Introduction
Advanced glycation end-products (AGEs) are a diverse
group of oxidant compounds with pathogenic significance in diabetes
and in several other chronic diseases (for reviews see refs. 1,2).
AGEs are generated by non-enzymatic reactions between reducing
sugars and free amino groups of proteins, lipids and nucleic acids
(3). Generation of AGEs has been
mainly studied in the context of food preparation, and dietary
intake of AGE forms a part of normal metabolism (4). However, when high levels of AGEs
accumulate in tissues or the blood stream, they cause oxidative
stress and promote inflammation by binding to cell surface
receptors or cross-linking with body proteins, altering their
structure and function (for review see ref. 5).
Peritoneal dialysis (PD) is applied in patients with
end-stage kidney disease to increase preservation of residual renal
function. Despite PD treatment, prevalence of cardiovascular events
is considerably increased in those patients, and they are the
leading cause of mortality (6).
Furthermore, inflammation and the resulting cumulative peritoneal
membrane injury contribute to the progression of chronic kidney
disease (7,8). Glucose degradation products (GDPs) in
the PD fluids have been identified as the major initiators of
inflammation and linked to the failure of ultrafiltration and
degradation of the peritoneal membrane following PD (9).
Previous studies have identified 3-deoxyglucosone
(3-DG), 3,4-dideoxyglucosone-3-ene (3,4-DGE) and methylglyoxal (MG)
as the most bioreactive GDPs in PD fluids (10,11).
Acting as reactive carbonyl compounds, these metabolites cause
protein modifications and ultimately the formation of AGEs
(12), which contribute to functional
impairment of human peritoneal mesothelial cells (HPMC) via AGE-AGE
specific receptor (AGE-RAGE) interaction (13). Indeed, the use of low-GDP PD fluids
decreased markers of inflammation: both local interleukin 6 levels
in dialysates (14) and systemic
serum C-reactive protein levels (15). While local damaging effects of GDPs on
peritoneal mesothelial cells are well studied, little is known of
the systemic effects (16).
Damaging effects of AGEs are physiologically
counteracted with various salvage pathways, among which
glyoxalase-1 (GLO1) is of outstanding importance. GLO1 detoxifies
MG, thus reducing the formation of AGEs (17–19).
Suppression of MG-mediated glycation by GLO1 is particularly
important in diabetes and uremia, where the blood plasma
concentration of MG is increased (20). Morcos et al (21) previously demonstrated that
overexpression of glod-4, the Caenorhabditis elegans (C.
elegans) ortholog of GLO1, decreased mitochondrial protein
modification and extended lifespan. Therefore, C. elegans
overexpressing glod-4 were used as a model organism to investigate
the effect of the degradation product MG on AGE formation (21).
In the present study, the systemic effects of the
glucose and GDP contents of PD fluids were analysed in the nematode
C. elegans, by using lifespan, neuronal integrity and
neuronal function as surrogate parameters for the damaging effects
of reactive metabolites. C. elegans is a well-established
model in the study of neurodegeneration as well as in aging
research, benefitting from an accessible and characterized nervous
system and a lifespan short enough to screen for ageing-related
degenerative damages (22).
More specifically, the current study focused on MG,
one of the major GDPs generated during heat sterilization of
conventional fluids used for PD (23). Previous studies have demonstrated that
increased GLO1 activity, acting as a detoxification system for MG,
has beneficial effects on GDP-mediated toxicity in HPMCs (24). To the best of our knowledge, the
present study is the first report using C. elegans with the
aim of demonstrating the protective capability of the GLO1
detoxifying pathway against PD fluid-induced damages and of
indicating its relevance as a potential therapeutic target.
Materials and methods
C. elegans maintenance
The wild-type strain N2 and the pan-neuronal green
fluorescent protein (GFP)-expressing strain NW1229 were provided by
the Caenorhabditis Genetics Center (College of Biological Sciences,
Saint Paul, MN, USA), which is funded by the National Institutes of
Health Office of Research Infrastructure Programs (program no. P40
OD010440). In NW1229, GFP is expressed as a rgef-1 (sequence
name, F25B3.3) reporter fusion in post-mitotic neurons throughout
the nervous system (pan-neuronally), beginning at the late comma
stage of embryo development (25).
