Introduction
Periodontitis is a common disease, with 5–30%
prevalence in the adult population (1). Periodontitis is an inflammatory process
of the periodontal tissues caused by bacterial infection, which
results in the destruction of periodontal connective tissue and the
reabsorption of the alveolar bone (2). Hylotelephium purpureum (also
known as Sedum purpureum) grows in the Far East, Japan,
Europe, North America and Northeast China (3). The grass is a herbal cure in traditional
Chinese medicine due to its anti-inflammatory, analgesic,
antispasmodic, antipyretic, antimicrobial, and antioxidant
properties (4,5). However, its efficacy in the treatment of
periodontal diseases has not yet been elucidated.
Based on previously reported favorable aspects of
Hylotelephium purpureum, we hypothesized that it would be a
beneficial antioxidant agent for the suppression of periodontal
inflammation and alveolar-bone destruction in periodontal disease.
Therefore, the present study aims to investigate the
anti-inflammatory activity and antinociceptive effects of a gel
form of the plant, and to assess the duration of activity and
efficacy of a Hylotelephium purpureum gel (HPG) in the
treatment of experimental periodontitis in a Chinese Kun Ming (KM)
mouse model.
Materials and methods
Preparation of HPG
A total of 10 g of Hylotelephium purpureum
whole grass extract was soaked in 500 ml of purified water,
neutralized to a pH of 8–9, and then dissolved. Following this, 160
g of poloxamer 407 was added to the filtrated drug solution.
Distilled water was then added to bring the quantity of the
solution to 1,000 ml. The gel was stored at ambient temperature.
The HPG formulation was prepared by the Academy of Traditional
Chinese Medicine (Changchun, China).
Animals
KM mice (nulliparous, 8–12 weeks) and Wistar rats
(5–10 weeks) of both sexes were obtained from the Changchun
Institute of Biological Products Co., Ltd. (Changchun, China). The
experimental procedures of the present study were approved by the
Animal Ethics Committee. The animals were housed in a
well-ventilated animal house with a 12 h light and dark schedule
and easy access to water and a standard pellet diet. Animals were
randomly selected, marked to permit individual identification, and
kept in their cages for at least 5–7 days prior to dosing to allow
for acclimatization to the laboratory conditions.
Acute toxicity experiment
Forty KM mice were randomly divided into two groups
of 20 mice/group (10 males and 10 females) and were used for the
acute oral toxicity study. Drinking water and food were provided
throughout the experiment, except for a short fasting period
wherein drinking water was still provided ad libitum, but no
food was provided for 16 h prior to treatment. A single high dose
of 40 ml/kg of 1.0% HPG was intragastrically administered to
mice in the treatment group. Meanwhile, the second group of mice
were allotted distilled water and were regarded as the control
group. All of the animals were weighed and visually observed for
mortality, behavioral patterns, changes in physical appearance,
injury, pain, or other signs of illness daily for 14 days (6). At the end of the acute toxicity study,
all mice were sacrificed. Vital organs such as the heart, kidneys,
liver, lung and spleen were isolated and examined for any lesions.
All of the individual organs were weighed, and their features were
compared between both the treated and control groups.
Determination of maximum
tolerance
Forty KM mice of both sexes were randomly divided
into two groups: An HPG-treated group and a control group. Mice in
the HPG-treated group were intragastrically administered HPG at a
maximal dose of 40 ml/kg, twice a day at a 4 h interval (total of
80 ml/kg per day), while the control group received an equal volume
of deionized water for 14 days. Following administration of HPG or
water, the responses of the mice, including toxic reactions and
mortality, were observed and recorded each successive day for a
2-week period. At the end of the experiment, animals were
sacrificed for gross-anatomy checks. Evaluations and recordings
were conducted to determine whether there were any obvious changes
in major organs under macroscopic observation.
Experimental periodontitis
A total of 50 Wistar albino rats (5–10 weeks old)
weighing 150–250 g were used in the present study. The animals were
anesthetized with ketamine. Preperiodontal examinations were
conducted, and the upper second molars were ligated using a 4-0
sterile braided-silk suture (Hangzhou Westlake Biological Materials
Co., Ltd), which was pretreated with Porphyromonas
gingivalis. Soft tissue indicators were measured. Four weeks
later, two rats were taken at random and sacrificed. Histological
examinations of the maxillary molars and their periodontal tissues
confirmed the model was established successfully (7).
Evaluation of the therapeutic effects
of experimental periodontitis
The remaining rats were divided into 6 groups, each
containing 8 animals: A non-ligated group; a ligature only group; a
ligature plus treatment with standard group (Su Xiao Ya Tong Ning
Ding, which is a proprietary Chinese medicine for the treatment of
stomatal toothache, dental caries and chronic pulpitis); and three
ligature plus treatment with HPG groups (1.0, 0.5 and 0.25%, twice
a day for 2 weeks). All rats were anesthetized following 2 weeks.
