Introduction
Multicolor flow cytometry provides detailed
phenotypic information on the cellular composition of a sample. It
allows for the identification of cellular subsets based on specific
markers both on the surface of and inside cells (1). With the development of novel classes of
fluorochromes and advancements in cytometer design, the use of more
than 8 colors for flow cytometry should become common practice in
clinical labs and increase the sensitivity of current assays
(2).
Paroxysmal nocturnal hemoglobinuria (PNH) is a rare
stem cell disorder caused by acquired mutation in the X-linked
phosphatidylinositol glycan complementation class A gene, with an
annual incidence rate of 1.3 new cases per million of the
population per year (3–8). The acquired mutation generates a defect
in the expression of glycol-phosphatidyl inositol (GPI)-linked
structures such as the complement regulatory proteins, CD55 and
CD59 (9–12). A lack of these GPI-linked complement
regulatory proteins on red blood cells (RBCs) may cause
intravascular complement-mediated hemolysis, resulting in the
release of free hemoglobin and leading to the symptoms of PNH,
which may include the development of anemia, hemoglobinuria,
thrombosis, dysphagia, abdominal pain, pulmonary hypertension,
renal impairment and erectile dysfunction (4,13–16). Previous PNH assays have focused on
loss of CD55 and CD59 on CD235a red cells or granulocytes (17,18).
However, pathological hemolysis of PNH red cells may generate
inaccurate results, as many patients who are screened for PNH have
received erythrocyte transfusions, which may contribute to
underestimating the size of the PNH clone population. As a result,
PNH erythrocytes may be undetectable even when a substantial PNH
population is present (19).
Furthermore, CD55 expression cannot distinguish intermediate
complement sensitivity (type II) and more abnormal PNH-type III RBC
clones (20), and the sensitivity of
CD55 alone is not high enough to detect PNH population sizes of
less than 1% (21). Despite there
being therapeutic support for patients with PNH, including
transfusions and anticoagulation management (22), the survival rate remains low, with 35%
of cases being fatal within 5 years of diagnosis (23–25). Small
PNH clones may grow over time and benefit from eculizumab treatment
(25). Thus, high-sensitivity PNH
assays are required to detect PNH populations of less than 1% in
order to follow up closely and to provide early treatments for
patients.
To improve the resolution of PNH detection, the
present study formulated an 8-color combination for the detection
of PNH white blood cells (WBCs), which included lineage-gating
antibodies (CD64 for monocytes and CD15 for granulocytes) together
with fluorescein-labeled proaerolysin (FLAER) and three GPI-linked
markers, namely CD157 [both granulocyte and monocyte-specific
(26)], CD24 [granulocyte-specific
(27)] and CD14 [monocyte-specific
(28)]. This combination matches the
International Clinical Cytometry Society (ICCS) guidelines for the
diagnosis and monitoring of PNH and related disorders by flow
cytometry (20).
To the best of our knowledge, this present report is
the first to identify a high-sensitivity 8-color antibody cocktail
for PNH flow detection with the use of a dump channel. It may be a
useful tool for PNH detection and has the potential to improve the
accuracy, work flow, cost and diagnostic process while saving the
labor and time of technicians and clinical pathologists.
Materials and methods
Blood samples and antibodies
Whole blood (WB) samples (100 µl) from PNH patients
were provided by the College of American Pathologists (CAP;
Northfield, IL, USA) and UK NEQAS (London, UK) between May 2017 and
August 2017. WB samples (100 µl) from 10 healthy donors (aged
58.4±11.1 years) were also collected. The samples were stored in BD
Vacutainer™ blood collection tubes containing EDTA as an
anti-coagulant (BD Biosciences, Franklin Lakes, NJ, USA) at 4°C and
tested within a month. For certain experiments, PNH proficiency
test samples were obtained from CAP and UK NEQAS. Alexa Fluor
(A)488-conjugated FLAER was purchased from Cedarlane (Burlington,
ON, Canada). Phycoerythrin (PE)-conjugated anti-CD157 (SY/11B5),
Allophycocyanin (APC)-conjugated anti-CD24 (ALB9) and
APC-A750-conjugated anti-CD64 (clone 22) were purchased from
Beckman Coulter, Inc. (Brea, CA, USA). Peridinin chlorophyll
protein complex (PerCP)-conjugated anti-CD14 (MfP9), PE-cyanine
(Cy)7-conjugated anti-CD5 (L17F12), PE-Cy7-conjugated anti-CD19
(SJ25C1), V450-conjugated anti-CD15 (MMA), V500-C-conjugated
anti-CD45 (2D1), PerCP-Cy5.5-conjugated anti-CD3 (SK7),
APC-conjugated anti-CD4 (SK3), APC-H7-conjugated anti-CD8 (SK1),
V450-conjugated anti-CD7 (M-T701), PE-conjugated anti-CD55 (IA10)
and APC-conjugated anti-CD14 (MfP9) were purchased from BD
Biosciences. All the antibodies were titrated to optimize the
cocktail performance. The present study was reviewed by the
Research Determination Office of Kaiser Permanente (Oakland, CA,
USA) and determined not to be human research due to the use of
secondary de-identified samples; therefore, the requirements of
informed patient consent and ethical approval were waived.
Cell staining with 8-color antibody
cocktail and acquisition
The 8-color antibody cocktail for PNH detection was
prepared according to the configuration listed in Table I. A total of 1×106 cells in
the EDTA-whole blood samples were transferred to 12×75 mm Falcon
tubes and stained with the 8-color cocktail antibody cocktail for
15 min at room temperature in dark. The whole blood was then lysed
with 2 ml BD FACS™ Lysing Solution (BD Biosciences) for 10 min at
room temperature. After washing the cells twice with
phosphate-buffered saline (PBS; Beckman Coulter, Inc.) and
resuspending in 0.5 ml 0.5% paraformaldehyde as fixation buffer
(Polysciences, Inc., Warrington, PA, USA), data were acquired on a
BD FACSCanto™ II flow cytometer with standard configuration (BD
Biosciences). A total of 100,000 events in the monocyte and
granulocyte cell gates were acquired. BD FACSDiva™ v.6.1.3 software
under standard 8-color configuration (BD Biosciences) was used for
acquisition and analysis. For compensation, a BD™ Comp Beads kit
was used to create cocktail-specific compensation matrices on whole
blood from the healthy donors utilizing batch-matched single color
reagents following the manufacturer's instructions. For a 4-color
control experiment, PE-CD55 (10 µl), APC-CD14 (5 µl), FLAER-A488
(10 µl) and V500-C-CD45 (5 µl) were used to stain four normal whole
blood samples (100 µl each). For certain experiments, whole blood
was stained with PerCP-Cy5.5-CD3, APC-CD4, V450-CD7 and
APC-H7-CD8.
 | Table I.Configuration of 8-color flow assay
cocktail for paroxysmal nocturnal hemoglobinuria detection. |
Table I.