The glod-4/GLO1 transgenic C. elegans (strain VH725,
GLO1-Tg) was generated by our group and has been described
previously (21).
Age-synchronized embryos were hatched, grown and
cultivated throughout their lifespan on nematode growth medium
(NGM) agar (A1296; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
in 60 mm Petri plates (25 animals per plate) and maintained at 20°C
in a temperature-controlled incubator at ambient air humidity
(75%). As a food source, 100 µl of a standardized Escherichia
coli OP50 (Caenorhabditis Genetics Center, Saint Paul, MN, USA)
overnight culture (optical density, 1.5) were added to the surface
of each NGM plate.
PD fluid treatment
PD fluids contained either minimal concentrations of
GDPs (low-GDP fluid, Gambrosol trio 10® double-chamber
PD fluid; Baxter International, Inc., Deerfield, IL, USA) or
conventional GDP concentrations (high-GDP fluid,
Gambrosol® single-chamber PD fluid; Baxter
International, Inc.). Low- and high-GDP fluids differed with
respect to pH (6.6 vs. 5.5) and concentrations of GDPs: 3-DG (12.3
vs. 118 µmol/l), 3,4-DGE (below detection limit vs. 10 µmol/l) and
MG (<2.8 vs. 5.3 µmol/l), determined as described previously
(26).
Glucose concentrations were adjusted to 1.5 or 4%
glucose by addition of GDP-free glucose stock solution to obtain
low-GDP PD fluid containing 1.5% (L-1.5) or 4% (L-4) glucose, or
high-GDP PD fluid containing 1.5% (H-1.5) or 4% (H-4) glucose. The
samples were sterile filtered (0.02 µm; Sarstedt AG & Co.,
Nümbrecht, Germany) and incubated without any preservatives at 37°C
for 7 days to ensure GDP formation.
For PD fluid treatment, the food source was
supplemented with 100 µl of L-1.5, L-4, H-1.5 or H-4. Feeding
procedures were repeated every 72 h for determination of lifespan
or daily for evaluation of neuronal integrity and function. For
control treatments, standard maintenance medium was used (M9
buffer, containing 22 mM KH2PO4, 42 mM
Na2HPO4, 86 mM NaCl, adjusted to pH 7.0,
autoclaved and supplemented with 1 mM MgSO4; all
reagents from Sigma-Aldrich; Merck KGaA).
Determination of lifespan
To evaluate effects on lifespan, Kaplan-Meier
survival curves were calculated. To prevent progeny production, NGM
plates containing 300 µg/ml 5-fluorodesoxyuridine (F0503;
Sigma-Aldrich; Merck KGaA) were used. For lifespan analysis, the
pre-fertile period of adulthood was defined as t=0. Animals were
regarded to have succumbed if they did not move following a
repeated mechanical stimulus. Animals were excluded if they had
crawled away from the plate or contained internally hatched larvae.
Experiments were performed in triplicates with 50 animals each.
Evaluation of neuronal integrity and
neuronal functions
Neuronal integrity and neuronal functions of C.
elegans were used as accessible surrogate parameters to
quantify the extent of glucose and GDP-induced damages (22). Neuronal integrity was assessed by
quantifying fluorescence of the pan-neuronal GFP-expressing C.
elegans (strain NW1229), as described previously (27–29).