We recorded the observed gingival index (GI) and gingival
sulcus-bleeding index in each animal, as previously described
(8,9).
Additionally, serum superoxide dismutase (SOD), glutathione
peroxidase (GPx) and malondialdehyde (MDA) levels were measured
(10,11). Alveolar bone loss in the first molars
was determined histologically. Periodontal tissues were
histopathologically examined to assess any differences among the
study groups.
The following classifications were used to score
periodontal tissue inflammation: 0, no inflammation; 1, periodontal
membrane vascular hyperemia, bleeding, mild inflammatory cell
infiltration, osteoclasts occasionally visible or not present; 2,
periodontal ligament vascular obviously dilated and congested,
bleeding, inflammatory cells with moderate infiltration, membrane
of increased width or partial disappearance, visible as a few
osteoclasts, and mild alveolar bone absorption; and 3, weak tooth
film, inflammatory cells and severe infiltration, abscess
formation, broken bone cell infiltration, cementum and an alveolar
bone with obvious absorption.
Anti-inflammatory activity
Xylene-induced mouse-ear edema
test
A total of 50 KM mice were randomly divided into
five groups of 10 each, as follows: Blank control group (equal
volume of saline); a positive control (treatment with standard Su
Xiao Ya Tong Ning Ding); and three groups receiving treatment with
HPG at various concentrations (1.0, 0.5 and 0.25%). Mice in each
group were intragastrically administered at the design dose
(capacity of 0.2 ml/10 g) for 7 consecutive days. After the last
administration, 0.05 ml of xylene was evenly applied on the right
ear of each mouse, and the left ear served as the control. After 45
min, the mice were euthanized and the left and right ears were
removed, round ear samples in the corresponding parts were removed
with a 4 mm radius punch, and the ears were weighed on an
electronic balance. The degree of edema was recorded as the weight
of the right ear sample subtracted from the weight of left
earpiece. The degree of edema among the various groups was
compared, and the edema inhibition rate was calculated: Edema
inhibition rate = (degree of edema of blank control group - degree
of edema of treatment group)/degree of edema of blank control group
× 100%.
Effects on acetic acid-induced mouse
peritoneal capillary permeability
A total of 50 KM mice were randomly divided into
five groups of 10 each: A blank control group (equal volume of
saline); a positive control group (treatment with standard Su Xiao
Ya Tong Ning Ding); and three treatment with HPG groups (1.0, 0.5
and 0.25%). Treatment groups were intragastrically administered
(0.2 ml/10 g) for 7 consecutive days. The blank control group was
given an equivalent volume of distilled water. After the last
administration, the mice were given a tail-vein injection of 0.1
ml/10 g of 0.5% Evans Blue solution in saline, as well as a 0.2 ml
intraperitoneal injection of 0.6% acetic acid per mouse. Thirty
minutes later, the animals were sacrificed, and the abdominal skin
and muscle were removed. The abdominal cavity was washed with 6 ml
of a 0.9% NaCl solution; the washing liquid was pipetted out and
combined. Then, a 0.9% NaCl solution was added to bring the volume
to 10 ml, followed by centrifugation at 500 × g. The supernatant
was collected, and the absorbance was measured at 590 nm.
Differences among groups were compared.
Effect on carrageenan-induced hind-paw
edema
The KM mice were randomly divided into five groups,
as described previously. Local treatment was conducted with saline,
standard drug (Su Xiao Ya Tong Ning Ding®) and HPG (1.0,
0.5 and 0.25%) twice a day for 5 days. At 1 h following the last
administration, the mice were administered a subcutaneous injection
of 0.1 ml of a 1% solution of carrageenan into the plantar side of
the left-hind paw (12). Local
treatment was conducted again on the injection site. The thickness
of the dorsoventral diameter in each animal was measured using a
pair of dial-thickness gauge calipers at 1, 2 and 4 h following the
induction of inflammation.
Antinociceptive analysis
Acetic-acid induced writhing
response
KM mice were locally treated with their respective
treatments as previously described in material and method twice a
day for 5 days. After 1 h treatment, 0.7% acetic acid (0.1 ml/10 g
body weight) was administered intraperitoneally to each mouse. The
mice were observed, and the number of abdominal constrictions and
stretching over a period of 5–15 min were counted.
Hot-plate test
HPG (1.0, 0.5 and 0.25%) was administered dermally
for 5 days. Following the last administration, mice were
individually placed on a heated plate at 55±1°C. The latency time
of forepaw licking or jumping was determined at 60, 120 and 240 min
following treatment.