Configuration of 8-color flow assay
cocktail for paroxysmal nocturnal hemoglobinuria detection.
| Fluorochrome | A488 | PE | PerCP | PE-Cy7 (dump) | APC | APC-A750 | V450 | V500-C |
|---|
| Marker | FLAER | CD157 | CD14 | CD5+CD19 | CD24 | CD64 | CD15 | CD45 |
| Clone | FLAER | SY/11B5 | MfP9 | L17F12+SJ25C1 | ALB9 | 22 | MMA | 2D1 |
| Volume/test
(µl) | 10 | 10 | 10 | 2.5+2.5 | 5 | 5 | 2.5 | 5 |
Sensitivity test
The percentage of PNH clones
[FLAER−/CD157− on CD64+
granulocytes (Grans)] was determined using the 8-color cocktail
listed in Table I. Three PNH samples
were spiked in 100 µl normal whole blood to obtain a final
concentration of PNH clones of 0.5–2%. They were then serial
diluted at 1:1 (v:v) using normal whole blood as diluent up to
0.005–0.01% expected PNH percentage (Table II). Unspiked normal whole blood was
used as a negative control. Following preparation of the serial
dilutions, the samples were stained with the 8-color cocktail
following the staining procedure described above. A total of
100,000 events in the monocyte and granulocyte gates were acquired
for each sample. CD15+ Grans
FLAER−/CD157− PNH clones were identified on
CD157/FLAER dot plots.
 | Table II.Target number of events for
paroxysmal nocturnal hemoglobinuria populations with high
sensitivity. |
Table II.
Target number of events for
paroxysmal nocturnal hemoglobinuria populations with high
sensitivity.
| No. of events
(FLAER−/CD157− on Grans) | Total events | Sensitivity
(%) |
|---|
| 5 | 100,000 | 0.005 |
| 10 | 100,000 | 0.010 |
| 20 | 100,000 | 0.050 |
Data acquisition
Data acquisition was performed with FCS Express 5
(De Novo Software, Glendale, CA, USA) and BD FACSDiva™ v.6.1.3
using gating strategy on dot plots, after which appropriate subsets
were identified.
Statistical analysis
To assess the correlation of CD15+ Grans
FLAER−/CD157− PNH clones between expected and
actual percentages, a linear regression analysis was performed. All
statistical calculations were performed with Excel 2016 (Microsoft
Corporation, Redmond, WA, USA) and Prism 7 (GraphPad Software,
Inc., La Jolla, CA, USA). R2>0.95 and P-value
<0.0001 were considered to indicate significant correlation.
Results
Normal blood exhibits positive
staining for CD55 using the 4-color PNH panel
In the present study, a whole blood sample from a
healthy donor was stained with a 4-color cocktail containing
FLAER-A488, PE-CD55, APC-CD14 and V500-C-CD45. From this, a minor
CD55− granulocyte population was observed (Fig. 1). However, this population was
FLAER+, suggesting that it was not representative of PNH
clones, which have been determined to be FLAER−
(26). Three other normal samples
exhibited the same FLAER−/CD55+ population of
granulocytes (data not shown).
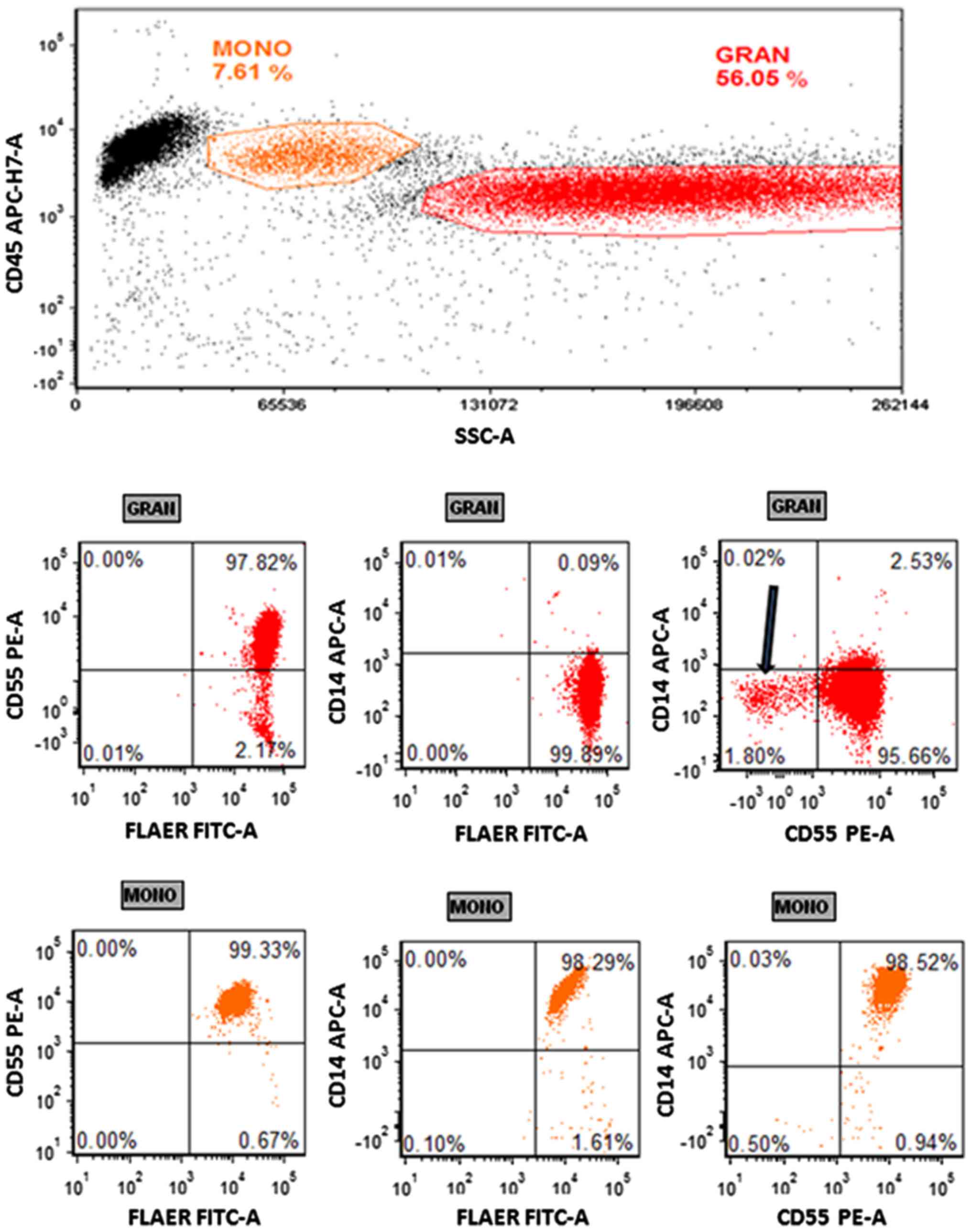 | Figure 1.Paroxysmal nocturnal hemoglobinuria
flow assay with a 4-color cocktail. Whole blood was stained with a
4-color cocktail (FLAER-A488/CD55-PE/CD14-APC/CD45-V500-C) and
lysed with FACSLyse. A total of 50,000 events were collected.
