Introduction
Numerous studies have described that oxidative
stress, induced by reactive oxygen species (ROS) and mitochondrial
dysfunction, is implicated in the etiology of neurodegenerative
disorders (1–3). Adenosine is a naturally occurring
nucleoside that is used to control heart arrhythmia (4). Adenosine is a neuromodulator that
suppresses neuronal excitability through activation of the
inhibitory adenosine A1 receptor (AA1R) (5). Therefore, AA1R has therapeutic potential
as a neuroprotective candidate.
Apoptosis, a form of programmed cell death, serves
an important role in neurodegenerative disorders (6). Studies on apoptosis and its role in
neurodegenerative disorders including Alzheimer's disease,
Parkinson's disease, Huntington's disease and ischemia have
elucidated the possible apoptotic mechanisms underlying these
disorders (7–9). Apoptosis is regulated by the expression
or activation of proapoptotic genes and proteins including
mammalian sterile 20-like kinase 1 (Mst1) (10). Mst1 is a stress-activated,
proapoptotic kinase that, following caspase-mediated cleavage,
enters the nucleus and induces chromatin condensation followed by
DNA fragmentation (11,12). Mst1 protein has been reported to
induce the mitochondrial-dependent pathway of apoptosis as well as
promote apoptosis via caspase-dependent and -independent pathways
(13,14).
In the present study, the in vitro
neuroprotective effects of adenosine in inhibiting apoptosis and
promoting cell survival were investigated in bone marrow-derived
neural stem cells (B-dNSCs) preexposed to hydrogen peroxide
(H2O2). The effects of adenosine on
expression of proapoptotic Mst1 and antiapoptotic nuclear factor
(erythroid-derived 2)-like 2 (Nrf2) and B-cell lymphoma 2
(Bcl-2) genes in the H2O2-induced
B-dNSCs were evaluated. Recent studies have demonstrated that Nrf1,
Nrf2 and Bcl-2 family coactivators control mitochondrial
transcription specificity factors (15,16).
Furthermore, Bcl-2 and Nrf2 are established as critical factors in
protecting cells against H2O2-induced
apoptosis (17). The results from
this study indicated that apoptosis rate and Mst1 expression
in the adenosine-treated B-dNSCs were significantly decreased while
Nrf2 and AAIR mRNA levels were increased. The present
study aimed to develop understanding of the neuroprotective effects
of adenosin in neurological disorders.
Materials and methods
Bone marrow-derived stromal cell
(BMSC) isolation and culture
In the present study, 5 male Wistar rats (weighing
150–200 g, aged 8 weeks old), purchased from Razi Vaccine and Serum
Research Institute (Karaj, Iran), were sacrificed under anesthesia
with 100 mg/kg ketamine and 10 mg/kg xylazine. All experimental
protocols were approved by the Zanjan University of Medical
Sciences (Zanjan, Iran) Ethics Committee. All rats were housed in a
temperature (25–27°C) and humidity (~50%) controlled environment
under a 12-h light/dark cycle. The rat tibia and femoral bone
marrow were aseptically aspirated with 2 ml Dulbecco's modified
Eagle's medium (DMEM; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) and then centrifuged for 5 min at 1,500 × g and 4°C. The
supernatant was removed, 1 ml fresh media was added and repeated
pipetting was performed to create a single cell suspension.
Subsequently, cells were transferred to a 25-cm2 flask
for tissue culture with 5 ml low-glucose DMEM containing 10% fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA), 100 U/ml penicillin and 100 mg/ml streptomycin
(Sigma-Aldrich; Merck KGaA). The isolated cells were incubated at
37°C in 5% CO2 for 2 days. The adherent cells were
collected and subcultured, and the culture medium was replaced
every 2–3 days until cells became 70–80% confluent.
The cells were harvested with trypsin-EDTA (0.25%;
Sigma-Aldrich; Merck KGaA) and passaged up to three times (P3). For
identifying BMSCs, immunocytochemical evaluation was performed for
cluster of differentiation (CD)-90 as a mesenchymal stem cell
marker (18). Briefly, BMSCs were
cultured on cover slides and fixed in 3% paraformaldehyde for 15
min at room temperature (RT), then permeabilized with 0.4% Triton
X-100. Blocking was performed in 10% FBS in phosphate-buffered
saline (PBS) for 30 min at RT. The cells were then incubated with
anti-CD90 monoclonal antibodies (ab225; 1:200; Abcam, Cambridge,
UK) overnight at 4°C, followed by incubation with a fluorescein
isothiocyanate (FITC)-conjugated rabbit anti-rat antibody (ab6730;
1:300; Abcam) for 4 h at RT. Nuclei were counterstained with
ethidium bromide for 30 sec at RT.
Neurosphere formation and
expansion
To form neurosphere-like structures, isolated BMSCs
at P3 were seeded (1×105 cells/ml) in 25-cm2
non-adherent plastic flasks and incubated with NSC expansion medium
containing DMEM/F12 supplemented with 2% B27 (Gibco; Thermo Fisher
Scientific, Inc.), 20 ng/ml basic fibroblast growth factor (bFGF;
Invitrogen; Thermo Fisher Scientific, Inc.), 20 ng/ml epidermal
growth factor (EGF; Invitrogen; Thermo Fisher Scientific, Inc.),
100 U/ml penicillin and 100 mg/ml streptomycin. The medium and
growth factors were added every 3 days for a week. For the
preparation of a single cells from the neurospheres, the floating
structures were isolated by centrifuging for 5 min at 300 × g and
4°C.
They were then dissociated enzymatically using
Trypsin-EDTA (0.25%) and mechanically (by pipetting) to single
cells, and subsequently expanded on 6-well adherent plates coated
with poly-l-lysine (Sigma-Aldrich; Merck KGaA). The cells
(105 cells/well) were then suspended in DMEM/F12
supplemented with 2% B27, 20 ng/ml bFGF and 20 ng/ml EGF and 5% FBS
and passaged up to three times (19).
For identifying B-dNSCs, immunocytochemical evaluation was
performed for nestin as an NSC/progenitor cell marker (20) with corresponding antibody (ab6142;
1:300; Abcam) as above. The FITC-conjugated rabbit anti-rat
antibody (ab6730; 1:300; Abcam) was used for detection (4 h at RT
in the dark). Ethidium bromide (20 sec) was used for nuclei
counterstaining at RT. Images were captured with an Olympus BX51
fluorescence microscope (Olympus Corporation, Tokyo, Japan).
