Introduction
Colorectal cancer is among the most prevalent
malignancies worldwide, and therefore effective and minimally
invasive procedures are required to reduce the incidence rate of
this malignancy (1,2). More recently, utilization of colorectal
endoscopic submucosal dissection (ESD) as a minimally invasive
treatment for en bloc resection of large superficial neoplasms has
become a favored method (3). However,
the risk of complication is higher than in gastric ESD as the wall
of the colon is thin and operability is limited. In addition,
colorectal ESD generally requires a long procedure time due to its
technical difficulty (4,5). Therefore, a high level of gas enters the
colonic lumen. This is associated with aggravation of subjective
symptoms, such as abdominal pain, discomfort and distention, and an
increased risk of severe problems, including pneumoderma,
pneumothorax, abdominal compartment syndrome and air embolism
(6–14).
The safety and efficacy of CO2
insufflation during ESD for lesions of the esophagus and stomach
have been demonstrated in several randomized controlled trials
(15–21). A pilot study also reported that
CO2 insufflation was safe and effective during
colorectal ESD (15). As
CO2 is absorbed faster than air and is rapidly
eliminated through the lungs, CO2 insufflation is
expected to reduce residual gas in both the small and large bowels
following ESD, and consequently reduce the abdominal symptoms and
complications associated with ESD (15,22–30).
However, to the best of our knowledge, detailed and quantitative
examinations evaluating the effects of CO2 insufflation
on residual gas in the gastrointestinal tract following colorectal
ESD have not been performed.
The aim of the present study was to assess whether
CO2 insufflation could decrease the level of residual
gas in the gastrointestinal tract following ESD in patients with
colorectal neoplasms. All patients received an abdominal computed
tomography (CT) examination immediately following colorectal ESD,
and the level of residual gas in the gastrointestinal tract
following ESD was objectively evaluated by measuring the axes of
the cecal lumen and the diameter of the terminal ileum lumen. The
safety of CO2 insufflation was also assessed by
measuring the levels of transcutaneous CO2 tension
(PtcCO2) and evaluating the development of ESD-related
complications such as air leaks.
Materials and methods
Patients
Between January and December 2009, all consecutive
patients undergoing colorectal ESD at Gifu University Hospital
(Gifu, Japan), were screened for the present study. Colorectal ESD
was indicated for lesions that required endoscopic en bloc
resection, despite this technique being difficult to use for
endoscopic mucosal resection or polypectomy.
Patients with one or more of the following
conditions were excluded: (i) They exhibited chronic pulmonary
dysfunction defined as a forced expiratory volume in 1.0 sec/forced
vital capacity of <70% or a vital capacity of <80%; (ii) they
were unable to understand the consent information required for
participation; and/or (iii) they refused to participate in the
study. The study protocol was approved by the institutional Ethics
Committee of Gifu University Hospital (ethical approval no.
28-104). All eligible individuals provided written informed consent
prior to study enrollment. Randomization was conducted using a
random number list, and patients were divided into two groups,
namely a CO2 insufflation group (CO2 group)
and an air insufflation group (air group).
ESD procedures and examination
schedule for study events prior to and following ESD
ESD was conducted in the afternoon on the day of
admission. Prior to inserting the colonoscope, 5.0–10.0 mg diazepam
(Cercine®; Takeda Pharmaceutical Company, Ltd., Tokyo,
Japan), 7.5–15.0 mg pentazocine (Pentagin®; Daiichi
Sankyo Co., Ltd., Tokyo, Japan) and 12.5–25.0 mg hydroxyzine
(Atarax-P®; Phizer Japan, Inc., Tokyo, Japan) were
injected intravenously for induction of anesthesia and analgesia.
As necessary, 5.0 mg diazepam or 7.5 mg pentazocine and 12.5 mg
hydroxyzine were administered repeatedly for deep sedation; when
patients opened their eyes or moved their body, the patients were
considered to be out of deep sedation and received the additional
injections. The ESD procedures were performed using a colonoscope
and water jet (PCF-Q260J; Olympus Medical Systems; Olympus
Corporation, Tokyo, Japan). The colorectal lesions were resected
using either a DualKnife (KD-650Q) or FlexKnife (KD-630L; both from
Olympus Corporation). A 0.4% high-molecular-weight hyaluronic acid
solution containing 0.001% epinephrine was injected into the
submucosal layer to raise the lesion. The mucosal layer around the
lesion was cut circumferentially; subsequently, the submucosal
layer was directly dissected with the DualKnife or FlexKnife. If
possible, the post-ESD ulcer was closed using clips; otherwise,
exposed blood vessels were clipped to reduce the risk of active
bleeding. Circulation vitals, including systolic and diastolic
blood pressures, heart rate and transcutaneous oxygen saturation
(SpO2) were measured with a bedside monitor (BSM-4101;
Nihon Kohden Corporation, Tokyo, Japan) at the start of the
procedure, every 5 min during the procedure, and at the end of the
procedure. On the second hospital day, blood tests for white blood
cell (WBC) count and C-reactive protein (CRP) were performed, and
the highest axillary temperature during the total hospital stay was
recorded. WBC count was measured with an automated hematology
analyzer (XE-2100; Sysmex Corporation, Kobe, Japan) and CRP was
measured with an automatic biochemical analyzer (JCA-BM2250; JEOL,
Ltd., Tokyo, Japan) with use of a CRP kit (CRP Latex X2
NX®; Denka Seiken Co. Ltd., Tokyo, Japan), according to
the manufacturer's instructions.