Monocytes and granulocytes were gated on a CD45/SSC plot. The top
panel exhibits the granulocytes on CD55/FLAER, CD14/FLAER and
CD14/CD55 plots. The bottom panel exhibits the same plots with
monocyte gating. The arrow indicates the CD55−
population with FLAER+ expression. Representative dot
plots are shown (n=3). MONO, monocyte; GRAN, granulocyte; CD,
cluster of differentiation; FLAER, fluorescein-labeled
proaerolysin; A488, Alexa Fluor 488; PE, phycoerythrin; APC,
allophycocyanin; FITC, fluorescein isothiocyanate; SSC, side
scatter; -A, area. |
Artifacts on granulocyte and monocyte
staining with fluorochrome-conjugated antibodies
To increase the sensitivity of multicolor flow
cytometry, the present study aimed to remove non-specific binding
and artifacts. A normal blood sample was stained with a multicolor
cocktail targeting T cell markers (CD3, CD4, CD8 and CD7). A number
of artifacts were identified in the granulocytes and monocytes
regions on CD3/SSC, CD4/SSC, CD8/SSC and CD7/SSC plots (Fig. 2, indicated in red). Other lymphocyte
markers including CD25 exhibited the same artifacts (data not
shown). Some of those artifacts were doublets and could be
eliminated by doublet discrimination (data not shown). However,
some artifacts remained following gating out of all doublets as
observed on the CD19+CD5/SSC plot in Fig.
3 (green arrow).
 | Figure 3.Gating strategy of 8-color paroxysmal
nocturnal hemoglobinuria flow assay. A PNH+ whole blood
sample was stained with the 8-color PNH cocktail. Doublets were
discriminated on a FSC-W/FSC-H plot. The monocytes and granulocytes
were gated together on a CD45/SSC plot. The
CD19−CD5− population was gated on a
CD5CD19/SSC plot; non-specific binding on granulocytes and
monocytes is indicated by the green arrow. The CD64+
Mono and CD15+ Grans were identified on CD64/SSC and
CD15/SSC plots, respectively. The PNH clone was identified on
CD157/FLAER, CD24/FLAER and CD14/FLAER plots as indicated in the
black circle. Mono, monocyte; Grans, granulocyte; CD, cluster of
differentiation; FLAER, fluorescein-labeled proaerolysin; PE,
phycoerythrin; Cy-, cyanine; APC, allophycocyanin; PerCP, peridinin
chlorophyll protein complex; FITC, fluorescein isothiocyanate; FSC,
forward scatter; SSC, side scatter; -W, width; -H, height; -A,
area. |
Granulocyte and monocyte PNH clones
were identified with the 8-color flow assay
To determine whether the 8-color PNH panel was able
to identify PNH clones, blood samples spiked with PNH clones were
stained with an 8-color PNH cocktail (Table I). The forward scatter width and
height (FSC-W and -H) were used to gate the singlets population.
Monocytes and granulocytes were gated together and non-specific
binding events were removed by gating the negative population on
the ‘dump’ channel (CD5 and CD19; Fig.
3). A number of artifacts were identified on the right side of
the granulocyte and monocyte region on the CD5+CD19/side scatter
(SSC) plot, and those events were eliminated by gating the negative
populations of monocytes and granulocytes, resulting in reduced
background (Fig. 3). The monocyte
antigen CD64 and granulocyte antigen CD15 further separated
monocytes and granulocytes, respectively. The PNH clones exhibited
negativity for FLAER and GPI-linked antigens (CD157, CD24 and CD14;
Fig. 3). The
CD5+CD19+ population was present together
with CD15+ Grans, CD64+ Mono and affected
FLAER−/CD157− quadrant as indicated in
Fig. 4 (top panel; green population).
There were FLAER−/CD157− differences that
ranged from 0.014 to 1.28% when excluding or including the
CD5+CD19+ populations (Fig. 4, lower panel). By contrast, normal
whole blood stained with the same 8-color PNH cocktail did not
exhibit any events on the lower left quadrant of FLAER/CD157,
FLAER/CD24 and FLAER/CD14 plots (Fig.
5, top panel). The samples of 10 normal donors were stained
with this 8-color PNH cocktail and exhibited an average of
0.002773±0.001743% PNH clones (FLAER−/CD157−)
in the CD15+ Gran population and 0.000764±0.002415% in
the CD64+ monocyte population, respectively (Fig. 5, lower panel). These results suggest
that the 8-color assay has high specificity.