Determination of minimum inhibitory
concentration (MIC)
The MICs of HPG, tinidazole and Su Xiao Ya Tong Ning
Ding against five bacterial strains were determined by the
test-tube continuous dilution method. HPG was serially diluted at
concentrations of 5.0, 2.5, 1.25, 0.62, 0.31, 0.15 and 0.08 mg/ml.
The concentrations of tinidazole in the medium was 128, 64, 32, 16,
8, 4, 2 and 1 µg/ml. The concentrations of Su Xiao Ya Tong Ning
Ding were 30, 15, 7.5, 3.7, 1.85 and 0.92%. A total of 0.05 ml of a
bacterial solution was added to each group and cultured at 37°C for
18 h, and a blank control was used. The concentration of drug in
the last clear test tube was taken as the minimum inhibitory
concentration.
Statistical analysis
The SPSS 17.0 software (SPSS, Inc., Chicago, IL,
USA) was used to analyse and process the data. All data are
represented as the means ± SD of three independent experiments.
Statistical significance was tested by Student's t-test and one-way
analysis of variance. P<0.05 was considered to represent a
statistically significant difference.
Results
HPG analysis
HPG containing a flavonoid extract was formulated as
a local delivery drug. The pH was within the acceptable range of
7.0–8.0, even at the end of 30 days. The product contained a total
amount of quercetin (C15H10O7) and
rhizoma kaempferiae (C15H10O6) was
>5.0 mg/ml, as evaluated by chromatograph (data not shown).
Acute toxicity and maximum
tolerance
In the acute toxicity test, administration of HPG
(40 ml/kg) to mice did not cause death or acute behavioral changes
during the observation periods, and we did not notice any
pathological changes in the mice. The LD50 was estimated
to be >40 ml/kg. For maximum tolerance, a further test of HPG
did not demonstrate any behavioral changes or mortality in mice at
doses of 80 ml/kg during the 14 days of the experiment. HPG was
safe at the given dose in mice.
The therapeutic effects of HPG in
experimental periodontitis
GI and sulcus bleeding index
(SBI)
The mean GI prior to treatment in the control, Su
Xiao Ya Tong Ning Ding, and 1.0, 0.5 and 0.25% HPG groups were
2.75±0.46, 2.63±0.52, 2.50±0.76, 2.50±0.53 and 2.50±0.53,
respectively. The mean GIs after 14 days in each group were
3.00±0.00, 1.13±0.64, 0.75±0.71, 1.13±0.64 and 1.50±0.53,
respectively. The mean percentage changes were −9.09, 57.03, 70.00,
54.80 and 40.00%, respectively. There was a statistically
significant change in the GI at the end of 14 days (P=0.001;
Table I). As shown in Table I, the mean sulcus bleeding index prior
to treatment in the control, Su Xiao Ya Tong Ning Ding, and 1.0,
0.5 and 0.25% HPG groups were 3.25±1.04, 3.50±1.41, 3.50±1.20,
3.38±1.19 and 3.13±1.13, respectively. The mean SBIs at the end of
14 days in each group were 3.38±1.19, 2.00±1.07, 1.50±1.20,
2.13±1.13 and 2.13±0.83, and the mean percentage changes were
−4.00, 42.86, 57.14, 36.98 and 31.95%, respectively. There was a
statistically significant difference in the SBI at the end of 14
days (P=0.01).
 | Table I.Comparisons of the gingival and sulcus
bleeding indexes in response to treatment. |
Table I.
Comparisons of the gingival and sulcus
bleeding indexes in response to treatment.
|
| GI | SBI |
|---|
|
|
|
|
|---|
| Group | Before | After | Before | After |
|---|
| Non-ligated | 0 | 0 | 0 | 0 |
| Ligature alone | 2.75±0.46 | 3.00±0.00 | 3.25±1.04 | 3.38±1.19 |
| Ligature + Su Xiao Ya
Tong Ning Ding | 2.63±0.52 |
1.13±0.64c | 3.50±1.41 |
2.00±1.07a |
| Ligature + 1.0%
HPG | 2.50±0.76 |
0.75±0.71c | 3.50±1.20 |
1.50±1.20b |
| Ligature + 0.5%
HPG | 2.50±0.53 |
1.13±0.64c | 3.38±1.19 |
2.13±1.13a |
| Ligature + 0.25%
HPG | 2.50±0.53 |
1.50±0.53b | 3.13±1.13 | 2.13±0.83 |
Serum levels of SOD, GPx and MAD
The serum levels of SOD in the non-ligated, ligature
alone, Su Xiao Ya Tong Ning Ding, and 1.0, 0.5 and 0.25% HPG groups
were 103.9±3.948, 91.10±4.102, 99.84±6.377, 99.11±5.112,
93.77±5.626 and 92.174±5.2005, respectively. The GPx levels in the
different groups were 196.2±6.735, 157.27±31.48, 190.34±5.101,
188.48±5.834, 187.55±15.81 and 182.70±19.69. The MDA levels were
3.741±0.691, 8.928±1.003, 6.231±1.099, 6.570±1.015, 7.133±0.778 and
7.949±1.495. As shown in Table II,
the levels of SOD and GPx in the Su Xiao Ya Tong Ning Ding group
and 1% HPG group were significantly heightened (P<0.01,
P<0.05). However, the levels of MDA in these groups were
decreased (P<0.001, P<0.01). There were no statistically
significant differences in the serum levels of SOD, GPx and MDA in
the 0.25% HPG group compared with the ligature alone group.