CO2 insufflation and
transcutaneous gas analysis
CO2 was administered using a
CO2 regulation unit (OLYMPUS UCRTM; Olympus
Corporation). PtcCO2 was continuously measured from the
time of insertion of the colonoscope until the end of the procedure
by using a CO2 sensor kit (TOSCA measurement system and
TOSCA 500 monitor; Linde Medical Sensors Ag, Basel, Switzerland).
The low-flow gas tube (MAJ-1742, Olympus Corporation) of the UCR
was used for CO2 insufflation, which was set at a
constant rate of 1.4 l/min for all patients, as reported previously
(31).
Evaluation of CT examination
CT examination of the abdomen and pelvis was
performed immediately following ESD using a 16-MDCT device
(LightSpeed 16; GE Healthcare Life Sciences, Little Chalfont, UK)
with a fixed tube voltage of 120 kVp and an automatic tube current
modulation program (3D mA modulation; GE Healthcare Life Sciences).
The operational parameters of the scanner were set to noise index,
10.0 HU at 5 mm slice thickness; collimation, 1.25 mm; detector
configuration, 16×1.25 mm; table feed, 27.5 mm/rotation; pitch,
1.37:1; field of view, 32×32 cm; gantry rotation time, 0.5 sec;
acquisition time, 12.7 sec. All transverse CT images were
reconstructed at 5 mm section thickness with a standard
reconstruction algorithm. CT images were analyzed using Advantage
Workstation v4.6 software (GE Healthcare Life Sciences). To
evaluate the amount of residual gas in the gastrointestinal tract
following ESD, a scan was performed at the level of the ileocecal
valve, and the major and minor axes of the cecal lumen and the
diameter of the terminal ileum lumen were measured. A small level
of focal free air close to the colonic wall of the resected lesion
was defined as a transmural air leak and a high level of free air
reaching the surface of the liver was defined as free air (32,33).
Definitions of outcome parameters and
complications
The operation time was measured from the start of
injection into the submucosal layer until the end of the procedure.
A diagnosis of perforation was made by direct endoscopic
observation during ESD. Post-procedure hemorrhage was diagnosed
with clinical evidence of bleeding following ESD, as shown by
repetitive bloody bowel discharges that required endoscopic
treatment.
Statistical analysis
All the variables in the present study are described
as the number of patients (%) or the median (range). Fisher's exact
test was used to compare differences in categorical variables