Adenosine dose response
B-dNSC viability and the protective effects of
adenosine were evaluated using the 3-(4, 5-dimethythiazol-2-yl)-2,
5-diphenyl-tetrazolium (MTT) bromide assay (21). B-dNSCs were cultured in 96-well plates
(105 cells/well) in NSC expansion medium. The cells were
then incubated with different concentrations of adenosine (0, 2, 4,
6, 8 and 10 µM) at 37°C for 48 h. To induce oxidative stress,
H2O2 was prepared from a 30% stock solution.
The pretreated NSCs were incubated with 125 µM
H2O2 for 30 min at 37°C. Subsequently, the
cells were incubated with 1 mg/ml MTT at 37°C for 4 h. The culture
medium was removed and 100 µl dimethyl sulfoxide was added to each
well to dissolve the formazan crystals. The amount of formazan
dissolved was quantified at absorbance (A)570 nm using a microplate
ELISA reader. The relative cell viability in percentage was
calculated as (A570 of treated samples/A570 of untreated samples) ×
100 (22).
Experimental groups
Based on results of the MTT assay, cells were
assigned to different experimental groups as follows: N (untreated
B-dNSCs), NA (B-dNSCs treated with 6 µM adenosine), NH (B-dNSCs
treated with 125 µM H2O2) and NAH (B-dNSCs
pretreated with 6 µM adenosine followed by 125 µM
H2O2).
Assessment of apoptosis by terminal deoxynucleotidyl
transferase dUTP nick-end labeling (TUNEL) staining. Cells were
fixed with 4% paraformaldehyde in PBS for 30 min at room
temperature (RT). DNA fragmentation was assessed with an
In-Situ Cell Death Detection kit (Roche Diagnostics GmbH,
Mannheim, Germany) according to the manufacturer's instructions.
TUNEL-positive cells were colored using diaminobenzidine as the
chromogen for 5 min at RT, and counterstained with hematoxylin for
1 min at RT. The percentage of TUNEL-positive cells was assessed in
five randomly selected microscopic fields of the Olympus BX51
fluorescence microscope per culture well.
Immunofluorescence staining
Cells were cultured on cover slides at a density of
1×105 cells/ml in NSC growth media as described above
and fixed in 4% paraformaldehyde for 15 min at RT, followed by
permeabilization in PBS-0.1% Triton X-100 for 30 min at RT.
Blocking was performed in 10% FBS in PBS for 30 min at RT. For
immunofluorescence staining, cells were incubated with primary
polyclonal antibodies against rat CD90 (ab225; 1:200), nestin
(ab6142; 1:300; both from Abcam), Nrf2 (sc-722; 1:1,200) and Mst1
(sc-100449; 1:300; both from Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) individually overnight at 4°C, then incubated with
a fluorescein isothiocyanate-conjugated rabbit anti-rat antibody
(ab6730; 1:300; Abcam) for 4 h at RT in the dark. DAPI (5 min) and
ethidium bromide (20 sec) were used for nuclei counterstaining at
RT. Images were captured with the Olympus BX51 fluorescence
microscope.
Real-time reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Real-time RT-qPCR was performed with cDNA from the
experimental groups following treatments. In all groups, 1,000 ng
purified RNA extracted using TRIzol (Invitrogen; Thermo Fisher
Scientific, Inc.) from cultured cells was used to synthesize 20 µl
cDNA, using a RevertAid™ First Strand cDNA Synthesis kit
(Fermentas; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. cDNA (25 ng of RNA samples) was used
to quantify Bcl-2, Nrf2, Mst1 and AA1R mRNA levels.
As an internal control, primers for GAPDH were used. The
primer sequences of all primers used are listed in Table I. The PCR reaction was performed in a
12.5-µl final volume containing forward and reverse primers (200 nM
each), cDNA (0.5 µl), SYBR®-Green I (6.5 µl; Fermentas;
Thermo Fisher Scientific, Inc.) and nuclease-free water up to final
volume for 40 cycles (Applied Biosystems 7500; Thermo Fisher
Scientific, Inc.) at 95°C for 15 sec followed by 60°C for 1 min.
For analyzing relative changes in mRNA levels, the
2−ΔΔCq method was employed (23).
 | Table I.PCR primer sequences. |
Table I.
PCR primer sequences.
| Gene | GenBank accession
no. | Forward, 5′-3′ | Reverse, 5′-3′ | Product size,
bp |
|---|
| Mst1 | NM_001107800.1 |
GCTAAAGTGAAGTGGACGGATACC |
GGAACAGTTGCTACCAGAGTGTCAG | 173 |
| Nrf2 | NM_031789.2 |
CACCAGTGGATCTGTCAGCTACTC |
GTGGTGAAGACTGAGCTCTCAACG | 168 |
| Bcl-2 | NM_016993.1 |
GTGGCCTTCTTTGAGTTCGGTG |
ATCCCAGCCTCCGTTATCCTG | 147 |
| GAPDH | NM_017008 |
AACCCATCACCATCTTCCAG |
GTGGTTCACACCCATCACAA | 197 |
| AA1R | XM_006249861 |
CCACAGACCTACTTCCACACC |
CTGTCTTGTACCGGAGAGGGA | 136 |
Statistical analysis
Statistical analysis of data was performed using
SPSS 15.0 software (SPSS, Inc., Chicago, IL, USA) based on a
minimum of three independent experiments. The results were
normalized with those of the experimental controls, and were
presented as the mean percentage ± standard error of the mean and
analyzed by one-way analysis of variance followed by the Tukey's
post hoc multiple group comparison test. A difference between
groups was considered statistically significant when P<0.05.