 | Table II.Comparisons of the serum levels of
SOD, GPx and MDA in different treatment groups. |
Table II.
Comparisons of the serum levels of
SOD, GPx and MDA in different treatment groups.
| Treatment group | SOD (U/ml) | GPx (U/ml) | MDA (nmol/l) |
|---|
| Non-ligated |
103.9±3.948 |
196.2±6.735 |
3.741±0.691 |
| Ligature alone |
91.10±4.102 |
157.27±31.48 |
8.928±1.003 |
| Ligature + Su Xiao Ya
Tong Ning Ding |
99.84±6.377b |
190.34±5.101a |
6.231±1.099c |
| Ligature + 1.0%
HPG |
99.11±5.112b |
188.48±5.834a |
6.570±1.015b |
| Ligature + 0.5%
HPG |
93.77±5.626 |
187.55±15.81a |
7.133±0.778b |
| Ligature + 0.25%
HPG |
92.174±5.2005 |
182.70±19.69 |
7.949±1.495 |
Exterior behavioral observations and
histopathological examinations
The animals in the non-ligated group increased
gradually in weight, with normal diet and activity. Rats in the
ligature-only group gradually showed loss of appetite and weight.
While after a 14-day administration, the rats were gradually
restored to activity and appetite in the administration groups, and
the weight of these rats also increased. In the Su Xiao Ya Tong
Ning Ding group, periodontal tissue was found to have
inflammatory-cell infiltration, but the rate was significantly
alleviated compared with the ligature-only group (Fig. 1). The rats in the high-dose HPG group
showed significant reductions in gingival inflammation and pocket
depth (Fig. 1D-F). The periodontal
bone loss difference was not statistically significant. These
results indicate that HPG of 1.0 and 0.5% and Su Xiao Ya Tong Ning
Ding can significantly reduce the degree of injury to periodontal
tissue in an experimental mouse model of periodontitis.
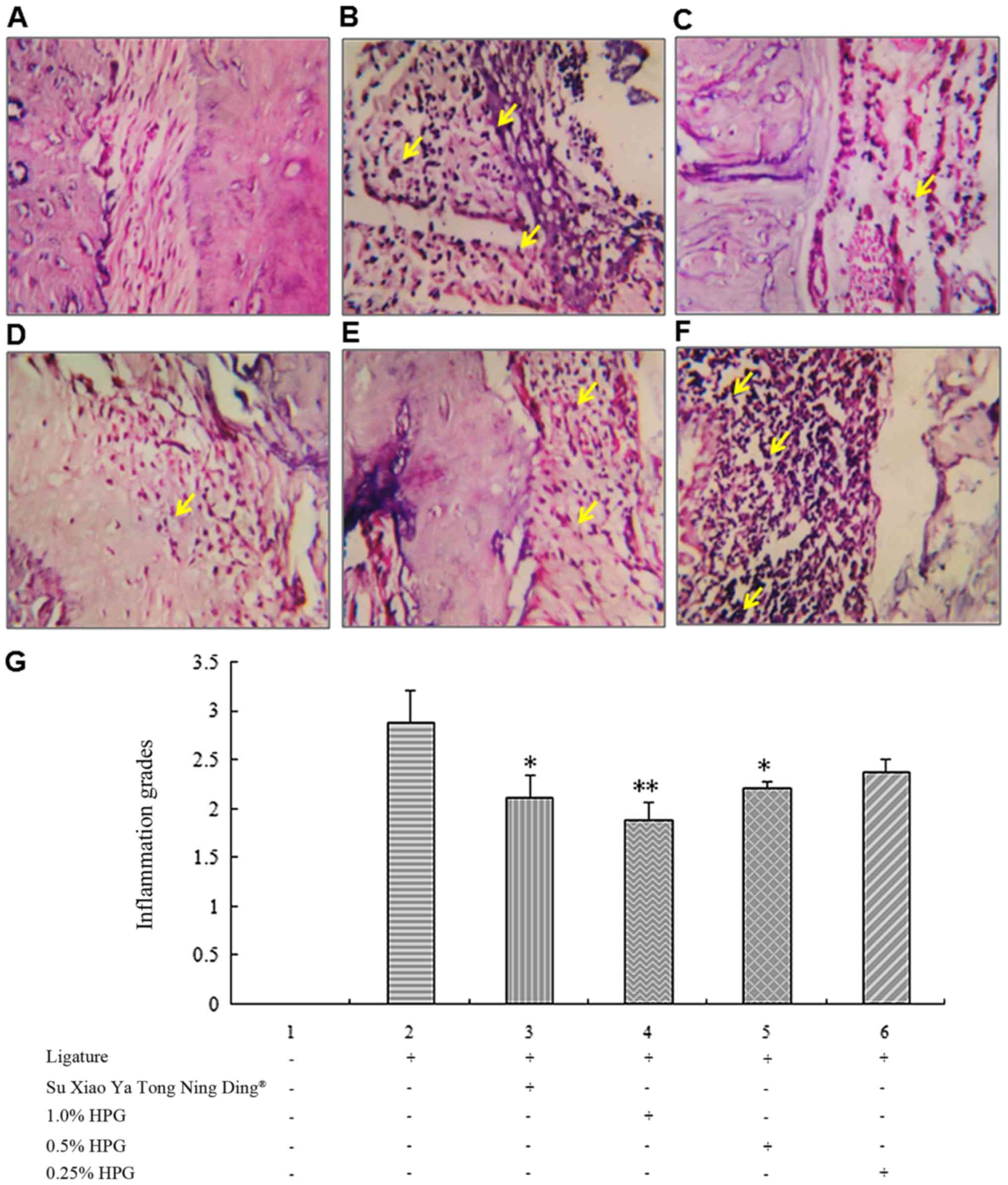 | Figure 1.Periodontal inflammation grade in
different treatment groups. (A) The histological images of the
non-ligated group showing normal periodontium (magnification,
×100). (B) The histological images of the ligature alone group,