between the two groups when required, and the non-parametric
Mann-Whitney U test was used to compare continuous variables.
Two-sided P<0.05 was considered to indicate statistically
significant differences for all tests. All statistical analyses
were performed using JMP v12 (SAS Institute, Inc., Cary, NC,
USA).
Results
Patient and procedure
characteristics
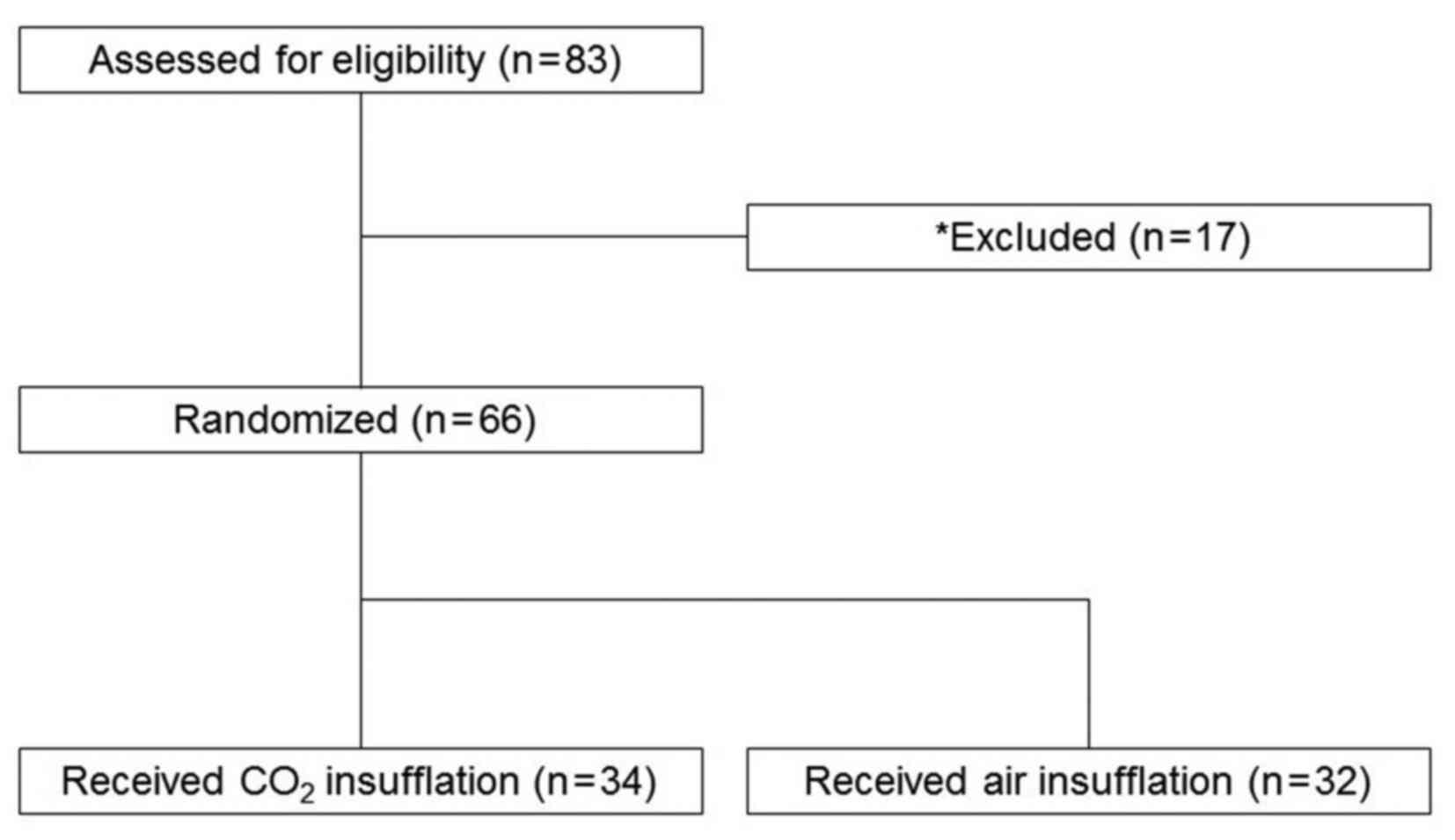
A total of 83 patients underwent colorectal ESD
during the study period. Among them, 17 patients were excluded due
to presentation of chronic pulmonary dysfunction; the remaining 66
patients were enrolled in the present study. The enrolled subjects
were randomized into two groups: A total of 34 patients received
CO2 insufflation (CO2 group) and 32 patients
received air insufflation (air group; Fig. 1).
Baseline characteristics and related factors for
each group are listed in Table I. The
median age was 65.0 (34.0–87.0) years in the CO2 group
and 65.5 (34.0–86.0) years in the air group. No significant
differences in age, sex, en bloc resection rate, location of the
colorectal lesion, histopathological type, tumor size, histological
depth, resection size or histopathologically curative resection
rate between the groups were identified. The median procedure time
was 35 (10–163) and 30 (9–94) min and the rate of clip closure for
the post-ESD ulcer was 44.1 and 53.1% in the CO2 and air
groups, respectively; neither of these differed significantly
between the groups.
 | Table I.Patient and procedure
characteristics. |
Table I.
Patient and procedure
characteristics.
| Variables | CO2
group (n=34) | Air group
(n=32) | P-value |
|---|
| Age, years | 65.0
(34.0–87.0) | 65.5
(34.0–86.0) | 0.78 |
| Sex, male/female,
n | 18/16 | 17/15 | 1.00 |
| Location of lesion,
C/A/T/D/S/R, n | 2/3/7/0/5/17 | 3/6/5/1/5/12 | 0.58 |
| En bloc resection,
n (%) | 33 (97.1) | 31 (96.9) | 1.00 |
| Histopathological
type, tub1/tub2/adenoma/carcinoid/hyperplastic/endocrine carcinoma,
n | 14/1/13/5/0/1 | 11/1/14/5/1/0 | 0.71 |
| Histological depth,
M/SM, n | 25/8 | 25/7 | 1.00 |
| Histopathologically
curative resection, n (%) | 30 (88.2) | 31 (96.9) | 0.36 |
| Tumor size, mm | 30 (12–72) | 30 (12–47) | 0.93 |
| Resection size,
mm | 23 (7–62) | 24 (3–46) | 0.73 |
| Procedure time,
min | 35 (10–163) | 30 (9–94) | 0.57 |
| Prophylactic clip
closure, n (%) | 15 (44.1) | 17 (53.1) | 0.62 |
Presence of residual gas in the
gastrointestinal tract evaluated by CT examination