Results
Isolation and culture of BMSCs
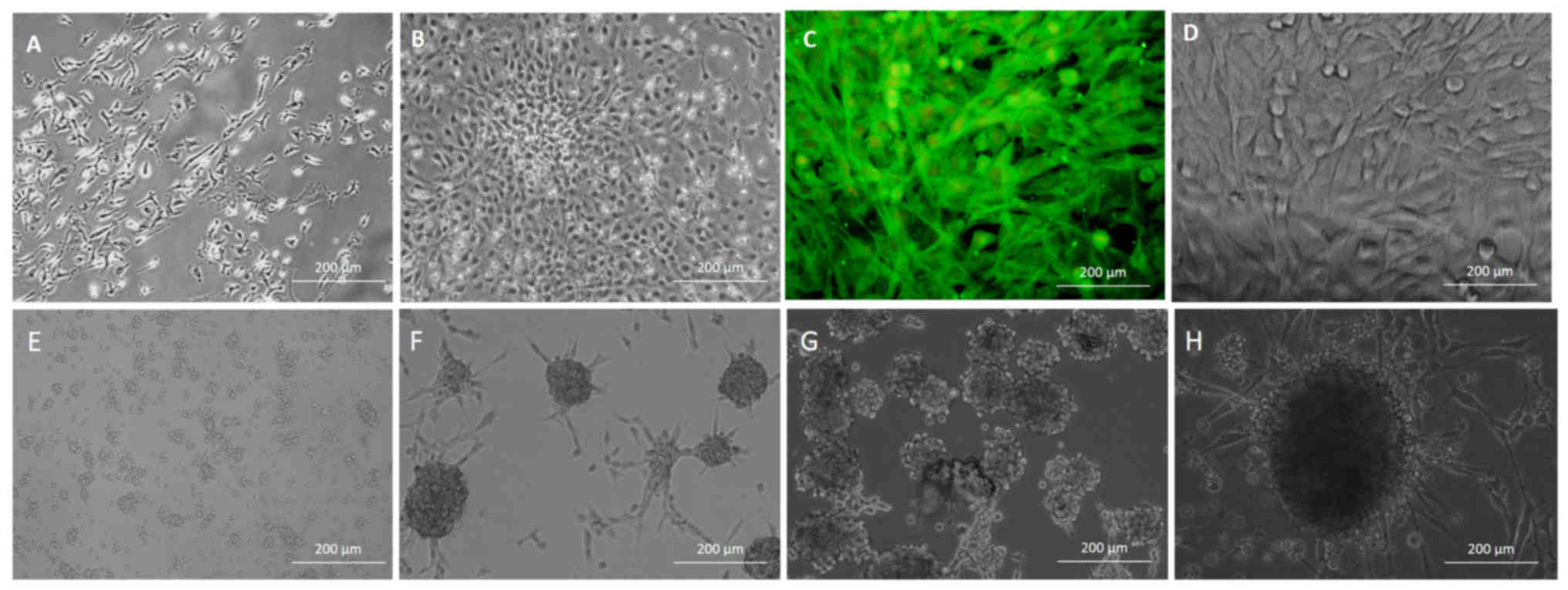
The results demonstrated that, after a 24-h culture,
BMSCs successfully attached to the dish surface. When adhered, the
BMSCs exhibited fibroblast-like or triangular shape (Fig. 1A). Following replacement of the
medium, the majority of non-adherent cells were eliminated and
adherent cells gradually proliferated. After 3–5 days, adherent
cells formed clones (Fig. 1B). At 5
days the clones enlarged and fused with other clones. After 10
days, the adherent cells became confluent and could be subcultured.
At 2 h after subculture the majority of BMSCs adhered, and after
3–4 days cells were confluent. The BMSCs were identified to express
CD90, which served to identify cells as MSCs (Fig. 1C and D), at a positive expression rate
of 97.30±0.68% (data not shown).
Induction of NSCs
Following culture in neural stem cell expansion
medium, a number of the BMSCs proliferated into floating spherical
aggregates, deemed as neurospheres with multipolar processes
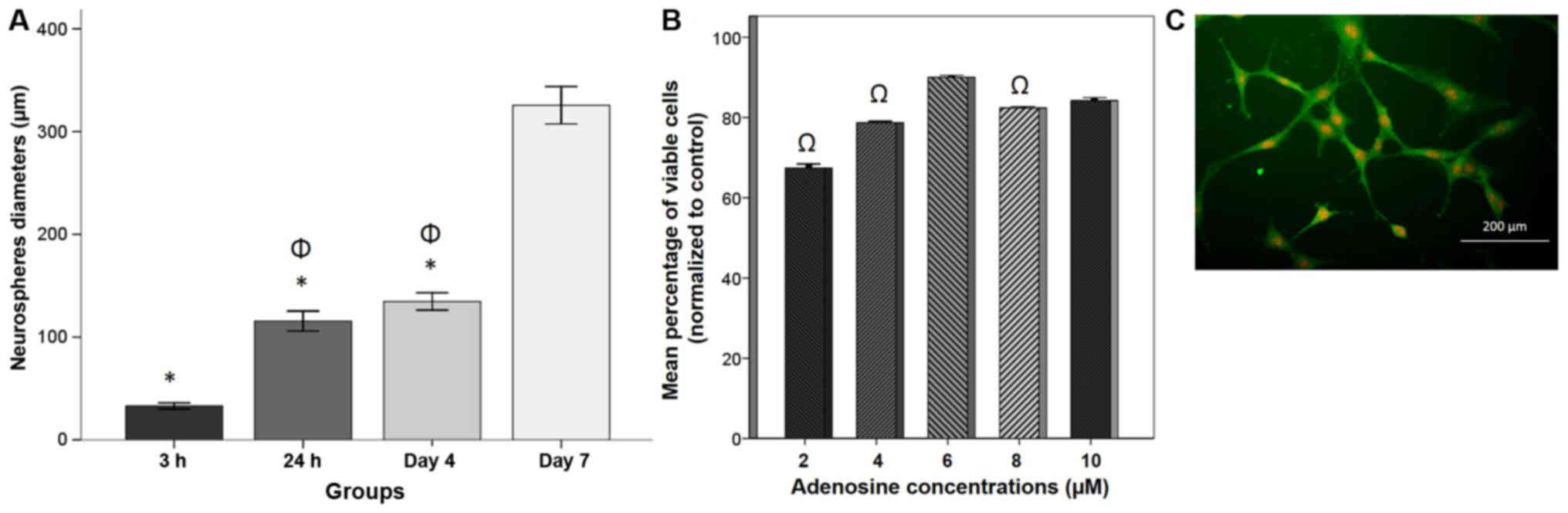
(Fig. 1E). On assessment of spheroid
diameter (Fig. 1F-H), diameters at
day 7 (325.61±9.09 µm) were significantly increased compared with
those at 3 h (32.92±1.47 µm), 24 h (115.59±4.88 µm) and 4 days
(134.69±4.21 µm; P<0.05; Fig. 2A).
The neurospheres were passaged and induced to differentiate by
neural stem cell expansion medium containing 5% FBS following
plating on poly-L-lysine-coated adherent plates. To confirm the
induced NSCs, the P3 cells were subjected to immunostaining
analysis. The B-dNSCs from neurospheres expressed a high level of
nestin (Fig. 2C) at a positive
immunostaining rate of 98.00±0.35% (data not shown).