with intense inflammatory cell infiltrate, dilated blood vessels,
and osteoclasts in their Howship's lacunae with multiple
reabsorption foci (magnification, ×100). (C) The histological
images of Su Xiao Ya Tong Ning Ding with moderate inflammatory cell
infiltrate in periodontal ligament and osteoclasts in their
Howship's lacunae with multiple reabsorption foci. (D-F)
Histological images of different doses of HPG (D, 1.0%; E, 0.5%; F,
0.25%). Yellow arrows indicate inflammatory cells (magnification,
×100). (G) Comparison of periodontal inflammation grade in
different groups. HPG, Hylotelephium purpureum gel.
*P<0.05; **P<0.01. |
Inhibitory effects of HPG on
xylene-induced ear edema in mice
The HPG high- and medium-dosage groups and the Su
Xiao Ya Tong Ning Ding group all antagonized xylene-induced
mouse-ear edema, when compared to the blank control group.
Following treatment with HPG at high- or medium-doses, or Su Xiao
Ya Tong Ning Ding the degree of ear edema was markedly reduced, and
the differences were statistically significant (P<0.01 or
P<0.05; Table III).
 | Table III.Inhibitory effect of HPG on
xylene-induced ear edema in mice. |
Table III.
Inhibitory effect of HPG on
xylene-induced ear edema in mice.
| Treatment group | Dose (ml) | Degree of ear edema
(mg) | Edema inhibition rate
(%) |
|---|
| Ligature alone | N/A |
18.7±2.79 | 85.3 |
| Su Xiao Ya Tong Ning
Ding | 0.3 |
11.2±4.42b | 42.6c |
| 1% HPG | 0.3 |
7.60±4.72c | 32.7c |
| 0.5% HPG | 0.3 |
10.3±3.92c | 46.8c |
| 0.25% HPG | 0.3 |
14.5±4.95a | 63.5a |
Inhibitory effects of HPG on
acetic-acid induced peritoneal capillary permeability in mice
Compared with the blank control group, the HPG
high-, medium- and low-dosage groups and the Su Xiao Ya Tong Ning
Ding group all had significantly inhibited 0.6%-acetic-acid induced
peritoneal capillary permeability in mice (P<0.05 or P<0.01;
Table IV).
 | Table IV.Inhibitory effects of HPG on acetic
acid-induced peritoneal capillary permeability. |
Table IV.