Briefly, GFP fluorescence of animals was recorded (BioTek FLx800)
and quantified (Gen5 v1 software; both from BioTek Instruments,
Inc., Winooski, VT, USA). Experiments were performed in
triplicates. To determine the impact on neuronal function, animals
were recorded (Moticam 1000; Beyersdörfer GmbH, Mandelbachtal,
Germany) and different parameters of animal motility were analyzed
as described previously (30). In
brief, a worm tracking software program (WormTracker 4.0; Thomas
Bornhaupt, Neustadt adW., Germany) was used to split the recorded
videos into single frames and to locate the head and tail positions
of individual animals. These data were then used to calculate the
head and tail velocities in relation to the plate surface
(absolute) as well as in relation to the center of the body
(relative), resulting in values for absolute head motility
(mm/sec), relative head motility (mm/sec), absolute tail motility
(mm/sec) and relative tail motility (mm/sec). In contrast to a
simple description of whole nematode motility, these values are
more specific parameters of anterior and posterior locomotor
ability (31).
Statistical analysis
StatView 5.0 software (SAS Institute, Inc., Cary,
NC, USA) was used for statistical analysis. Log-rank tests were
used to analyze differences between Kaplan-Meier survival curves.
Data on neuronal integrity and function are expressed as the mean ±
standard error of the mean. Student's t-tests were used to analyze
differences between values of two groups. Differences with
P<0.05 were considered statistically significant.
Results
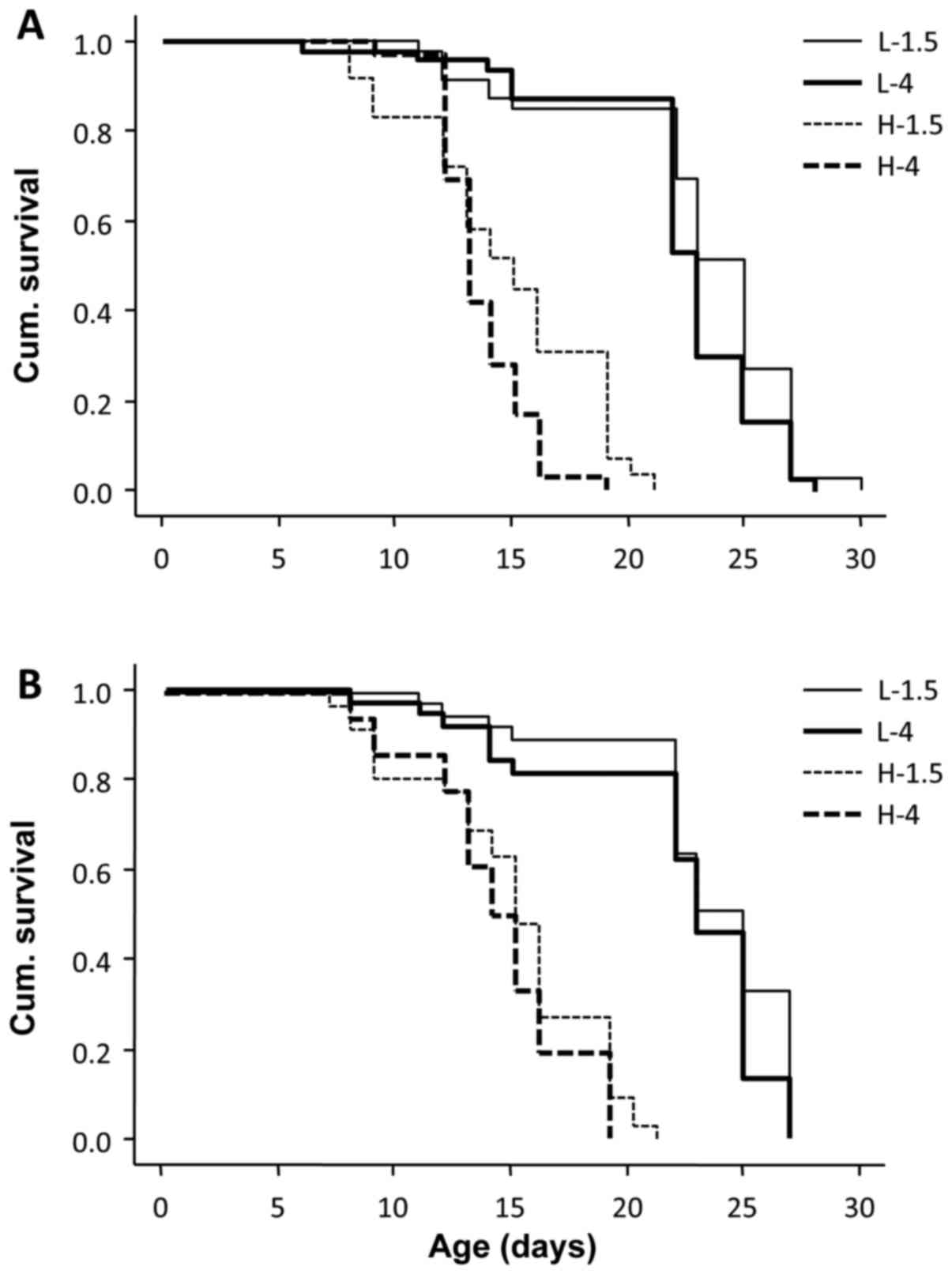
Lifespan is inversely affected by the
glucose/GDP content of PD fluids and GLO-1
To evaluate the effects of glucose and GDPs on the
lifespan of C. elegans, four treatments groups were
established: Low-GDP, low glucose (1.5%); low-GDP, high glucose
(4%); high-GDP, low glucose; and high-GDP, high-glucose. In
preliminary experiments in the current study, it was confirmed that
low-GDP, low glucose PD fluid (L-1.5) did not significantly affect
lifespan when compared with animals treated with standard