Dose response and cell viability
To evaluate the protective effect of adenosine
against H2O2-induced cytotoxicity, B-dNSCs
were treated with various concentrations of adenosine (2, 4, 6, 8
and 10 µM) for 48 h prior to H2O2 exposure.
Cell viability was then evaluated by MTT assay (Fig. 2B). The pretreatment of B-dNSCs with
adenosine increased cell viability in an apparent dose dependent
manner, with the exception of 6 µM adenosine, which induced the
greatest cell viability (90.02±0.24%) relative to the other
concentrations tested, to a significant extent compared with 2, 4
and 8 µM adenosine (P<0.05). Therefore, 6 µM adenosine was
selected as a suitable dose for further studies.
TUNEL assay
A TUNEL assay was performed to determine the
antiapoptotic effect of adenosine in B-dNSCs treated with
H2O2. As depicted in Fig. 3A-D, TUNEL-positive nuclei staining
demonstrated that H2O2 induced apoptotic cell
death. The percentage of TUNEL-positive cells was significantly
decreased in the NAH group compared with that in the NH group
(P<0.05; Fig. 3E). The mean
percentages of TUNEL positive cells in the N, NA, NH and NAH groups
were 5.47±0.48, 9.43±0.45, 53.19±1.12 and 30.07±1.16%,
respectively.
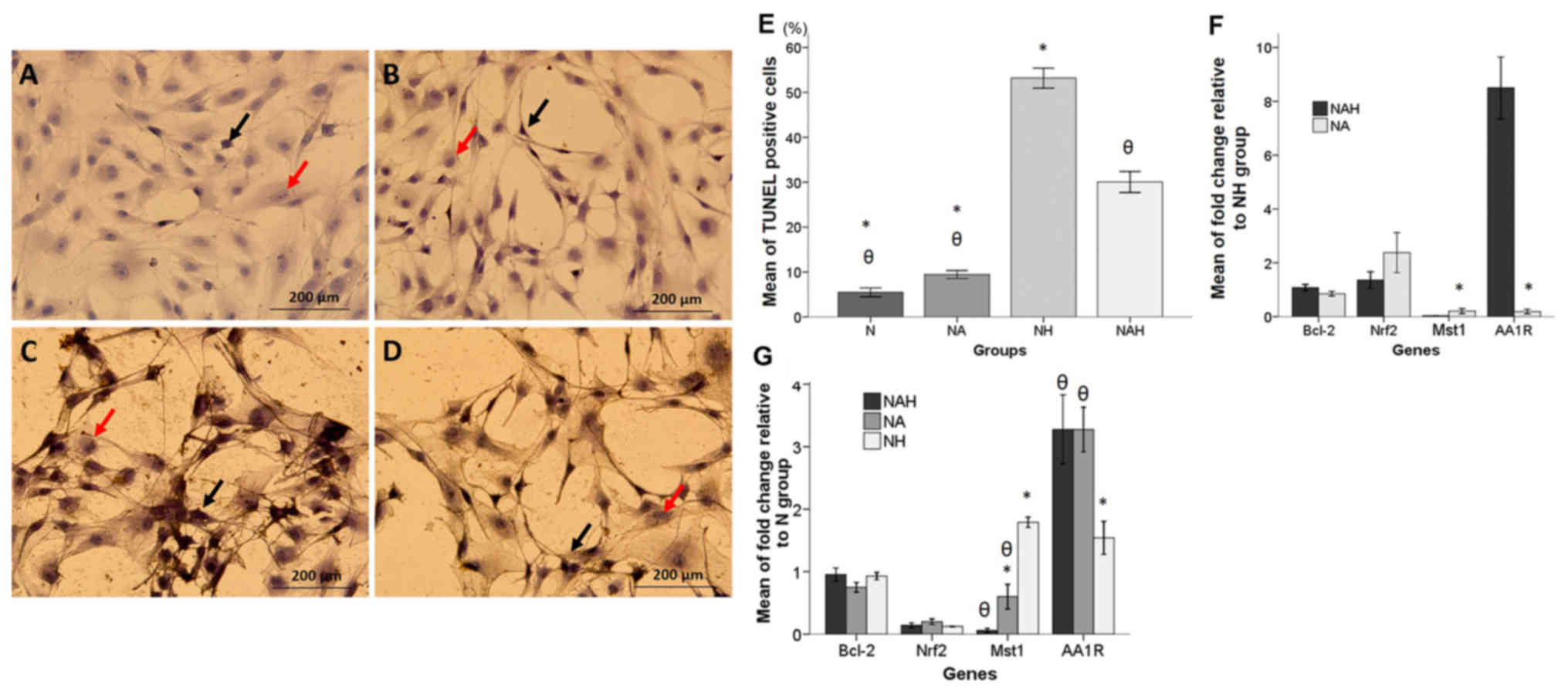 | Figure 3.TUNEL assay and real-time RT-qPCR
results. (A-D) Detection of apoptosis by TUNEL assay in the N, NA,
NH and NAH experimental groups. TUNEL staining was performed in
B-dNSCs exposed to 6 µM adenosine for 48 h then 125 µM
H2O2 for 30 min. Photomicorgraphs are shown
indicating the (A) B-dNSCs without treatment (N group); (B) NA
group; (C) NH group; and (D) NAH group. Black and red arrows
indicate TUNEL-positive and non-fragmented nuclei, respectively.
(E) Histogram of the mean percentages of apoptotic cells in the
experimental groups. (F and G) RT-qPCR results (F) relative to the
NH group and (G) relative to the N group. mRNA level is presented
as relative expression normalized to GAPDH mRNA
amplification. The bars indicate the mean ± standard error of the
mean. θP<0.05 vs. NH group; *P<0.05 vs. NAH group.
Magnification, ×200. TUNEL, terminal deoxynucleotidyl transferase
dUTP nick-end labeling; B-dNSCs, bone marrow-derived neural stem
cells; N, untreated B-dNSCs; NA, B-dNSCs treated with 6 µM
adenosine; NH, B-dNSCs treated with 125 µM
H2O2; NAH, B-dNSCs pretreated with 6 µM
adenosine + 125 µM H2O2; Bcl-2, B-cell
lymphoma 2; Nrf2, nuclear factor (erythroid-derived 2)-like 2;
Mst1, mammalian sterile 20-like kinase 1; AA1R, adenosine A1
receptor; H2O2, hydrogen peroxide; RT-qPCR,
reverse transcription-quantitative polymerase chain reaction. |
Gene expression
The changes in expression of Bcl-2, Nrf2,
Mst1 and AA1R in the experimental groups were examined
using real-time RT-qPCR. The results were presented relative to the
NH and N groups individually (Fig.