Inhibitory effects of HPG on acetic
acid-induced peritoneal capillary permeability.
| Treatment
group | Dose (ml) | Absorbance |
|---|
| Non-ligated | N/A |
0.048±0.020c |
| Ligature alone | N/A |
0.421±0.093 |
| Su Xiao Ya Tong
Ning Ding | 0.3 |
0.276±0.113b |
| 1.0% HPG | 0.3 |
0.226±0.103c |
| 0.5% HPG | 0.3 |
0.262±0.101b |
| 0.25% HPG | 0.3 |
0.301±0.119a |
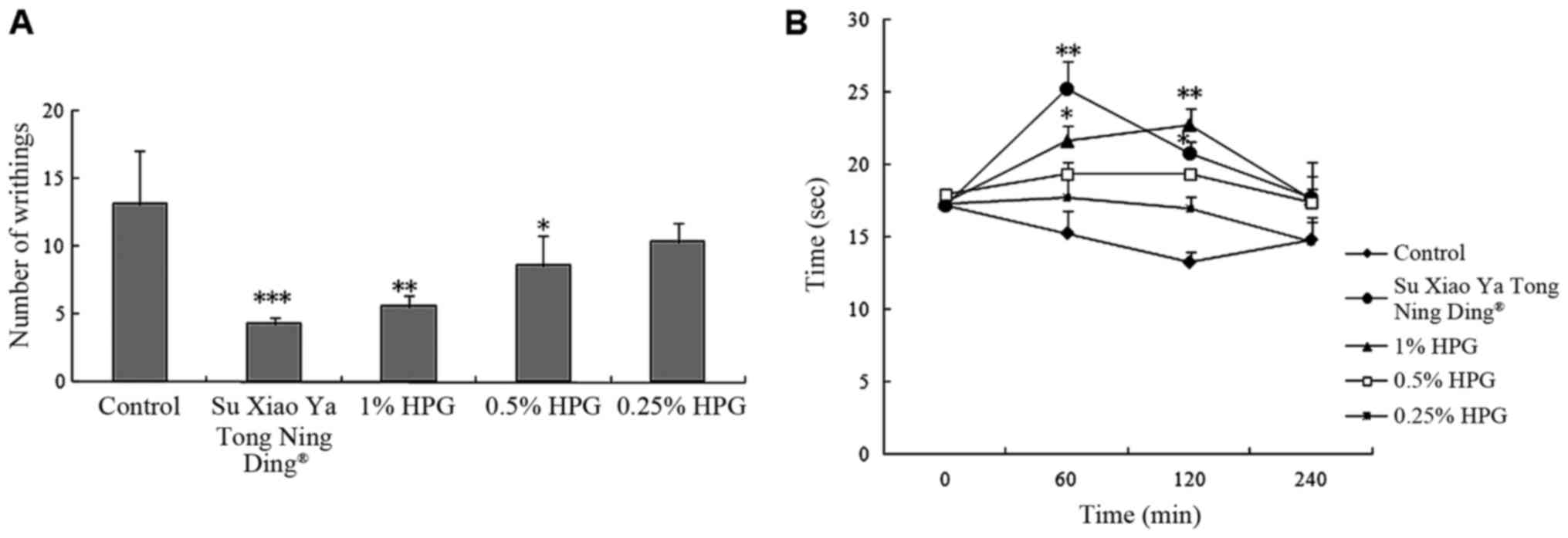
Evaluation of antinociceptive activity
of HPG
As shown in Fig. 2,
HPG exhibited antinociceptive activity in acetic acid-induced
writhing response and hot-plate test. Local administration of the
HPG (1.0, 0.5 and 0.25%) significantly decreased the number of
writhes in mice induced by acetic acid, with inhibition rates of
57.25, 34.35 and 20.61%, respectively, (P<0.01) in a
dose-dependent manner. As the positive drug, Su Xiao Ya Tong Ning
Ding produced a 67.18% reduction compared to the control.
Meanwhile, compared to the control group at 60 and 120 min,
high-dose HPG could prolong the latency times of mice (P<0.05,
P<0.01, respectively). Su Xiao Ya Tong Ning Ding markedly
increased the pain threshold of mice in the first 60 min
(P<0.01), but it decreased it thereafter.
MIC determination results of HPG
The determination of the MIC of the different
treatments is shown in Table V. The
results demonstrate that HPG had relatively good bacteriostatic and
bactericidal effects on Bacteroides melaninogenicus,
Porphyromonas gingivalis, Fusobacterium nucleatum, Streptococcus
mutans, Aggregatibacter actinomycetemcomitans and
Bacteroides melaninogenicus.
 | Table V.Minimum inhibitory concentrations of
HPG, tinidazole and Su Xiao Ya Tong Ning Ding among bacterial
species. |
Table V.