maintenance medium (M9 buffer; P>0.05; data not shown). The
higher concentration of GDPs significantly reduced the maximum
lifespan of C. elegans, under both low-glucose (from 30 to
21 days, P<0.001) and high-glucose conditions (from 28 to 19
days, P<0.001). Similarly, increased glucose reduced the maximum
lifespan further, under both low-GDP (from 30 to 28 days;
P<0.01) and high-GDP conditions (from 21 to 19 days; P<0.05;
Fig. 1A).
To assess the effect of endogenous GLO1, animals
overexpressing this enzyme were used, and again lifespan was
measured following culture in high or low GDP, and high or low
glucose concentrations. The higher concentrations of GDPs reduced
the maximum lifespan of GLO1 transgenic C. elegans to a
similar extent as observed in wild-type animals (from 27 to 21 days
under low-glucose conditions, P<0.001; and from 27 to 19 days
under high-glucose conditions, P<0.001). However, in the low-GDP
group, overexpression of GLO1 abolished the effect of high glucose
(P>0.05; Fig. 1B).
Reduction of neuronal integrity by PD
fluids is prevented by GLO-1
To investigate the effect of glucose and GDP content
on neuronal integrity, C. elegans with neuronal GFP
expression were used. In this setting, quantification of GFP
fluorescence allows assessment of neuronal integrity as described
previously (29). In preliminary
experiments in the current study, it was confirmed that standard M9
buffer did not significantly affect neuronal fluorescence over the
study period of 7 days (P>0.05; data not shown). Exposure of
wild-type animals to PD fluids led to a decrease in signal
intensity of neuronal GFP at all GDP and glucose concentrations
(P<0.05), indicative of neuronal damage. Fluorescence reduction
ranged from 34% in low-GDP fluids to 41% in high-GDP fluids. The
presence of additional glucose had no further effect (Control;
Fig. 2A). GLO1 overexpression
protected animals from neurostructural damage in the low-GDP group,
but not in the high-GDP group (GLO1-Tg; Fig. 2A). Representative images of animals
without neuronal damage and with reduced neuronal integrity are
presented in Fig. 2B and C,
respectively.