3F-G). In the groups treated with adenosine (relative to the NH
group), all genes exhibited increased expression except Mst1
and AAR1. The mean fold-changes relative to the NH group
were as follows: For Mst1, NA: 0.20±0.04, NAH: 0.03±0.00;
for Bcl-2, NA: 0.85±0.04, NAH: 1.08±0.05; for Nrf2,
NA: 2.37±0.37.4, NAH: 1.35±0.15; and for AA1R, NA:
0.20±0.04, NAH: 8.50±0.57 (Fig.
3F).
The mean values of fold-change in the Bcl-2,
Nrf2, Mst1 and AA1R genes relative to levels in the N
group are presented in Fig. 3G. mRNA
expression of proapoptotic Mst1 in the NH group was
significantly upregulated compared with that in the NAH and NA
groups (P<0.05). Additionally, the relative expression ratio of
AA1R was significantly increased in the NAH and NA groups
compared with that in the NH group (P<0.05). The mean
fold-changes relative to the N group were as follows: For
Mst1, NH: 1.91±0.09, NA: 0.60±0.09, NAH: 0.06±0.01; for
Bcl-2, NH: 0.92±0.02, NA: 0.75±0.03, NAH: 0.95±0.05; for
Nrf2, NH: 0.12±0.00, NA: 0.20±0.02, NAH: 0.13±0.01; and for
AA1R, NH: 1.36±0.05, NA: 3.27±0.17, NAH: 3.27±0.27 (Fig. 3G).
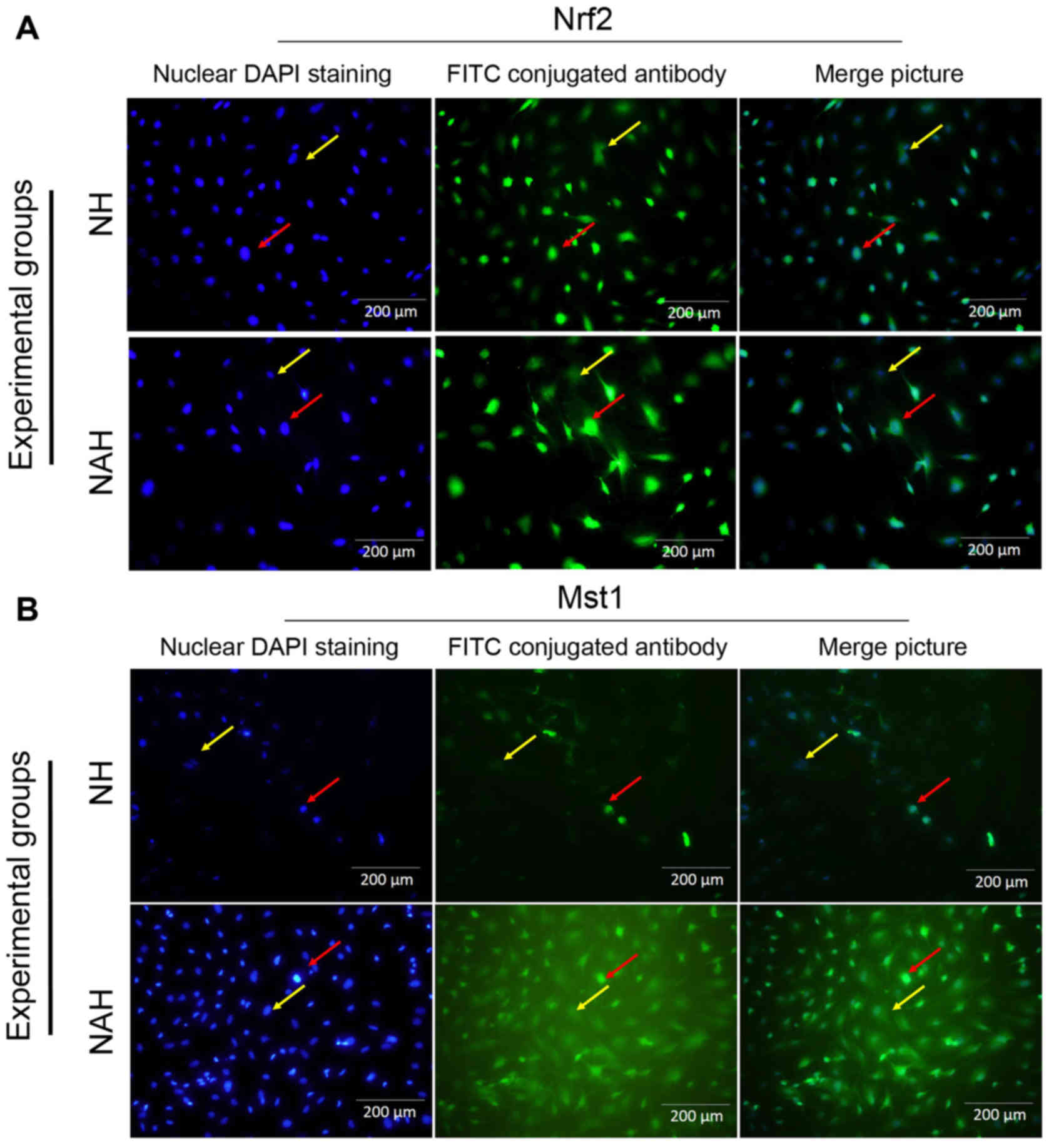
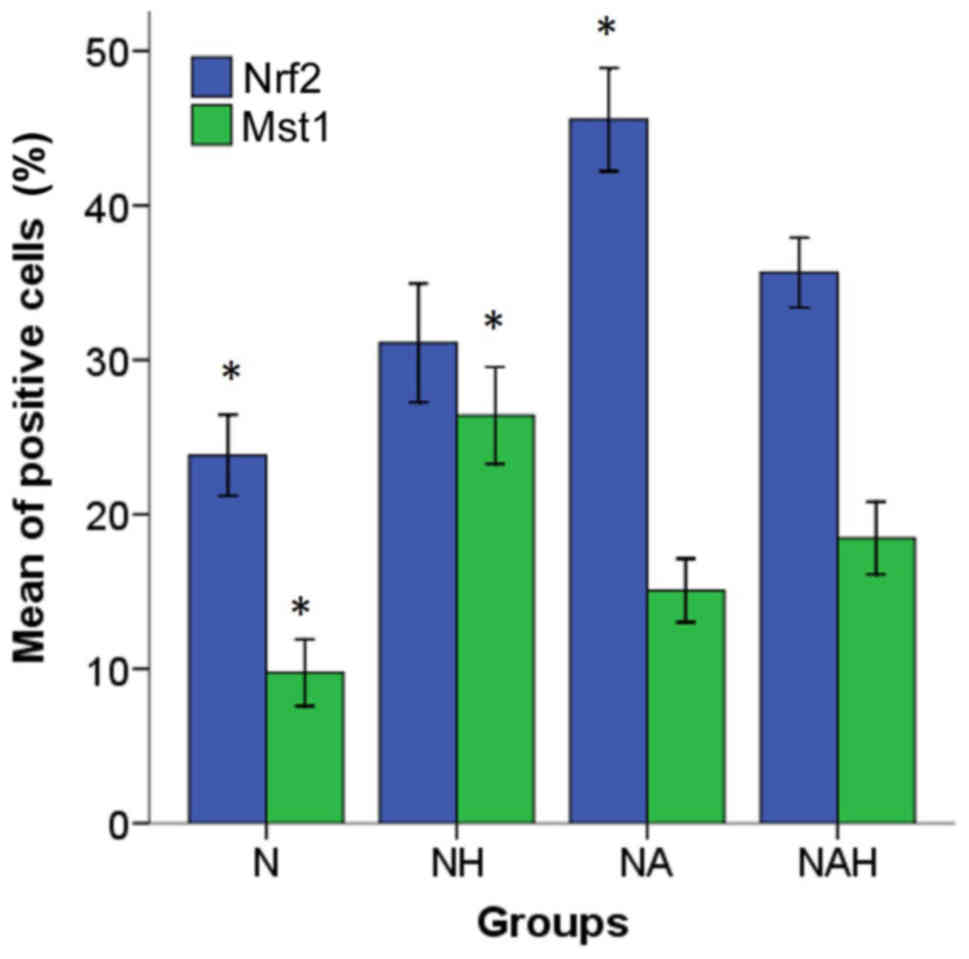
Mst1 and Nrf2 protein expression
To determine the neuroprotective effect of
adenosine, Mst1 and Nrf2 protein expression was detected by
immunocytochemistry (Figs. 4 and
5). The percentages of Nrf2 and Mst1
positive cells were calculated in five microscopic fields (Fig. 4A and B). The control group (N)
exhibited Mst1 and Nrf1 positive cells at rates of 9.75±1.08 and
23.82±1.31%, respectively. By contrast, a significantly increased
percentage of Mst1 positive cells (26.4±1.56%) was
identified in the NH group compared with the NAH group (P<0.05).
The percentage of Mst1 positive cells was significantly
decreased in the NAH group (18.46±1.17%) compared with NH group
(P<0.05). Additionally, the percentage of Nrf2 positive cells
was significantly increased in the NA group (45.56±1.67) compared
with the NH group (31.1±1.92; P<0.05; Fig. 5).
Discussion
The results of the present study indicate that
adenosine upregulates antiapoptotic Bcl-2 and Nrf2
and downregulates Mst1 in B-dNSCs, and thus protects the
cells against H2O2-induced apoptosis.
Additionally, adenosine may increase the mRNA expressions of
AA1R, as one of the adenosine receptors activated in NSCs.
The results further demonstrated that adenosine attenuated the
apoptotic and necrotic effects of H2O2 at the
cellular level. While adenosine is currently only used to treat
cardiac arrhythmias, research increasingly suggests various
combinations of novel clinical applications of this drug (24,25).
The current study reports a novel cytoprotective
mechanism of adenosine involving Nrf2 and
Bcl-2-mediated induction of oxidative stress-related
proteins. Oxidative damage is among the causes of neurodegenerative