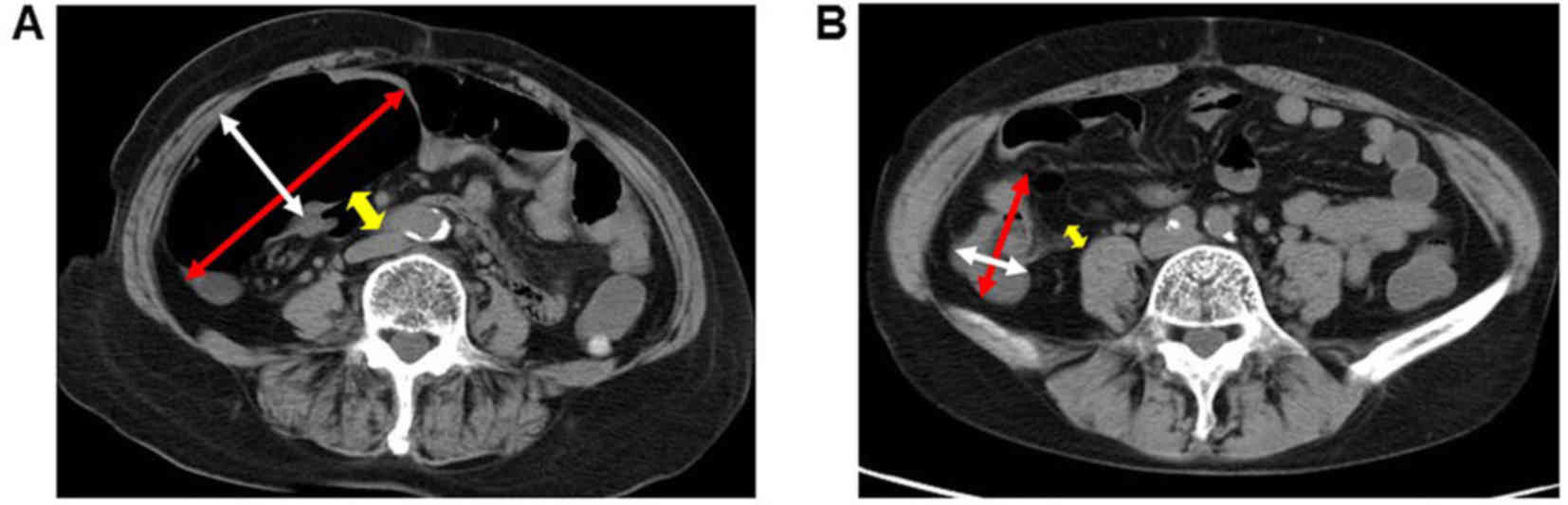
Representative CT images of patients in the two
groups are presented in Fig. 2. In
the air group, marked intestinal dilatation of the cecum and
terminal ileum was observed compared with the CO2 group.
The findings of the CT examination following ESD are summarized in
Table II. The median major (21.8 vs.
56.3 mm, P<0.001) and minor (13.4 vs. 36.6 mm, P<0.001) axes
of the cecal lumen at the level of the ileocecal valve were
significantly lower in the CO2 group compared with the
air group. In addition, the median diameter of the terminal ileum
lumen was significantly lower in the CO2 group compared
with that in the air group (5.0 vs. 16.4 mm, P<0.001). These
findings demonstrated the effects of CO2 insufflation on
the diminution of residual gas in the bowel following colorectal
ESD.
 | Table II.Post-ESD computed tomography
results. |
Table II.
Post-ESD computed tomography
results.
| Variables | CO2
group (n=34) | Air group
(n=32) | P-value |
|---|
| Major axis of cecal
lumen after ESD, mm | 21.8
(6.1–59.0) | 56.3
(28.9–126.9) | <0.001 |
| Minor axis of cecal
lumen after ESD, mm | 13.4
(4.2–39.8) | 36.6
(11.9–62.3) | <0.001 |
| Diameter of
terminal ileum lumen after ESD, mm | 5.0 (0.0–15.9) | 16.4
(3.5–28.1) | <0.001 |
| Free air after ESD,
n (%) | 1 (2.9) | 3 (9.4) | 0.340 |
| Transmural air leak
after ESD, n (%) | 8 (23.5) | 2 (6.3) | 0.080 |
The presence of free air was indicated in 1 patient
(2.9%) in the CO2 group and 3 patients (9.4%) in the air
group. Transmural air leak was also observed in 8 patients (23.5%)
in the CO2 group and 2 patients (6.3%) in the air group;
however, no significant differences were identified in the
incidence rates of these air leaks between the groups.
PtcCO2 and vital signs
prior to and following ESD
The PtcCO2 and vital signs (blood
pressure, heart rate and SpO2) of patients recorded
during ESD are listed in Table III.
The median PtcCO2 prior to and following ESD was 38.5
(22.0–51.0) and 46.5 (30.0–58.0) mmHg in the CO2 group
and 40.0 (27.0–48.0) and 47.0 (36.0–55.0) mmHg in the air group,
respectively; no significant differences in these values between
the groups were determined. The median peak PtcCO2
during ESD was 49.0 (34.0–111.0) mmHg in the CO2 group
and 49.0 (40.0–55.0) mmHg in the air group; no significant
difference was observed between these values.
 | Table III.PtcCO2 and vital signs
during ESD. |
Table III.
PtcCO2 and vital signs
during ESD.