Minimum inhibitory concentrations of
HPG, tinidazole and Su Xiao Ya Tong Ning Ding among bacterial
species.
|
| Minimum inhibitory
concentration |
|---|
|
|
|
|---|
| Bacterial
species | 1% HPG (mg/ml) | Tinidazole
(µg/ml) | Su Xiao Ya Tong
Ning Ding (%) |
|---|
| Aggregatibacter
actinomycetemcomitans 1 | 5.00 | 64 | 15.0 |
| Aggregatibacter
actinomycetemcomitans 2 | 2.50 | 32 | 15.0 |
| Aggregatibacter
actinomycetemcomitans 3 | 2.50 | 32 |
7.5 |
| Aggregatibacter
actinomycetemcomitans 4 | 2.50 | 16 |
7.5 |
| Aggregatibacter
actinomycetemcomitans 5 | 5.00 | 32 | 15.0 |
| Bacteroides
melaninogenicus 1 | 2.50 | 16 |
7.5 |
| Bacteroides
melaninogenicus 2 | 2.50 | 16 | 15.0 |
| Bacteroides
melaninogenicus 3 | 1.25 | 8 |
7.5 |
| Bacteroides
melaninogenicus 4 | 2.50 | 16 |
7.5 |
| Streptococcus
mutans A | 5.00 | 64 | 15.0 |
| Streptococcus
mutans B | 2.50 | 32 |
7.5 |
| Streptococcus
mutans C | 2.50 | 32 |
7.5 |
| Streptococcus
mutans D | 2.50 | 32 |
7.5 |
| Streptococcus
mutans E | 5.00 | 64 | 15.0 |
| Streptococcus
mutans F | 5.00 | 64 | 15.0 |
| Streptococcus
mutans G | 5.00 | 64 | 15.0 |
| Porphyromonas
gingivalis 1 | 2.50 | 32 |
7.5 |
| Porphyromonas
gingivalis 2 | 1.25 | 16 |
3.7 |
| Porphyromonas
gingivalis 3 | 5.00 | 32 | 15.0 |
| Porphyromonas
gingivalis 4 | 5.00 | 32 | 15.0 |
| Porphyromonas
gingivalis 5 | 5.00 | 64 | 15.0 |
| Porphyromonas
gingivalis 6 | 2.50 | 32 | 15.0 |
| Porphyromonas
gingivalis 7 | 2.50 | 32 | 15.0 |
| Porphyromonas
gingivalis 8 | 2.50 | 32 | 15.0 |
| Fusobacterium
nucleatum 1 | 1.25 | 16 |
3.7 |
| Fusobacterium
nucleatum 2 | 2.50 | 16 |
3.7 |
| Fusobacterium
nucleatum 3 | 2.50 | 16 |
3.7 |
Discussion
Periodontitis is a chronic inflammatory disease
caused by bacterial infection of the supporting tissues surrounding
the teeth. The concept of local delivery of chemotherapeutic agents
to the periodontal pocket as a method to treat periodontal disease
has been studied for over the past few decades. Although various
locally delivered antimicrobial agents are commercially available,
the need for safe, effective, and economical agents has motivated
the use of various natural extracts. Various herbal products and
their extracts such as guava, pomegranate, neem, propolis, tulsi,
green tea, cranberry, grapefruit, etc., in the form of mouthwashes
and gels have shown significant advantages over the chemical ones
in the treatment of periodontal diseases (13–15).
Periodontal disease can be induced in rodents by
tying a ligature of 2-0-5-0 braided silk around the cervix of the
maxillary or mandibular molars, or by injecting lipopolysaccharides
into the papilla, or a combination of both (16). Souza et al (17) used a period of 4 weeks for
periodontitis induction in the maxilla. This timeframe was similar
to the study period used in our study.
Hylotelephium purpureum is an herbal cure in
traditional medicine because of its anti-inflammatory, analgesic,
antispasmodic, antipyretic, antimicrobial, and antioxidant
properties (4,5). However, its efficacy in the treatment of
periodontal diseases has not yet been elucidated. In the present
study, we squeezed the juice of the Hylotelephium purpureum
from the whole grass, and then extracted and separated its
effective ingredients, filtering the effective parts of the plant.
We found that the extract contained 76% quercetin and kaempferide.
Quercetin is a flavonol found in many fruits, vegetables, leaves
and grains. Kaempferide is an O-methylated flavonol, a type of
chemical compound. HPG was produced from the extract and used to
investigate the anti-inflammatory activity and antinociceptive
effects, as well as assessed the durations of the action and the
efficacy of iHPG, in the treatment of experimental periodontitis in
a KM mouse model.