diseases (26). Increased production
of cellular oxidants and defective protective mechanisms against
oxidants are two major factors in the development of oxidative
damage (21). However, physiological
concentrations of free radicals in cells are essential for normal
function and have key importance in the regulation of signaling
pathways (14) including those
associated with antiapoptotic transcription of genes and DNA repair
proteins (17). The main targets of
ROS during oxidative stress are considered to be DNA, RNA, proteins
and lipids (22). In apoptosis, a
series of events occurs systematically and repeatedly. The process
is regulated by interventional genes including caspases and
Fas-associated protein with death domain, and also molecular
systems including Bcl-2/Bcl-2-associated X protein and Fas/Fas
ligand (27). Therefore, interference
in antiapoptotic and proapoptotic gene expression may be used to
establish alterations in apoptosis (23).
Studies have indicated that adenosine may protect
stem cells against oxidative damage and thus reduce apoptosis in
these cells (28,29). The results of the current study
confirmed previous findings regarding the protective effect of
adenosine (30). The concept that
purines, including ATP, ADP and adenosine, serve as extracellular
signaling molecules was first suggested in 1929 by DeMaagd and
Philip (31). Depending on the
specific receptor activated, extracellular purines mediate
biological functions including transmission of nerve impulses,
muscle contraction, secretion from endocrine and exocrine glands
and inflammation (31). Zhai et
al (32) demonstrated that the
protein level of AA1R was significantly increased in an
intracerebral hemorrhage condition, while there was no significant
change in the protein levels of the other adenosine receptors.
Furthermore, activation of AA1R attenuates neuronal
apoptosis in cortical neurons (7).
The present results demonstrated that treatment with adenosine
during oxidative damage caused overexpression of the antiapoptotic
Bcl-2 and Nrf2 genes, as well as increased expression
of AA1R and decreased expression of proapoptotic
Mst1.
Mst1, also known as serine/threonine kinase
4, is a stress-activated, proapoptotic kinase that is involved in
the Hippo pathway (33). Despite
extensive studies, the mechanisms underlying the regulation of
apoptosis by Mst1 are not well understood, though
Mst1-induced caspase-3 cleavage may be involved (34). It has also been suggested that
increased expression of Mst1 initiates apoptosis through
activation of p53, but the molecular mechanisms are not fully
understood (35). The Nrf2
gene affects cell proliferation, cell growth and regulation of cell
metabolism via the phosphatidylinositol-3-kinase/Akt pathway
(36). Sarkar et al
demonstrated that Nrf2 enhanced the expression of
anti-apoptotic Bcl-2 (37).