| Variables | CO2
group (n=34) | Air group
(n=32) | P-value |
|---|
| Baseline
PtCO2, mmHg | 38.5
(22.0–51.0) | 40.0
(27.0–48.0) | 0.13 |
| PtCO2
after ESD, mmHg | 46.5
(30.0–58.0) | 47.0
(36.0–55.0) | 0.38 |
| Peak
PtCO2, mmHg | 49.0
(34.0–111.0) | 49.0
(40.0–55.0) | 0.95 |
| Baseline systolic
blood pressure, mmHg | 132.5
(94.0–187.0) | 133.5
(98.0–180.0) | 0.78 |
| Systolic blood
pressure after ESD, mmHg | 134.5
(100.0–180.0) | 135.5
(85.0–174.0) | 0.83 |
| Maximum systolic
blood pressure, mmHg | 150.5
(112.0–229.0) | 145.0
(102.0–182.0) | 0.80 |
| Maximum systolic
blood pressure elevation value, mmHg | 3.5 (0.0–92.0) | 4.5 (0.0–42.0) | 0.64 |
| Baseline diastolic
blood pressure, mmHg | 74.0
(38.0–103.0) | 69.5
(50.0–95.0) | 0.48 |
| Diastolic blood
pressure after ESD, mmHg | 74.5
(56.0–104.0) | 75.0
(45.0–94.0) | 0.89 |
| Maximum systolic
blood pressure, mmHg | 84.0
(64.0–121.0) | 83.0
(53.0–112.0) | 0.90 |
| Maximum systolic
blood pressure elevation value, mmHg | 7.5 (0.0–43.0) | 7.5 (0.0–34.0) | 0.73 |
| Baseline heart
rate, n/min | 76.5
(51.0–125.0) | 71.0
(50.0–116.0) | 0.30 |
| Heart rate after
ESD, n/min | 72.0
(53.0–97.0) | 69.5
(53.0–93.0) | 0.31 |
| Maximum heart rate,
n/min | 87.5
(61.0–125.0) | 80.0
(57.0–125.0) | 0.07 |
| Maximum heart rate
elevation value, n/min | 3.5 (0.0–48.0) | 1.5 (0.0–37.0) | 0.74 |
| Minimum
SpO2, % | 98.0
(94.0–100.0) | 98.5
(95.0–100.0) | 0.81 |
Circulation vitals, including systolic and diastolic
blood pressures and heart rate prior to and following ESD, did not
differ significantly between the groups. In addition, significant
elevation or lowering of blood pressures and heart rate during ESD
was not observed in either group. The median minimum
SpO2 levels did not differ significantly between the
groups, being 98.0 (94.0–100.0) % in the CO2 group and
98.5 (95.0–100.0) % in the air group (Table III).
Incidence of post-ESD complications
and hospital stay
ESD-related complications and the length of hospital
stay are summarized in Table IV. No
significant differences were observed in body temperature,
incidence of post-ESD hemorrhage or the length of stay between the
CO2 and air groups. Furthermore, no significant
differences were identified in serum CRP levels or WBC counts on
day 1 after ESD. A patient in the CO2 group presented a
complication of perforation during ESD, but the lesion was closed
using clips. The patient did not require emergency surgery and was
discharged 4 days after ESD. No cases of cardiopulmonary adverse
events occurred in either group.
 | Table IV.ESD-related complications and length
of hospital stay. |
Table IV.
ESD-related complications and length
of hospital stay.
| Variables | CO2
group (n=34) | Air group
(n=32) | P-value |
|---|
| Body temperature,
°C | 36.8
(36.3–38.5) | 36.8
(36.3–38.4) | 0.94 |
| Post-procedure
hemorrhage, n (%) | 2 (5.9) | 1 (3.1) | 1.00 |
| WBC on day 1 after
ESD, n/µl | 6,800
(4,220–14,890) | 7,500
(3,940–18,900) | 0.80 |
| CRP on day 1 after
ESD, mg/dl | 0.27
(0.02–6.17) | 0.20
(0.02–11.03) | 0.98 |
| Perforation, n
(%) | 1 (2.9) | 0 (0) | 1.00 |
| Emergency surgery,
n (%) | 0 (0) | 0 (0) | 1.00 |
| Hospital stay,
days | 3 (2–9) | 3 (2–10) | 0.34 |
Discussion
ESD of colorectal tumors can provide clinical
benefits as it enables en bloc resection of large superficial
neoplasms (32,34). However, one of the problems with this
procedure is the severe intraoperative and post-operative abdominal
discomfort and distention caused by air infusion (15). CO2 is absorbed by the bowel
mucosa approximately 100 times faster than air and is rapidly
eliminated through the lungs (35).
This may be associated with the superior recovery quality of
CO2 insufflation compared with room air insufflation in
colonoscopy (36). Therefore, when
compared with air insufflation, CO2 insufflation is
expected to reduce the volume of residual gas following ESD, which
is a primary cause of patient discomfort associated with this
procedure, and consequently prevent the development of abdominal
symptoms and problems associated with ESD (5,22–30).
The results of the present randomized trial
indicated that CO2 insufflation during colorectal ESD
significantly reduced the volume of residual bowel gas compared
with air insufflation. Several studies have reported the efficacy
of CO2 insufflation during various types of endoscopic
procedures (24,25,28,37,38).
However, few studies have objectively evaluated the level of
residual bowel gas following this procedure. Chen et al
(39), observed that CO2
insufflation significantly reduced the volume of residual bowel gas
compared with air insufflation following colonoscopy by using
abdominal radiography. To the best of our knowledge, the present
study is the first to objectively evaluate the degree of bowel
distention following colorectal ESD using CT examination. The
present study identified the median major and minor axes of the
cecal lumen at the level of the ileocecal valve to be significantly
lower in the CO2 group than in the air group
(P<0.001). In addition, the median diameter of the terminal
ileum lumen was significantly lower in the CO2 group
than in the air group (P<0.001). These findings suggest that
CO2 insufflation significantly reduced the volume of
residual bowel gas compared with air insufflation following
colorectal ESD.