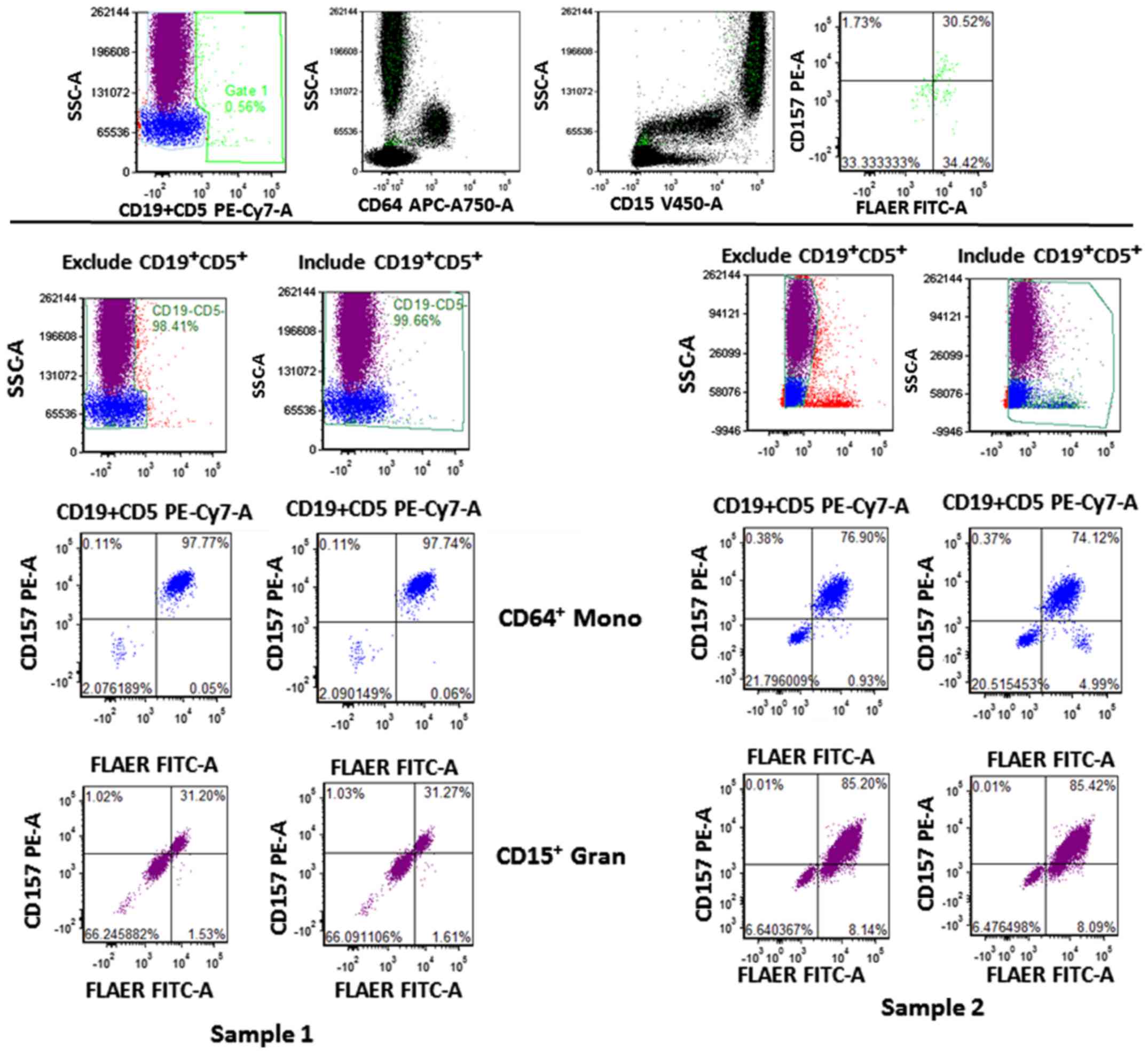 | Figure 4.Gating strategy of 8-color paroxysmal
nocturnal hemoglobinuria flow assay. The
CD19+CD5+ population was gated on gate 1 and
its distribution was shown on the CD15/SSC, CD64/SSC and
FLAER/CD157 dot plots, indicated in green (top panel). The
percentages of FLAER−/CD157− with exclusion
(left) and inclusion (right) of CD19+CD5+
cells in CD64+ Mono and CD15+ Grans from two
samples (lower panel). Mono, monocyte; Grans, granulocyte; CD,
cluster of differentiation; FLAER, fluorescein-labeled
proaerolysin; PE, phycoerythrin; Cy-, cyanine; APC,
allophycocyanin; FITC, fluorescein isothiocyanate; FSC, forward
scatter; SSC, side scatter; -A, area. |
 | Figure 5.Gating strategy of 8-color paroxysmal
nocturnal hemoglobinuria flow assay. A normal whole blood sample
was stained with the 8-color PNH cocktail as a control and PNH
clones (FLAER−/CD157−) were not observed
(upper panel). The representative dot plots from two normal donors
(lower left) are shown (n=10). The table showing the percentage of
PNH clones in CD15+ Grans and CD64+ Mono is
shown (lower right). Mono, monocyte; Grans, granulocyte; CD,
cluster of differentiation; FLAER, fluorescein-labeled
proaerolysin; PE, phycoerythrin; APC, allophycocyanin; PerCP,
peridinin chlorophyll protein complex; FITC, fluorescein
isothiocyanate; SSC, side scatter; -A, area. |
Sensitivity of the WBC 8-color PNH
flow assay
The percentage of PNH clones in three PNH samples
were determined using the 8-color panel. The PNH samples were then
spiked into normal whole blood to attain a final PNH concentration
of 0.5–2%, and serial dilutions were performed. The first tube of
sample 1 exhibited 2.2% PNH clones
(FLAER−/CD157−), and was subsequently diluted
in 50% increments until it reached 0.01% (Fig. 6). The normal whole blood control did
not exhibit a PNH population on the left lower quadrant of the
FLAER/CD157 plot (Fig. 6). The actual
number of FLAER−/CD157− events in sample 1
was 10/69,216 CD15+ Grans at the lower limit of
detection (LOD) of 0.014% (Fig. 6 and
Table III). The number of
FLAER−/CD157− events in samples 2 and 3 at
the corresponding LODs were 10/73,469 CD15+ Grans and
8/77,229 CD15+ Grans, respectively (Table III); samples 1–3 exhibited an LOD
for FLAER−/CD157−CD15+ Grans of
0.01% (Table III) and 0.05% for
CD64+ Mono (data not shown) on the 8-color flow assay.