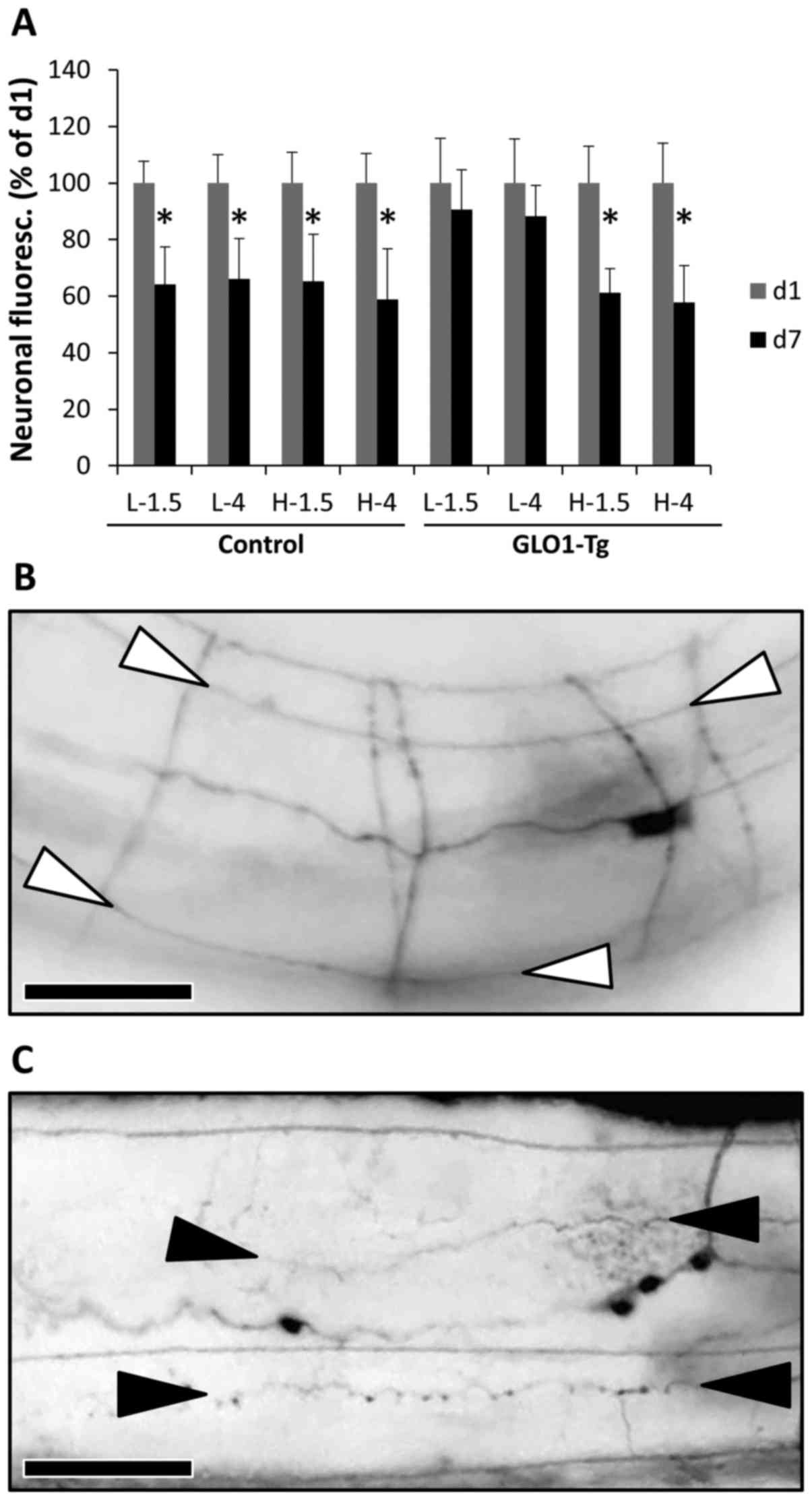 | Figure 2.Neuronal integrity. (A) Neuronal
integrity was assessed by fluorescence measurement of pan-neuronal
green fluorescent protein-expressing Caenorhabditis elegans,
either without (Control) or with GLO-1 transgenic overexpression
(GLO1-Tg), exposed over a period of 1 day (d1, grey columns) or 7
days (d7, black columns) to low-GDP PD fluid containing 1.5%
(L-1.5) or 4% glucose (L-4), or high-GDP PD fluid containing 1.5%
(H-1.5) or 4% glucose (H-4). Fluorescence was reduced as follows:
by 36% (Control, L-1.5), 34% (Control, L-4), 35% (Control, H-1.5),
41% (Control, H-4), 9% (GLO1-Tg, L-1.5), 12% (GLO1-Tg, L-4), 39%
(GLO1-Tg, H-1.5) and 42% (GLO1-Tg, H-4). Results are the means with
standard error of the mean of three independent experiments.