Pingle et al reported that AA1R, protects against
human immunodeficiency virus type 1 toxicity by inhibiting nu clear
factor-κB and thereby reducing the expression of inducible nitric
oxide (NO) synthase and NO radicals and neuronal apoptosis
(38).
In addition, the use of AA1R agonist has been
reported to lead to upregulation of Bcl-2 and Nrf2
gene expression (39). Nrf2
expression also leads to increased expression of mitochondrial
transcription factor A, as a key regulator of antioxidant signaling
(40). The current results indicated
that Nrf2-mediated upregulation of antiapoptotic protein Bcl-2 may
lead to decreased apoptotic cell death and increased cell survival
in response to H2O2 exposure. Nrf2 is
a transcription factor that controls the expression of a variety of
antioxidant genes (41). A previous
study demonstrated that Nrf2 was retained in the cytoplasm
by the inhibitor kelch-like ECH-associated protein 1, which
functions as an adapter for cullin 3/ring-box 1-mediated
degradation of Nrf2 (42). The
expression of the mitochondrial protein uncoupling protein 3 is
driven by the Nrf2 transcription factor, which decreases ROS
production and prevents cell death (43). Additionally, Nrf2 may increase
the expression of certain antioxidant enzymes, including heme
oxygenase-1, NAD(P)H:quinone oxidoreductase 1 and manganese
superoxide dismutase (44). Taken
together, the Nrf2 transcription factor may control the
expression of a number of protective genes in response to oxidative
stress (45). Thus, according to
studies on adenosine, manipulating the adenosine system as a novel
strategy for the management of brain disorders may have promise
(31). However, to achieve successful
clinical application in the treatment of brain disorders, further
studies are required to evaluate the use of adenosine and its
receptor agonists as neuroprotective drugs.
In conclusion, the results of the current study
demonstrated that adenosine, as a receptor-specific agonist of
AA1R, protected NSCs derived from BMSCs against oxidative
damage. Adenosine can cross the blood-brain barrier and therefore
may be considered as a suitable drug for the treatment of diseases
of the nervous system caused by oxidative stress.
Acknowledgements
The authors are grateful to Dr Soghrat Faghihzadeh
at the Department of Biostatistics and Epidemiology of Zanjan
University of Medical Sciences (Zanjan, Iran), who provided
valuable feedback on the manuscript.
Funding
The current study was funded by Zanjan University of
Medical Sciences (Zanjan, Iran; grant no. A-12-973-2).
Availability of data and materials
All primary data are archived at the Zanjan
University of Medical Sciences and available on request.
Authors' contributions
MG performed the experiments. IJA acted as study
advisor and was responsible for technical aspects in the
immunocytochemistry. AT is the co-corresponding author, analyzed
data regarding gene expression, was responsible for primer design
and supervised the project. AA designed and directed the project,
supervised the project, monitored experiments, is the
co-corresponding author and wrote the manuscript. All authors
discussed the results and contributed to the final manuscript.
Ethics approval and consent to
participate
All experimental protocols were approved by the
Zanjan University of Medical Sciences Ethics Committee.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yeh LK, Liu CY, Chien CL, Converse RL, Kao
WW, Chen MS, Hu FR, Hsieh FJ and Wang IJ: Molecular analysis and
characterization of zebrafish keratocan (zKera) gene. J Biol Chem.
283:506–517. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen X, Guo C and Kong J: Oxidative stress
in neurodegenerative diseases. Neural Regen Res. 7:376–385.
2012.PubMed/NCBI
|
|
3
|
Uttara B, Singh AV, Zamboni P and Mahajan
RT: Oxidative stress and neurodegenerative diseases: A review of
upstream and downstream antioxidant therapeutic options. Curr
Neuropharmacol. 7:65–74. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu Z, Liu Y, Gao R, Li H, Dunn T, Wu P,
Smith RG, Sarkar PS and Fang X: Ethanol suppresses PGC-1α
expression by interfering with the cAMP-CREB pathway in neuronal
cells. PLoS One. 9:e1042472014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cunha RA: Adenosine as a neuromodulator
and as a homeostatic regulator in the nervous system: Different
roles, different sources and different receptors. Neurochem Int.
38:107–125. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xu L, Xu H, Cao Y, Yang P, Feng Y, Tang Y,
Yuan S and Ming J: Validation of reference genes for quantitative
real-time PCR during bicolor tepal development in asiatic hybrid
lilies (Lilium spp.). Front Plant Sci. 8:6692017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gorman AM: Neuronal cell death in
neurodegenerative diseases: Recurring themes around protein
handling. J Cell Mol Med. 12(6A): 2263–2280. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gibson RM: Does apoptosis have a role in
neurodegeneration? BMJ. 322:1539–1540. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mattson MP: Apoptosis in neurodegenerative
disorders. Nat Rev Mol Cell Biol. 1:120–129. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Abdanipour A, Tiraihi T and
Mirnajafi-Zadeh J: Improvement of the pilocarpine epilepsy model in
rat using bone marrow stromal cell therapy. Neurol Res. 33:625–632.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Racine RJ: Modification of seizure
activity by electrical stimulation. II. Motor seizure.
Electroencephalogr Clin Neurophysiol. 32:281–294. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Collak FK, Yagiz K, Luthringer DJ, Erkaya
B and Cinar B: Threonine-120 phosphorylation regulated by
phosphoinositide-3-kinase/Akt and mammalian target of rapamycin
pathway signaling limits the antitumor activity of mammalian
sterile 20-like kinase 1. J Biol Chem. 287:23698–23709. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ardestani A, Paroni F, Azizi Z, Kaur S,
Khobragade V, Yuan T, Frogne T, Tao W, Oberholzer J, Pattou F, et
al: MST1 is a key regulator of beta cell apoptosis and dysfunction
in diabetes. Nat Med. 20:385–397. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ura S, Masuyama N, Graves JD and Gotoh Y:
MST1-JNK promotes apoptosis via caspase-dependent and independent
pathways. Genes Cells. 6:519–530. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Strom J, Xu B, Tian X and Chen QM: Nrf2
protects mitochondrial decay by oxidative stress. FASEB J.
30:66–80. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Murata H, Takamatsu H, Liu S, Kataoka K,
Huh NH and Sakaguchi M: NRF2 regulates PINK1 expression under
oxidative stress conditions. PLoS One. 10:e01424382015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhong J and Li L: Skin-derived precursors
against UVB-induced apoptosis via Bcl-2 and Nrf2 upregulation.