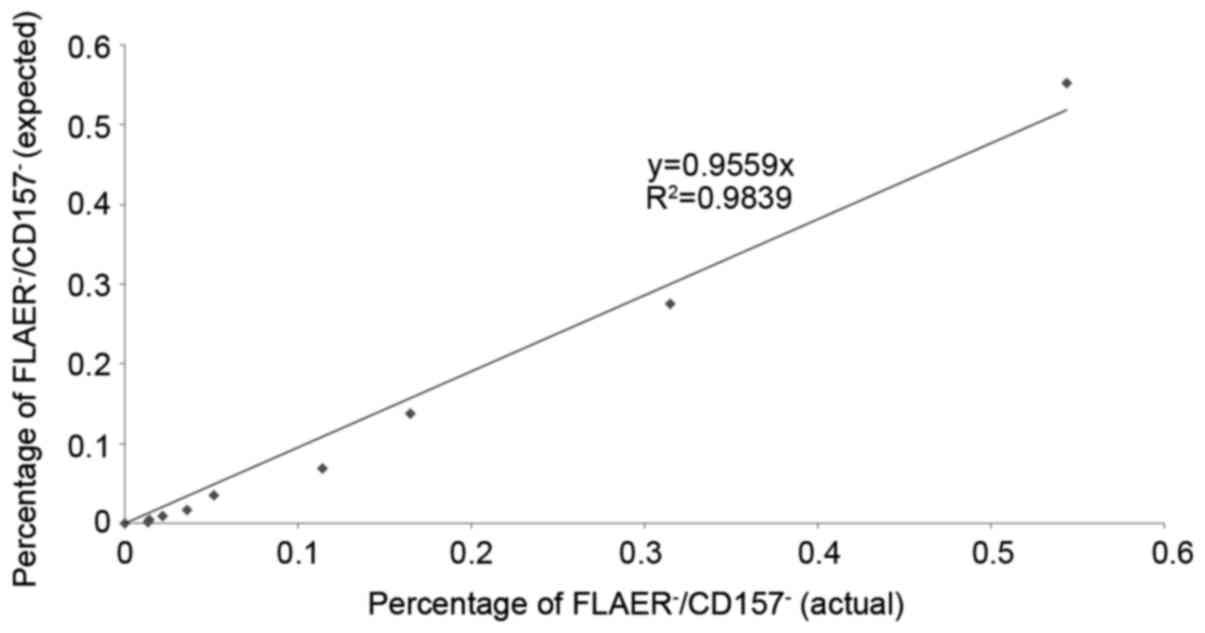
The expected PNH clone percentage was significantly correlated with
the actual value, R2 value of 0.9839 and p value less
than 0.0001 on a linear curve (Fig.
7), suggesting that the 8-color PNH flow assay had high
sensitivity, detecting a PNH clone population as low as 0.01% in
CD15+ Grans.
 | Table III.LOD of the 8-color PNH cocktail. |
Table III.
LOD of the 8-color PNH cocktail.
| Samples | Dilution | 1X | 0.5X | 0.25X | 0.125X | 0.06X | 0.03X | 0.015X | 0.008X | 0.004X | 0.002X | 0.001X | 0 |
|---|
| Sample 1 | No. of events
(FLAER−/CD157−) CD15+ Grans | 1,617 | 745 | 369 | 216 | 112 | 79 | 36 | 25 | 15 | 10 | 8 | 0 |
|
| Total
CD15+ Grans events | 73,188 | 69,461 | 67,875 | 68,534 | 67,772 | 69,034 | 69,834 | 69,065 | 68,350 | 69,216 | 60,254 | 61,597 |
|
| Percentage (%) | 2.20938 | 1.07254 | 0.54365 | 0.31517 | 0.16530 | 0.11444 | 0.05155 | 0.03620 | 0.02195 | 0.01445 | 0.01328 | 0 |
| Sample 2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| No. of events
(FLAER−/CD157−) CD15+ Grans | 1,190 | 524 | 263 | 153 | 89 | 49 | 28 | 16 | 10 | 9 | 0 | 0 |
|
| Total
CD15+ Grans events | 75,740 | 74,577 | 75,410 | 75,091 | 74,779 | 74,441 | 74,709 | 74,890 | 73,469 | 74,970 | 75,969 | 76,020 |
|
| Percentage (%) | 1.57117 | 0.70263 | 0.34876 | 0.20375 | 0.11902 | 0.06582 | 0.03748 | 0.02137 | 0.01361 | 0.01201 | 0 | 0 |
| Sample 3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| No. of events
(FLAER−/CD157−) CD15+ Grans | 344 | 201 | 84 | 49 | 20 | 8 | 5 | 5 | 2 | 4 | – | 0 |
|
| Total
CD15+ Grans events | 76,735 | 77,581 | 78,092 | 78,376 | 77,988 | 77,229 | 77,288 | 77,217 | 77,917 | 77,935 | – | 77,741 |
|
| Percentage (%) | 0.44830 | 0.25908 | 0.10757 | 0.06252 | 0.02565 | 0.01036 | 0.00647 | 0.00648 | 0.00257 | 0.00513 | – | 0 |
Accuracy of the WBC 8-color PNH flow
assay
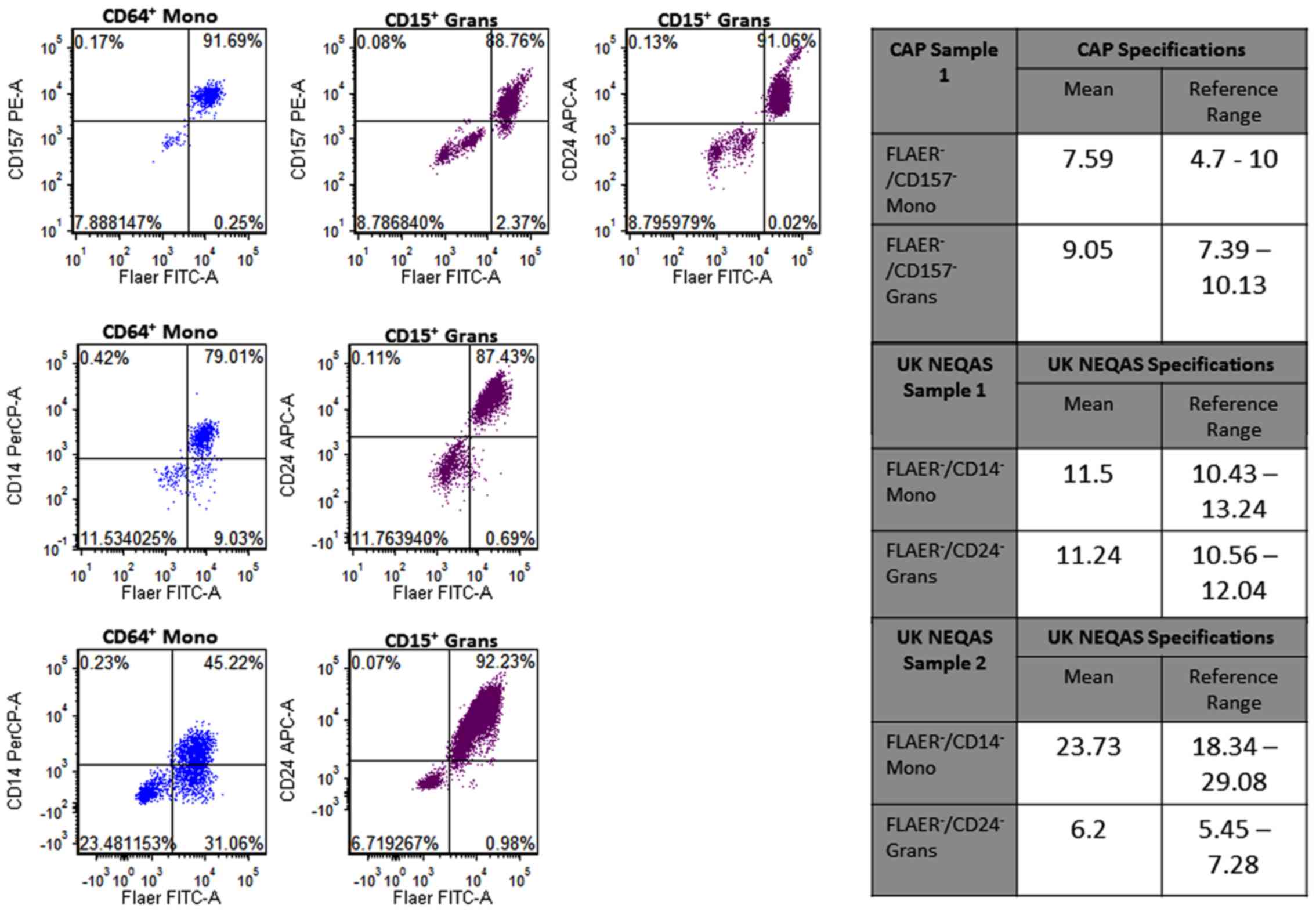
The percentages of PNH clones in the CAP and
UK-NEQAS samples were determined using the 8-color panel. The
percentage of FLAER−/CD157− Grans in the CAP
sample was 8.79%, which matched the CAP FLAER/CD157 granulocytes
specification (4.70–10.00%; Fig. 8).
The percentage of FLAER−/CD157− Mono in the
CAP sample was 7.89%, which matched the CAP
FLAER−/CD157− monocytes specification
(7.39–10.13%; Fig. 8). Furthermore,
the percentage of two UK-NEQAS samples identified by the 8-color
assay matched the UK-NEQAS specifications (Fig. 8), suggesting that the 8-color flow
assay had high accuracy for PNH clone identification.
Discussion
Flow cytometry has become a standard tool for the
analysis of cellular phenotype and function within the immune
system, among other applications. As more fluorochromes have been
developed with advances in cytometer design and lasers, the
identification of more rare cell populations has become achievable,
and the sensitivity of existing assays has increased (1,2). For PNH,
as a rare disease, a high sensitivity assay with short acquisition
time is required.
In the present study, it was demonstrated that the
4-color combination (FLAER, CD55, CD14 and CD45) was not accurate
in identifying PNH populations in whole blood samples. Earlier
methods of PNH detection have relied on the loss of CD55 and CD59
on RBCs and granulocytes (17,18,20).
However, the present study identified that CD55 negative