*P<0.05 for d1 vs. d7 under each treatment condition (calculated
using the unpaired Student's t-test). (B and C) Representative
images of animals without neuronal damage and with reduced neuronal
integrity. Shown are corresponding sections of the central body
region from (B) animals with intact nerve cords (ends are marked
with white arrowheads), and (C) animals with damaged nerve cords,
indicated by beading-like lesions (ends are marked by black
arrowheads). Scale bars, 0.03 mm (magnification, ×50). PD,
peritoneal dialysis; GLO-1, glyoxalase-1; GDP, glucose degradation
product. |
Neuronal function
To correlate PD fluid-induced defects in neuronal
structure with impairment of neuronal functions, different
parameters of animal motility were quantified. As GLO1
overexpression was indicated to prevent neuronal damages only under
low-GDP conditions, these experiments were performed under low-GDP
conditions and low or high glucose only.
Firstly, movement of the head, relative to the body
(relative head motility) and to the surface (absolute head
motility), was determined, since it is the most descriptive
parameter for overall animal motility (30,31). No
significant changes were observed in the relative and absolute head
motilities of a control group cultivated in GDP-free medium over a
period of 7 days (C-4, M9 buffer containing 4% glucose) (Fig. 3A and B). Exposure of wild-type C.
elegans to low-GDP fluids at 1.5% glucose (L-1.5) did not
reduce relative and absolute head motilities significantly
(Fig. 3A and B). When exposed to
low-GDP fluids with 4% glucose (L-4), a significant decline in mean
relative head motility, from 0.146 to 0.061 mm/sec (58.5%;
P<0.01) was detected (Fig. 3A).
Similarly, mean absolute head motility was decreased from 0.119 to
0.051 mm/sec (56.7%; P<0.01; Fig.
3B). Therefore, a rise in glucose content to 4% promoted
GDP-induced neuronal damages to a degree not only affecting
structural integrity, but also functional parameters. Similar to
their influence on neuronal structure, glucose and GDPs appeared to
contribute concertedly to the detrimental effects of PD fluids on
neuronal function, since control groups treated with GDP-free
medium at 4% glucose did not experience functional impairment.