In the present study, there was no significant
difference in the peak PtCO2, which is a useful marker
for evaluating CO2 retention (40), between the CO2 and air
insufflation groups. No marked adverse events, such as
CO2 narcosis, air embolism, SpO2 depression
or hemodynamic abnormality, occurred in either group. In addition,
post-procedure CT demonstrated no significant difference in the
incidence of free air or transmural air leak between the groups. As
CO2 insufflation can reduce the volume of residual bowel
gas, it may avoid an increase in intra-bowel pressure and improve
patient safety. These results suggest that CO2
insufflation during colorectal ESD is a safer alternative to air
insufflation.
In the present study, patients with chronic
pulmonary dysfunction were excluded, as the safety of
CO2 insufflation during colorectal ESD has not been
established for such patients. However, a recent study demonstrated
that CO2 insufflation during gastric ESD was safe for
patients with pulmonary dysfunction under conscious sedation
(31). CO2 insufflation
during colorectal ESD is also safe for patients with obstructive
ventilatory disturbance (41). The
number of patients, particularly elderly patients, suffering from
complications including chronic pulmonary dysfunction is
increasing; therefore, a clinical trial that clarifies the safety
and efficacy of CO2 insufflation during colorectal ESD
in such patients should be conducted.
The present study had a number of limitations.
First, the study was a single-center study with a relatively small
sample size. Therefore, multi-center studies with larger sample
sizes should be performed to confirm the present results. These
studies may also be useful for assessing whether CO2
insufflation may reduce the risk of ESD-related complications, such
as transmural air leak and perforation, compared with conventional
air insufflation. It should be also verified in future studies
whether CO2 insufflation relieved abdominal pain and
improved the degree of patients satisfaction. Second, the volume of
insufflated gas during the ESD procedure could not be measured.
However, it is probable that there was not much difference in the
volume of insufflated gas between the CO2 and air groups
since the flow volume was the same (1.4 L/min) in both groups and
no significant difference was observed in the median procedure time
between the groups.
Despite these limitations, it should be emphasized
that reduction of the patient's residual gastrointestinal gas
following ESD can decrease abdominal fullness; this is associated
with a high level of patient satisfaction (35). In conclusion, the present results
suggest that CO2 insufflation during colorectal ESD is
effective, as it may significantly reduce residual gas in the
gastrointestinal tract and therefore increase the satisfaction and
comfort of patients who have undergone ESD.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors contributions
TS and HA contributed to the study design,
acquisition and interpretation of data, and in the writing of the
manuscript; NO and JT acquired the data; MK and TI analyzed and
interpreted the data; MS wrote the manuscript and approved the
final contents of the manuscript. The final version of the
manuscript has been read and approved by all authors.
Ethics approval and consent to
participate
The study protocol was approved by the institutional
ethics committee of Gifu University Hospital (ethical approval
code: 28-104). All eligible individuals provided written informed
consent prior to study enrollment.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
References
|
1
|
Zauber AG, Winawer SJ, O'Brien MJ,
Lansdorp-Vogelaar I, van Ballegooijen M, Hankey BF, Shi W, Bond JH,
Schapiro M, Panish JF, et al: Colonoscopic polypectomy and
long-term prevention of colorectal-cancer deaths. N Engl J Med.
366:687–696. 2012. View Article : Google Scholar
|
|
2
|
Rex DK, Johnson DA, Lieberman DA, Burt RW
and Sonnenberg A: American College of Gastroenterology: Colorectal
cancer prevention 2000: Screening recommendations of the American
College of Gastroenterology. Am J Gastroenterol. 95:868–877. 2000.
View Article : Google Scholar
|
|
3
|
Saito Y, Kawano H, Takeuchi Y, Ohata K,
Oka S, Hotta K, Okamoto K, Homma K, Uraoka T, Hisabe T, et al:
Current status of colorectal endoscopic submucosal dissection in
Japan and other Asian countries: Progressing towards technical
standardization. Dig Endosc. 24 Suppl 1:67–72. 2012. View Article : Google Scholar
|
|
4
|
Fujishiro M: Endoscopic submucosal
dissection for colorectal neoplasms. World J Gastrointest Endosc.
1:32–38. 2009. View Article : Google Scholar
|
|
5
|
Saito Y, Uraoka T, Yamaguchi Y, Hotta K,
Sakamoto N, Ikematsu H, Fukuzawa M, Kobayashi N, Nasu J, Michida T,
et al: A prospective, multicenter study of 1111 colorectal
endoscopic submucosal dissections (with video). Gastrointest
Endosc. 72:1217–1225. 2010. View Article : Google Scholar
|
|
6
|
Morley AP, Lau JY and Young RJ: Tension
pneumothorax complicating a perforation of a duodenal ulcer during
ERCP with endoscopic sphincterotomy. Endoscopy. 29:3321997.
View Article : Google Scholar
|
|
7
|
Katzgraber F, Glenewinkel F, Fischler S
and Rittner C: Mechanism of fatal air embolism after
gastrointestinal endoscopy. Int J Legal Med. 111:154–156. 1998.
View Article : Google Scholar
|
|
8
|
Rai A and Iftikhar S: Tension pneumothorax
complicating diagnostic upper endoscopy: A case report. Am J
Gastroenterol. 94:845–847. 1999. View Article : Google Scholar
|
|
9
|
Nayagam J, Ho KM and Liang J: Fatal
systemic air embolism during endoscopic retrograde
cholangio-pancreatography. Anaesth Intensive Care. 32:260–264.