populations were not representative of PNH clones, suggesting that
a novel assay is required to aid more accurate clinical diagnosis
of PNH. Additionally, CD55 is not an adequate marker for PNH as it
cannot distinguish PNH type II (partially deficient GPI-anchored
proteins) from type III (lacking expression of GPI-anchored
proteins) RBCs (20).
The improved methods in flow cytometry may enable
the measurement of increased markers at a single cell level in less
time (2,29,30). In
the present study, an 8-color configuration was designed for PNH
identification. With increased photomultiplier tubes in the assay,
it allowed the inclusion of other antigens that could increase
sensitivity of the flow assay. However, artifacts were observed on
monocyte and granulocyte surface staining even when using
lymphocytes markers such as CD3 and CD7. These artifacts were
composed of doublets and non-specific staining signals, which were
present on the positive side of each channel. To generate a high
sensitivity flow assay for PNH detection, the aim was to eliminate
all potential non-specific binding events, as only 10 events in a
100,000 total population (granulocytes and monocytes) was required
to achieve 0.01% sensitivity. A minority of false positive events
would markedly decrease the sensitivity. The
CD5+CD19+ populations were combined with
CD15+ Grans, CD64+ monocytes and were even
identified on the lower left quadrant of the CD157/FLAER plot used
for PNH clone identification. The percentage differences in
FLAER−/CD157− clones by excluding and
including CD5+CD19+ populations were
0.014–1.28%, which are significant numbers when considering a 0.01%
PNH clone population was being targeted. However, it was
demonstrated using doublet discrimination (FSC-W/FSC-H) and a
‘dump’ channel (CD5 and CD19) to gate the negative population that
the background staining was lowered markedly to detect 0.002773%
(CD15+ Grans) and 0.000764% (CD64+ Mono) PNH
populations (FLAER−/CD157−) in whole blood
from 10 normal donors. Sutherland et al (31) described a 4-color combination of
FLAER, CD14, CD64 and CD45 for high-resolution detection of PNH
monocytes with a background rate of 0.0033%. Our current 8-color
combination further improved the background rate for monocytes and
granulocytes to 0.00076% and 0.00277%, respectively, with only
100,000 events of monocyte and granulocyte acquisition.
Furthermore, the gating strategy using granulocyte antigen (CD15)
and monocyte antigen (CD64) served a key role in separating the
monocyte and granulocyte populations prior to assessment of the
GPI-linked antigens. As a result, the 8-color panel improved
sensitivity and specificity with a shorter acquisition time in PNH
identification.
FLAER-A488 is a fluorescent aerolysin that directly
binds GPI-linked anchors and has been suggested as an optimum
reagent for PNH assays (20). In the
current study, three additional GPI-linked antigens were included
to improve specificity. CD157 is a GPI-linked protein expressed on
both granulocytes and monocytes (32); CD14 is a GPI-protein expressed on
monocytes (27); and CD24 is a
GPI-protein expressed on granulocytes (28). The combination of FLAER, CD157, CD24
and CD14 provided a comprehensive panel for targeting of all major
GPI-linked antigens on PNH samples.