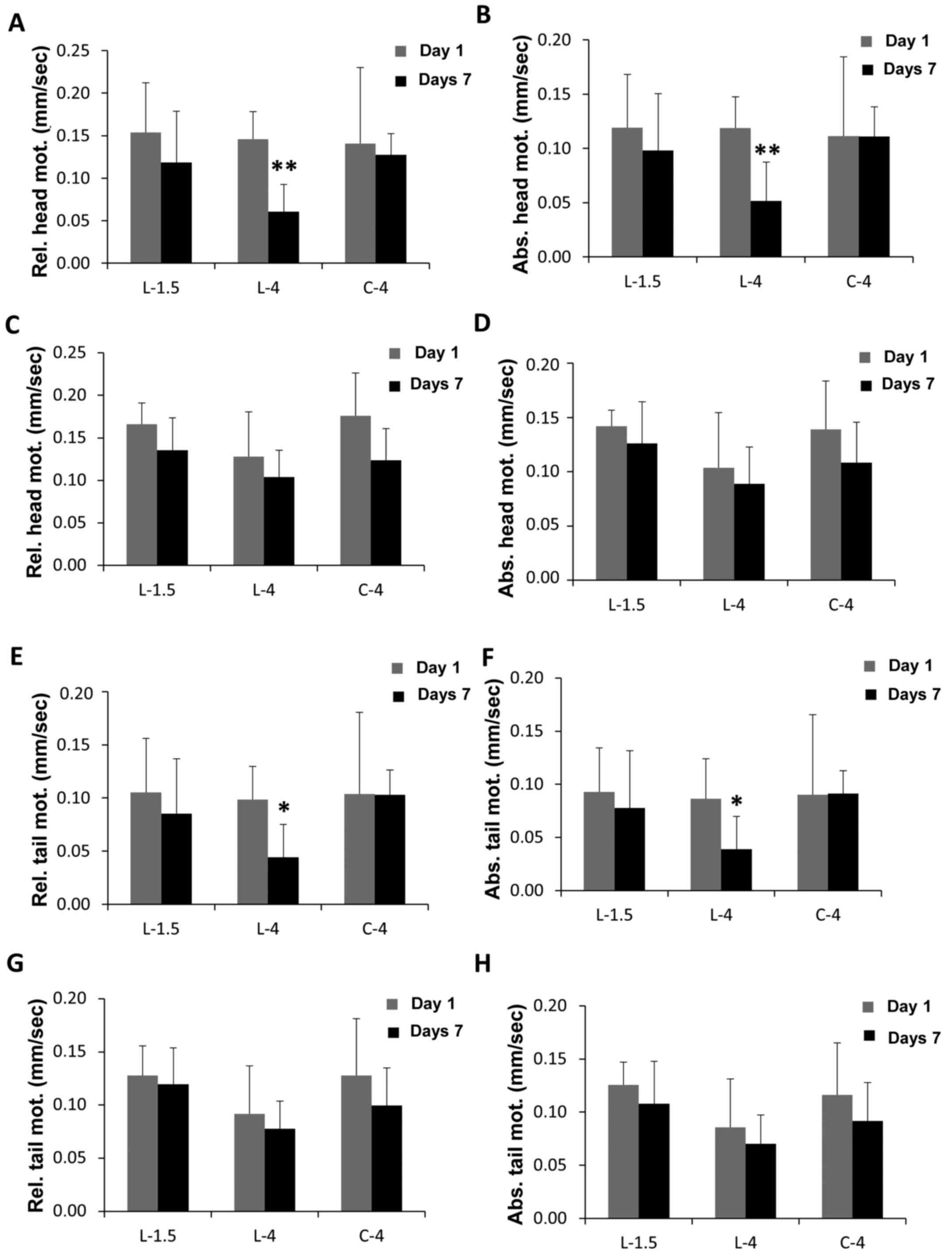 | Figure 3.Neuronal function. (A-H) Neuronal
function was measured in (A, B, E and F) wild-type and (C, D, G and
H) GLO-1-overexpressing animals treated for 1 day (grey columns) or
7 days (black columns) with low-GDP PD fluid containing 1.5%
(L-1.5) or 4% glucose (L-4), or GDP-free control medium (M9 buffer
containing 4% glucose, C-4), by quantification of (A and C)
relative and (B and D) absolute head motility, and (E and G)
relative and (F and H) absolute tail motility. Motility was reduced
as follows: by 23.1% (A, L-1.5), 58.5% (A, L-4), 9.1% (A, C-4),
17.4% (B, L-1.5), 56.7% (B, L-4), 0.3% (B, C-4), 18.3% (C, L-1.5),
18.5% (C, L-4), 29.7% (C, C-4), 11.3% (D, L-1.5), 14.3% (D, L-4),
22.1% (D, C-4), 18.9% (E, L-1.5), 55.1% (E, L-4), 0.9% (E, C-4),
16.3% (F, L-1.5), 55.0% (F, L-4), −1.0% (F, C-4), 6.7% (G, L-1.5),
15.3% (G, L-4), 22.1% (G, C-4), 13.9% (H, L-1.5), 18.0% (H, L-4),
and 21.1% (H, C-4). Results are shown as mean velocities with
standard error of the mean of three independent experiments, each
with 10 animals per group. *P<0.05 and **P<0.01 for d1 vs. d7
under each treatment condition (calculated using the unpaired
Student's t-test). PD, peritoneal dialysis; GLO-1, glyoxalase-1;
GDP, glucose degradation product. |
GLO1 overexpression protected animals from glucose
and GDP-induced neurofunctional damage (Fig. 3C and D). This confirmed the
neuroprotective effect of GLO1 against damage induced by low-GDP PD
fluids containing up to 4% glucose. Again, no significant changes
were measured in the head motilities of the control groups (C-4,
P>0.05; Fig. 3C and D).