2004.
|
|
10
|
Green BT and Tendler DA: Cerebral air
embolism during upper endoscopy: Case report and review.
Gastrointest Endosc. 61:620–623. 2005. View Article : Google Scholar
|
|
11
|
Stabile L, Cigada M, Stillittano D,
Morandi E, Zaffroni M, Rossi G and Lapichino G: Fatal cerebral air
embolism after endoscopic retrograde cholangiopancreatography. Acta
Anaesthesiol Scand. 50:648–649. 2006. View Article : Google Scholar
|
|
12
|
Bisceglia M, Simeone A, Forlano R,
Andriulli A and Pilotto A: Fatal systemic venous air embolism
during endoscopic retrograde cholangiopancreatography. Adv Anat
Pathol. 16:255–262. 2009. View Article : Google Scholar
|
|
13
|
Finsterer J, Stöllberger C and Bastovansky
A: Cardiac and cerebral air embolism from endoscopic retrograde
cholangio-pancreatography. Eur J Gastroenterol Hepatol.
22:1157–1162. 2010. View Article : Google Scholar
|
|
14
|
van Boxel GI, Hommers CE, Dash I, Goodman
AJ, Green J and Orme RM: Myocardial and cerebral infarction due to
massive air embolism following endoscopic retrograde
cholangiopancreatography (ERCP). Endoscopy. 42 Suppl 2:E80–E81.
2010. View Article : Google Scholar
|
|
15
|
Saito Y, Uraoka T, Matsuda T, Emura F,
Ikehara H, Mashimo Y, Kikuchi T, Kozu T and Saito D: A pilot study
to assess the safety and efficacy of carbon dioxide insufflation
during colorectal endoscopic submucosal dissection with the patient
under conscious sedation. Gastrointest Endosc. 65:537–542. 2007.
View Article : Google Scholar
|
|
16
|
Nonaka S, Saito Y, Takisawa H, Kim Y,
Kikuchi T and Oda I: Safety of carbon dioxide insufflation for
upper gastrointestinal tract endoscopic treatment of patients under
deep sedation. Surg Endosc. 24:1638–1645. 2010. View Article : Google Scholar
|
|
17
|
Maeda Y, Hirasawa D, Fujita N, Obana T,
Sugawara T, Ohira T, Harada Y, Yamagata T, Suzuki K, Koike Y, et
al: A prospective, randomized, double-blind, controlled trial on
the efficacy of carbon dioxide insufflation in gastric endoscopic
submucosal dissection. Endoscopy. 45:335–341. 2013. View Article : Google Scholar
|
|
18
|
Kikuchi T, Fu KI, Saito Y, Uraoka T,
Fukuzawa M, Fukunaga S, Sakamoto T, Nakajima T and Matsuda T:
Transcutaneous monitoring of partial pressure of carbon dioxide
during endoscopic submucosal dissection of early colorectal
neoplasia with carbon dioxide insufflation: A prospective study.
Surg Endosc. 24:2231–2235. 2010. View Article : Google Scholar
|
|
19
|
Suzuki T, Minami H, Komatsu T, Masusda R,
Kobayashi Y, Sakamoto A, Sato Y, Inoue H and Serada K: Prolonged
carbon dioxide insufflation under general anesthesia for endoscopic
submucosal dissection. Endoscopy. 42:1021–1029. 2010. View Article : Google Scholar
|
|
20
|
Kim SY, Chung JW, Park DK, Kwon KA, Kim KO
and Kim YJ: Efficacy of carbon dioxide insufflation during gastric
endoscopic submucosal dissection: A randomized, double-blind,
controlled, prospective study. Gastrointest Endosc. 82:1018–1024.
2015. View Article : Google Scholar
|
|
21
|
Li X, Dong H, Zhang Y and Zhang G:
CO2 insufflation versus air insufflation for endoscopic
submucosal dissection: A meta-analysis of randomized controlled
trials. PLoS One. 12:e01779092017. View Article : Google Scholar
|
|
22
|
Hussein AM, Bartram CI and Williams CB:
Carbon dioxide insufflation for more comfortable colonoscopy.
Gastrointest Endosc. 30:68–70. 1984. View Article : Google Scholar
|
|
23
|
Stevenson GW, Wilson JA, Wilkinson J,
Norman G and Goodacre RL: Pain following colonoscopy: Elimination
with carbon dioxide. Gastrointest Endosc. 38:564–567. 1992.
View Article : Google Scholar
|
|
24
|
Church J and Delaney C: Randomized,
controlled trial of carbon dioxide insufflation during colonoscopy.
Dis Colon Rectum. 46:322–326. 2003. View Article : Google Scholar
|
|
25
|
Sumanac K, Zealley I, Fox BM, Rawlinson J,
Salena B, Marshall JK, Stevenson GW and Hunt RH: Minimizing
postcolonoscopy abdominal pain by using CO2
insufflation: A prospective, randomized, double blind, controlled
trial evaluating a new commercially available CO2
delivery system. Gastrointest Endosc. 56:190–194. 2002. View Article : Google Scholar
|
|
26
|
Rogers BH: The safety of carbon dioxide
insufflation during colonoscopic electrosurgical polypectomy.