Furthermore, PNH samples provided by CAP and UK
NEQAS were also tested by the 8-color PNH flow assay. The
percentage of PNH clones identified by the 8-color assay matched
the specifications provided by CAP and UK NEQAS, suggesting that
the assay is able to identify PNH populations with sufficient
accuracy.
In conclusion, flow cytometry is an important tool
for leukemia/lymphoma diagnosis (33–36).
Abnormal cells may exhibit aberrant expression of certain markers
and downregulate other markers, in comparison to normal samples
(37). It is important that a
pre-mixed reagent generates an accurate expression profile of the
sample using a combination of multicolor cocktails, to enable
pathologists to correctly identify different types of
leukemia/lymphoma and also PNH in patient samples (26,38–42).
However, the current strategies employing CD55 and CD59-based
assays in certain laboratories do not have adequate sensitivity to
detect PNH population sizes of less than 1%, and thus the 30–40%
PNH samples that typically exhibit a lower than 1% PNH clone
population go undetected (21,43). A
high sensitivity flow assay is required to identify those
populations, to allow early and suitable treatments to be provided
for patients. The present results demonstrate that the 8-color PNH
antibody cocktail is sensitive in distinguishing small PNH
populations from normal samples with low background and short
acquisition time. It may provide an effective tool for pathologists
in the diagnosis of PNH, including in those cases with small
percentages of PNH clones.
Acknowledgements
The authors are thankful to CAP and UK NEQAS for
providing PNH samples for the study. All authors declare no
conflicts of interest regarding the study described in this
manuscript.
References
|
1
|
Chattopadhyay PK, Perfetto SP and Roederer
M: The colorful future of cell analysis by flow cytometry. Discov
Med. 4:255–262. 2004.PubMed/NCBI
|
|
2
|
Chan RC, Kotner JS, Chuang CM and Gaur A:
Stabilization of pre-optimized multicolor antibody cocktails for
flow cytometry applications. Cytometry B Clin Cytom. 92:508–524.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hill A, Platts PJ, Smith A, Richards SJ,
Cullen MJ, Hill QA, Roman E and Hillmen P: The incidence and
prevalence of paroxysmal nocturnal hemoglobinuria (PNH) and
survival of patients in Yorkshire. Blood. 108:9852006.
|
|
4
|
Parker C, Omine M, Richards S, Nishimura
J, Bessler M, Ware R, Hillmen P, Luzzatto L, Young N, Kinoshita T,
et al International PNH Interest Group, : Diagnosis and management
of paroxysmal nocturnal hemoglobinuria. Blood. 106:3699–3709. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hosokawa K, Kajigaya S, Keyvanfar K, Qiao
W, Xie Y, Townsley DM, Feng X and Young NS: T Cell transcriptomes
from paroxysmal nocturnal hemoglobinuria patients reveal novel
signaling pathways. J Immunol. 199:477–488. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hill A, Ridley SH, Esser D, Oldroyd RG,
Cullen MJ, Kareclas P, Gallagher S, Smith GP, Richards SJ, White J,
et al: Protection of erythrocytes from human complement-mediated
lysis by membrane-targeted recombinant soluble CD59: A new approach
to PNH therapy. Blood. 107:2131–2137. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Al-Ani F, Chin-Yee I and Lazo-Langner A:
Eculizumab in the management of paroxysmal nocturnal
hemoglobinuria: Patient selection and special considerations. Ther
Clin Risk Manag. 12:1161–1170. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fletcher M, Whitby L, Whitby A and Barnett
D: Current international flow cytometric practices for the
detection and monitoring of paroxysmal nocturnal haemoglobinuria
clones: A UK NEQAS survey. Cytometry B Clin Cytom. 92:266–274.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hill A, DeZern AE, Kinoshita T and Brodsky
RA: Paroxysmal nocturnal haemoglobinuria. Nat Rev Dis Primers.
3:170282017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brodsky RA: Paroxysmal nocturnal
hemoglobinuria. Blood. 124:2804–2811. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nafa K, Bessler M, Castro-Malaspina H,
Jhanwar S and Luzzatto L: The spectrum of somatic mutations in the
PIG-A gene in paroxysmal nocturnal hemoglobinuria includes large
deletions and small duplications. Blood Cells Mol Dis. 24:370–384.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Canalejo K, Riera Cervantes N, Felippo M,
Sarandría C and Aixalá M: Paroxysmal nocturnal haemoglobinuria.
Experience over a 10 years period. Int J Lab Hematol. 36:213–221.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yamashina M, Ueda E, Kinoshita T, Takami
T, Ojima A, Ono H, Tanaka H, Kondo N, Orii T, Okada N, et al:
Inherited complete deficiency of 20-kilodalton homologous
restriction factor (CD59) as a cause of paroxysmal nocturnal
hemoglobinuria. N Engl J Med. 323:1184–1189. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Motoyama N, Okada N, Yamashina M and Okada
H: Paroxysmal nocturnal hemoglobinuria due to hereditary nucleotide
deletion in the HRF20 (CD59) gene. Eur J Immunol. 22:2669–2673.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rother RP, Bell L, Hillmen P and Gladwin
MT: The clinical sequelae of intravascular hemolysis and
extracellular plasma hemoglobin: A novel mechanism of human
disease. JAMA. 293:1653–1662. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hill A, Richards SJ and Hillmen P: Recent
developments in the understanding and management of paroxysmal
nocturnal haemoglobinuria. Br J Haematol. 137:181–192. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
van der Schoot CE, Huizinga TW, van't
Veer-Korthof ET, Wijmans R, Pinkster J and von dem Borne AE:
Deficiency of glycosyl-phosphatidylinositol-linked membrane
glycoproteins of leukocytes in paroxysmal nocturnal hemoglobinuria,
description of a new diagnostic cytofluorometric assay. Blood.
76:1853–1859. 1990.PubMed/NCBI
|
|
18
|
Hall SE and Rosse WF: The use of
monoclonal antibodies and flow cytometry in the diagnosis of
paroxysmal nocturnal hemoglobinuria. Blood. 87:5332–5340.