Biomed Res Int. 2016:68947432016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Maleki M, Ghanbarvand F, Behvarz Reza M,
Ejtemaei M and Ghadirkhomi E: Comparison of mesenchymal stem cell
markers in multiple human adult stem cells. Int J Stem Cells.
7:118–126. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang Q, Mu J, Li Q, Li A, Zeng Z, Yang J,
Zhang X, Tang J and Xie P: A simple and efficient method for
deriving neurospheres from bone marrow stromal cells. Biochem
Biophys Res Commun. 372:520–524. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Suzuki S, Namiki J, Shibata S, Mastuzaki Y
and Okano H: The neural stem/progenitor cell marker nestin is
expressed in proliferative endothelial cells, but not in mature
vasculature. J Histochem Cytochem. 58:721–730. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Albrecht-Küpper BE, Leineweber K and Nell
PG: Partial adenosine A1 receptor agonists for cardiovascular
therapies. Purinergic Signal. 8 Suppl 1:91–99. 2012. View Article : Google Scholar
|
|
22
|
Abbasnia K, Ghanbari A, Abedian M,
Ghanbari A, Sharififar S and Azari H: The effects of repetitive
transcranial magnetic stimulation on proliferation and
differentiation of neural stem cells. Anat Cell Biol. 48:104–113.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee JH, Cheon YH, Woo RS, Song DY, Moon C
and Baik TK: Evidence of early involvement of apoptosis inducing
factor-induced neuronal death in Alzheimer brain. Anat Cell Biol.
45:26–37. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mori M, Nishizaki T and Okada Y:
Protective effect of adenosine on the anoxic damage of hippocampal
slice. Neuroscience. 46:301–307. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cunha RA: Neuroprotection by adenosine in
the brain: From A(1) receptor activation to A (2A) receptor
blockade. Purinergic Signal. 1:111–134. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Migita H, Kominami K, Higashida M,
Maruyama R, Tuchida N, McDonald F, Shimada F and Sakurada K:
Activation of adenosine A1 receptor-induced neural stem cell
proliferation via MEK/ERK and Akt signaling pathways. J Neurosci
Res. 86:2820–2828. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huang NK: Adenosine A2A receptors regulate
oxidative stress formation in rat pheochromocytoma PC12 cells
during serum deprivation. Neurosci Lett. 350:127–131. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ramkumar V, Hallam DM and Nie Z:
Adenosine, oxidative stress and cytoprotection. Jpn J Pharmacol.
86:265–274. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Vitolo OV, Ciotti MT, Galli C, Borsello T
and Calissano P: Adenosine and ADP prevent apoptosis in cultured
rat cerebellar granule cells. Brain Res. 809:297–301. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
DeMaagd G and Philip A: Parkinson's
disease and its management: Part 1: Disease entity, risk factors,
pathophysiology, clinical presentation, and diagnosis. P T.
40:504–532. 2015.PubMed/NCBI
|
|
32
|
Zhai W, Chen D, Shen H, Chen Z, Li H, Yu Z
and Chen G: A1 adenosine receptor attenuates intracerebral
hemorrhage-induced secondary brain injury in rats by activating the
P38-MAPKAP2-Hsp27 pathway. Mol Brain. 9:662016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Meng Z, Moroishi T and Guan KL: Mechanisms
of Hippo pathway regulation. Genes Dev. 30:1–17. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wrann CD, White JP, Salogiannnis J,
Laznik-Bogoslavski D, Wu J, Ma D, Lin JD, Greenberg ME and
Spiegelman BM: Exercise induces hippocampal BDNF through a
PGC-1α/FNDC5 pathway. Cell Metab. 18:649–659. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Youdim MB: The path from anti Parkinson
drug selegiline and rasagiline to multifunctional neuroprotective
anti Alzheimer drugs ladostigil and m30. Curr Alzheimer Res.
3:541–550. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Alexander GE: Biology of Parkinson's
disease: Pathogenesis and pathophysiology of a multisystem
neurodegenerative disorder. Dialogues Clin Neurosci. 6:259–280.
2004.PubMed/NCBI
|
|
37
|
Sarkar S, Raymick J and Imam S:
Neuroprotective and therapeutic strategies against Parkinson's
disease: Recent Perspectives. Int J Mol Sci. 17:172016. View Article : Google Scholar
|
|
38
|
Pingle SC, Jajoo S, Mukherjea D, Sniderhan
LF, Jhaveri KA, Marcuzzi A, Rybak LP, Maggirwar SB and Ramkumar V:
Activation of the adenosine A1 receptor inhibits HIV-1 tat-induced
apoptosis by reducing nuclear factor-kappaB activation and
inducible nitric-oxide synthase. Mol Pharmacol. 72:856–867. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Han J, Talorete TP, Yamada P and Isoda H:
Anti-proliferative and apoptotic effects of oleuropein and
hydroxytyrosol on human breast cancer MCF-7 cells. Cytotechnology.
59:45–53. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang Z, Zhou S, Jiang X, Wang YH, Li F,
Wang YG, Zheng Y and Cai L: The role of the Nrf2/Keap1 pathway in
obesity and metabolic syndrome. Rev Endocr Metab Disord. 16:35–45.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ishikawa T: Genetic polymorphism in the
NRF2 gene as a prognosis marker for cancer chemotherapy. Front
Genet. 5:3832014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Nuydens R, Dispersyn G, Van Den Keiboom G,
de Jong M, Connors R, Ramaekers F, Borgers M and Geerts H: Bcl-2
protects against apoptosis-related microtubule alterations in
neuronal cells. Apoptosis. 5:43–51. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Anedda A, López-Bernardo E, Acosta-Iborra
B, Suleiman Saadeh M, Landázuri MO and Cadenas S: The transcription
factor Nrf2 promotes survival by enhancing the expression of
uncoupling protein 3 under conditions of oxidative stress. Free
Radic Biol Med. 61:395–407. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Jeong YH, Park JS, Kim DH and Kim HS:
Lonchocarpine increases Nrf2/ARE-mediated antioxidant enzyme
expression by modulating AMPK and MAPK signaling in brain
astrocytes. Biomol Ther (Seoul). 24:581–588. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Birben E, Sahiner UM, Sackesen C, Erzurum
S and Kalayci O: Oxidative stress and antioxidant defense. World
Allergy Organ J. 5:9–19. 2012. View Article : Google Scholar : PubMed/NCBI
|