Gastrointest Endosc. 20:115–117. 1974. View Article : Google Scholar
|
|
27
|
Bretthauer M, Lynge AB, Thiis-Evensen E,
Hoff G, Fausa O and Aabakken L: Carbon dioxide insufflation in
colonoscopy: Safe and effective in sedated patients. Endoscopy.
37:706–709. 2005. View Article : Google Scholar
|
|
28
|
Bretthauer M, Thiis-Evensen E,
Huppertz-Hauss G, Gisselsson L, Grotmol T, Skovlund E and Hoff G:
NORCCAP (Norwegian colorectal cancer prevention): A randomised
trial to assess the safety and efficacy of carbon dioxide versus
air insufflation in colonoscopy. Gut. 50:604–607. 2002. View Article : Google Scholar
|
|
29
|
Nakajima K, Lee SW, Sonoda T and Milsom
JW: Intraoperative carbon dioxide colonoscopy: A safe insufflation
alternative for locating colonic lesions during laparoscopic
surgery. Surg Endosc. 19:321–325. 2005. View Article : Google Scholar
|
|
30
|
Wu J and Hu B: The role of carbon dioxide
insufflation in colonoscopy: A systematic review and meta-analysis.
Endoscopy. 44:128–136. 2012. View Article : Google Scholar
|
|
31
|
Takada J, Araki H, Onogi F, Nakanishi T,
Kubota M, Ibuka T, Shimizu M and Moriwaki H: Safety of carbon
dioxide insufflation during gastric endoscopic submucosal
dissection in patients with pulmonary dysfunction under conscious
sedation. Surg Endosc. 29:1963–1969. 2015. View Article : Google Scholar
|
|
32
|
Tamegai Y, Saito Y, Masaki N, Hinohara C,
Oshima T, Kogure E, Liu Y, Uemura N and Saito K: Endoscopic
submucosal dissection: A safe technique for colorectal tumors.
Endoscopy. 39:418–422. 2007. View Article : Google Scholar
|
|
33
|
Coriat R, Leblanc S, Pommaret E,
Chryssostalis A, Prat F and Chaussade S: Transmural air leak
following endoscopic submucosal dissection: A non-useful computed
tomography finding. Endoscopy. 42:1117, author reply 11182010.
View Article : Google Scholar
|
|
34
|
Terasaki M, Tanaka S, Oka S, Nakadoi K,
Takata S, Kanao H, Yoshida S and Chayama K: Clinical outcomes of
endoscopic submucosal dissection and endoscopic mucosal resection
for laterally spreading tumors larger than 20 mm. J Gastroenterol
Hepatol. 27:734–740. 2012. View Article : Google Scholar
|
|
35
|
Saltzman HA and Sieker HO: Intestinal
response to changing gaseous environments: Normobaric and
hyperbaric observations. Ann N Y Acad Sci. 150(1 Gastrointesti):
1–39. 1968. View Article : Google Scholar
|
|
36
|
Wang WL, Wu ZH, Sun Q, Wei JF, Chen XF,
Zhou DK, Zhou L, Xie HY and Zheng SS: Meta-analysis: The use of
carbon dioxide insufflation vs. room air insufflation for
gastrointestinal endoscopy. Aliment Pharmacol Ther. 35:1145–1154.
2012. View Article : Google Scholar
|
|
37
|
Dellon ES, Velayudham A, Clarke BW, Isaacs
KL, Gangarosa LM, Galanko JA and Grimm IS: A randomized,
controlled, double-blind trial of air insufflation versus carbon
dioxide insufflation during ERCP. Gastrointest Endosc. 72:68–77.
2010. View Article : Google Scholar
|
|
38
|
Hirai F, Beppu T, Nishimura T, Takatsu N,
Ashizuka S, Seki T, Hisabe T, Nagahama T, Yao K, Matsui T, et al:
Carbon dioxide insufflation compared with air insufflation in
double-balloon enteroscopy: A prospective, randomized, double-blind
trial. Gastrointest Endosc. 73:743–749. 2011. View Article : Google Scholar
|
|
39
|
Chen SW, Hui CK, Chang JJ, Lee TS, Chan
SC, Chien CH, Hu CC, Lin CL, Chen LW, Liu CJ, et al: Carbon dioxide
insufflation during colonoscopy can significantly decrease
post-interventional abdominal discomfort in deeply sedated
patients: A prospective, randomized, double-blinded, controlled
trial. J Gastroenterol Hepatol. 31:808–813. 2016. View Article : Google Scholar
|
|
40
|
Chhajed PN, Kaegi B, Rajasekaran R and
Tamm M: Detection of hypoventilation during thoracoscopy: Combined
cutaneous carbon dioxide tension and oximetry monitoring with a new
digital sensor. Chest. 127:585–588. 2005. View Article : Google Scholar
|
|
41
|
Yoshida M, Imai K, Hotta K, Yamaguchi Y,
Tanaka M, Kakushima N, Takizawa K, Matsubayashi H and Ono H: Carbon
dioxide insufflation during colorectal endoscopic submucosal
dissection for patients with obstructive ventilatory disturbance.
Int J Colorectal Dis. 29:365–371. 2014. View Article : Google Scholar
|