1996.PubMed/NCBI
|
|
19
|
Brodsky RA, Mukhina GL, Li S, Nelson KL,
Chiurazzi PL, Buckley JT and Borowitz MJ: Improved detection and
characterization of paroxysmal nocturnal hemoglobinuria using
fluorescent aerolysin. Am J Clin Pathol. 114:459–466. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Borowitz MJ, Craig FE, Digiuseppe JA,
Illingworth AJ, Rosse W, Sutherland DR, Wittwer CT and Richards SJ;
Clinical Cytometry Society, : Guidelines for the diagnosis and
monitoring of paroxysmal nocturnal hemoglobinuria and related
disorders by flow cytometry. Cytometry B Clin Cytom. 78:211–230.
2010.PubMed/NCBI
|
|
21
|
Movalia M and Illingworth A: Distribution
of PNH clone sizes within high risk diagnostic categories among 481
PNH positive patients identified by high sensitivity flow
cytometry. Blood. 120:12712012.
|
|
22
|
Hillmen P, Muus P, Röth A, Elebute MO,
Risitano AM, Schrezenmeier H, Szer J, Browne P, Maciejewski JP,
Schubert J, et al: Long-term safety and efficacy of sustained
eculizumab treatment in patients with paroxysmal nocturnal
haemoglobinuria. Br J Haematol. 162:62–73. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sugimori C, Chuhjo T, Feng X, Yamazaki H,
Takami A, Teramura M, Mizoguchi H, Omine M and Nakao S: Minor
population of CD55CD59 blood cells predicts response to
immunosuppressive therapy and prognosis in patients with aplastic
anemia. Blood. 107:1308–1314. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hillmen P, Lewis SM, Bessler M, Luzzatto L
and Dacie JV: Natural history of paroxysmal nocturnal
hemoglobinuria. N Engl J Med. 333:1253–1258. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kelly RJ, Hill A, Arnold LM, Brooksbank
GL, Richards SJ, Cullen M, Mitchell LD, Cohen DR, Gregory WM and
Hillmen P: Long-term treatment with eculizumab in paroxysmal
nocturnal hemoglobinuria: Sustained efficacy and improved survival.
Blood. 117:6786–6792. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sutherland DR, Acton E, Keeney M, Davis BH
and Illingworth A: Use of CD157 in FLAER-based assays for
high-sensitivity PNH granulocyte and PNH monocyte detection.
Cytometry B Clin Cytom. 86:44–55. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gatti A, Del Vecchio L, Geuna M, Della
Porta MG and Brando B: Multicenter validation of a simplified
method for paroxysmal nocturnal hemoglobinuria screening. Eur J
Haematol. 99:27–35. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Marinov I, Illingworth AJ, Benko M and
Sutherland DR: Performance characteristics of a non-fluorescent
aerolysin-based paroxysmal nocturnal hemoglobinuria (PNH) assay for
simultaneous evaluation of PNH neutrophils and PNH monocytes by
flow cytometry, following published PNH guidelines. Cytometry B
Clin Cytom. Jun 13–2016.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kusenda J, Fajtova M and Kovarikova A:
Monitoring of minimal residual disease in acute leukemia by
multiparametric flow cytometry. Neoplasma. 61:119–127. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Edwards BS, Oprea T, Prossnitz ER and
Sklar LA: Flow cytometry for high-throughput, high-content
screening. Curr Opin Chem Biol. 8:392–398. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sutherland DR, Keeney M and Illingworth A:
Practical guidelines for the high-sensitivity detection and
monitoring of paroxysmal nocturnal hemoglobinuria clones by flow
cytometry. Cytometry B Clin Cytom. 82:195–208. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hernández-Campo PM, Almeida J, Sánchez ML,
Malvezzi M and Orfao A: Normal patterns of expression of
glycosylphosphatidylinositol-anchored proteins on different subsets
of peripheral blood cells: A frame of reference for the diagnosis
of paroxysmal nocturnal hemoglobinuria. Cytometry B Clin Cytom.
70:71–81. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Seifert RP, Bulkeley W III, Zhang L, Menes
M and Bui MM: A practical approach to diagnose soft tissue myeloid
sarcoma preceding or coinciding with acute myeloid leukemia. Ann
Diagn Pathol. 18:253–260. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mayson E, Saverimuttu J and Cartwright K:
CD5-positive follicular lymphoma: Prognostic significance of this
aberrant marker? Intern Med J. 44:417–422. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pati HP and Jain S: Flow cytometry in
hematological disorders. Indian J Pediatr. 80:772–778. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Krause DS, Delelys ME and Preffer FI: Flow
cytometry for hematopoietic cells. Methods Mol Biol. 1109:23–46.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Huh YO and Ibrahim S: Immunophenotypes in
adult acute lymphocytic leukemia. Role of flow cytometry in
diagnosis and monitoring of disease. Hematol Oncol Clin North Am.
14:1251–1265. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chung NG, Buxhofer-Ausch V and Radich JP:
The detection and significance of minimal residual disease in acute
and chronic leukemia. Tissue Antigens. 68:371–385. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rawstron AC: Monoclonal B-cell
lymphocytosis. Hematology Am Soc Hematol Educ Program. 430–439.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Del Principe MI, Maurillo L, Buccisano F,
Sconocchia G, Cefalo M, De Santis G, Di Veroli A, Ditto C, Nasso D,
Postorino M, et al: Central nervous system involvement in adult
acute lymphoblastic leukemia: Diagnostic tools, prophylaxis, and
therapy. Mediterr J Hematol Infect Dis. 6:e20140752014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hayashi D, Lee JC, Devenney-Cakir B, Zaim
S, Ounadjela S, Solal-Céligny P, Juweid M and Guermazi A:
Follicular non-Hodgkin's lymphoma. Clin Radiol. 65:408–420. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Narita A, Muramatsu H, Okuno Y, Sekiya Y,
Suzuki K, Hamada M, Kataoka S, Ichikawa D, Taniguchi R, Murakami N,
et al: Development of clinical paroxysmal nocturnal haemoglobinuria
in children with aplastic anaemia. Br J Haematol. 178:954–958.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Timeus F, Crescenzio N, Longoni D, Doria
A, Foglia L, Pagliano S, Vallero S, Decimi V, Svahn J, Palumbo G,
et al: Paroxysmal nocturnal hemoglobinuria clones in children with
acquired aplastic anemia: A multicentre study. PLoS One.
9:e1019482014. View Article : Google Scholar : PubMed/NCBI
|