In the present study, we formulated and evaluated
the anti-inflammatory activity and antinociceptive effects of
Hylotelephium purpureum and assessed the duration of action
and efficacy of Hylotelephium purpureum in the treatment of
experimental periodontitis. The results demonstrated that
HPG obviously changed the GI and SBI in our model of
experimental periodontitis. The serum levels of SOD and GPx were
significantly heightened, while the level of MAD was decreased. The
gel showed 32.7% inhibition of edema, and it changed the peritoneal
capillary permeability in mice. Meanwhile, it had relatively good
bacteriostatic and bactericidal effects, as well as antinociceptive
activity. Hence, HPG can be a useful adjunct to enhance the results
of standard periodontal therapy.
In conclusion, within the limitations of the present
study, HPG appears to be an attractive alternating agent that can
be used for effective and safe local drug delivery as an adjunct to
mechanical nonsurgical periodontal therapy.
Acknowledgements
The present study was supported by the grants from
science and technology development projects of Jilin Province
Department of Traditional Chinese Medicine (grant no.
20100919).
References
|
1
|
Miyazaki H, Pilot T, Leclercq MH and
Barmes DE: Profiles of periodontal conditions in adults measured by
CPITN. Int Dent J. 41:74–80. 1991.PubMed/NCBI
|
|
2
|
Ryan ME: Nonsurgical approaches for the
treatment of periodontal diseases. Dent Clin North Am. 49:611–636,
vii. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xinqi Chen HC, Dai L and Xia Z: The flora
of China. Science Press; China: 2004
|
|
4
|
Winekenstädde D, Angelis A, Waltenberger
B, Schwaiger S, Tchoumtchoua J, König S, Werz O, Aligiannis N,
Skaltsounis AL and Stuppner H: Phytochemical profile of the aerial
parts of Sedum sediforme and anti-inflammatory activity of
myricitrin. Nat Prod Commun. 10:83–88. 2015.PubMed/NCBI
|
|
5
|
Sendl A, Mulinacci N, Vincieri FF and
Wagner H: Anti-inflammatory and immunologically active
polysaccharides of Sedum telephium. Phytochemistry. 34:1357–1362.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Peichl P: Health, safety and environmental
protection in a biological research laboratory. Int Arch Occup
Environ Health. 73:S8–S13. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu R, Li N, Liu N, Zhou X, Dong ZM, Wen
XJ and Liu LC: Effects of systemic ornidazole, systemic and local
compound ornidazole and pefloxacin mesylate on experimental
periodontitis in rats. Med Sci Monit. 18:BR95–BR102. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Loe H and Silness J: Periodontal disease
in pregnancy. I. Prevalence and severity. Acta Odontol Scand.
21:533–551. 1963. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ainamo J and Bay I: Problems and proposals
for recording gingivitis and plaque. Int Dent J. 25:229–235.
1975.PubMed/NCBI
|
|
10
|
Shapira L, Gordon B, Warbington M and Van
Dyke TE: Priming effect of Porphyromonas gingivalis
lipopolysaccharide on superoxide production by neutrophils from
healthy and rapidly progressive periodontitis subjects. J
Periodontol. 65:129–133. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Borges I Jr, Moreira EA, Filho DW, de
Oliveira TB, da Silva MB and Fröde TS: Proinflammatory and
oxidative stress markers in patients with periodontal disease.
Mediators Inflamm. 2007:457942007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pereira SL, de Oliveira JW, Angelo KK, da
Costa AM and Costa F: Clinical effect of a mouth rinse containing
Ocimum gratissimum on plaque and gingivitis control. J Contemp Dent
Pract. 12:350–355. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kukreja BJDV: Herbal mouthwashes - a gift
of nature. Int J Pharma Bio Sci. 3:46–52. 2012.
|
|
14
|
Desai AAM and Debnath S: A clinical trial
to evaluate the effects of triphala as a mouthwash in comparison
with chlorhexidine in chronic generalized periodontitis patient.
IJDA Arch. 2:243–247. 2010.
|
|
15
|
Reddy PDST, Swarna LD and Purushothaman M:
Local drug delivery of herbs for treatment of periodontitis. J
Innov Trends Pharma Sci. 1:245–251. 2010.
|
|
16
|
Struillou X, Boutigny H, Soueidan A and
Layrolle P: Experimental animal models in periodontology: A review.
Open Dent J. 4:37–47. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Souza DM, Prado FA, Prado MA, Rocha RF and
Carvalho YR: Evaluation of two morphometric methods of bone loss
percentages caused by periodontitis in rats in different locations.
J Appl Oral Sci. 18:493–497. 2010. View Article : Google Scholar : PubMed/NCBI
|