355. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hase K, Takahashi D, Ebisawa M, Kawano S,
Itoh K and Ohno H: Activation-induced cytidine deaminase deficiency
causes organ-specific autoimmune disease. PLoS One. 3:e30332008.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jiang C, Zhao ML, Waters KM and Diaz M:
Activation-induced deaminase contributes to the
antibody-independent role of B cells in the development of
autoimmunity. Autoimmunity. 45:440–448. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wu X, Darce JR, Chang SK, Nowakowski GS
and Jelinek DF: Alternative splicing regulates activation-induced
cytidine deaminase (AID): Implications for suppression of AID
mutagenic activity in normal and malignant B cells. Blood.
112:4675–4682. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Douglas RS, Woo EY, Capocasale RJ, Tarshis
AD, Nowell PC and Moore JS: Altered response to and production of
TGF-beta by B cells from autoimmune NZB mice. Cell Immunol.
179:126–137. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Vitale G, Mion F and Pucillo C: Regulatory
B cells: Evidence, developmental origin and population diversity.
Mol Immunol. 48:1–8. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rawlings DJ, Schwartz MA, Jackson SW and
Meyer-Bahlburg A: Integration of B cell responses through Toll-like
receptors and antigen receptors. Nat Rev Immunol. 12:282–294. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nadler LM, Stashenko P, Hardy R, van
Agthoven A, Terhorst C and Schlossman SF: Characterization of a
human B cell-specific antigen (B2) distinct from B1. J Immunol.
126:1941–1947. 1981.PubMed/NCBI
|
|
42
|
Moore-Connors JM, Kim HS, Marshall JS,
Stadnyk AW, Halperin SA and Wang J: CD43-, but not CD43+,
IL-10-producing CD1dhiCD5+ B cells suppress type 1 immune responses
during Chlamydia muridarum genital tract infection. Mucosal
Immunol. 8:94–106. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Barrat FJ, Cua DJ, Boonstra A, Richards
DF, Crain C, Savelkoul HF, de Waal-Malefyt R, Coffman RL,
Hawrylowicz CM and O'Garra A: In vitro generation of interleukin
10-producing regulatory CD4(+) T cells is induced by
immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and
Th2-inducing cytokines. J Exp Med. 195:603–616. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Romani L, Mencacci A, Cenci E, Spaccapelo
R, Del Sero G, Nicoletti I, Trinchieri G, Bistoni F and Puccetti P:
Neutrophil production of IL-12 and IL-10 in candidiasis and
efficacy of IL-12 therapy in neutropenic mice. J Immunol.
158:5349–5356. 1997.PubMed/NCBI
|
|
45
|
Morbach H, Eichhorn EM, Liese JG and
Girschick HJ: Reference values for B cell subpopulations from
infancy to adulthood. Clin Exp Immunol. 162:271–279. 2010.
View Article : Google Scholar : PubMed/NCBI
|