Next, tail motility, both relative to the body
(relative tail motility) and absolute to the surface (absolute tail
motility), was determined. Low-GDP fluids with 1.5% glucose did not
affect relative and absolute tail motilities over a period of 7
days (Fig. 3E and F). However,
low-GDP fluid with 4% glucose significantly reduced the mean
relative tail motility from 0.098 to 0.044 mm/sec motility (55.1%;
P<0.05; Fig. 3E), and mean
absolute tail motility from 0.086 to 0.039 mm/sec (55.0%;
P<0.05; Fig. 3F). In the control
group, following exposure to GDP-free medium with 4% glucose, no
significant changes were observed in the relative and absolute tail
motilities (Fig. 3E and F). The
protective capacity of GLO1 overexpression against neurofunctional
damage induced by low-GDP PD fluids containing up to 4% glucose was
also confirmed by tail motility: motility at 1.5 and 4% glucose was
not reduced significantly in these animals (Fig. 3G and H).
Thus, a second neurofunctional parameter of tail
motility appeared to be affected by GDP-induced damages, resulting
from PD fluids containing an elevated glucose level of 4%.
Confirmed by the observations regarding neuronal structure and head
motilities, both glucose and GDP content in PD fluids affected
neuronal functions in C. elegans.
Discussion
PD fluids contain glucose at different
concentrations, dependent on the intended ultrafiltration rate.
Breakdown of glucose gives rise to the formation of glucose
degradation products (GDPs), a mechanism accelerated during the
heat sterilization process and particularly in a low-pH environment
(32). Single-chamber PD fluids,
undergoing heat sterilization in the presence of glucose,
consequently contain high concentrations of GDPs. The high-GDP
fluid used in the current study contained 5.3 µmol/l MG at a pH of
5.5 (26). PD fluids with high GDP
content are considered to be less biocompatible, as indicated by
their inhibitory effect on cell growth (10). In order to reduce GDP content,
double-chamber PD fluids have been implemented where glucose is
added only following the heat sterilization process to limit GDP
formation (16). The low-GDP fluid
used in the current study contained lower MG concentrations
(<2.8 µmol/l) at a higher pH (6.6) (26). Additional approaches to decrease GDP
levels of PD fluids, besides reduction of glucose content and
adjustment of pH, include the use of filter sterilization (33).
3-DG, 3,4-DGE and MG have been previously identified
as the most bioreactive GDPs formed in PD fluids (10,11), and
lead to formation of AGEs, which indirectly mediates GDP toxicity
via RAGE activation (13,34). GDP toxicity correlates with functional
and morphological changes in peritoneal membrane integrity, thus
degrading ultrafiltration quality and ultimately limiting use of
the peritoneal membrane as a dialysis substrate membrane (33).
Previous studies have not only illustrated that GDPs
affect the peritoneal membrane, but also depicted the consequences
on systemic circulation. It is apparent that systemic circulation
of GDPs promotes chronic kidney disease, which is mediated via
AGE-RAGE interaction (35). In
vivo studies have indicated resorption of GDPs from the
peritoneal cavity, accompanied by an increase in plasma AGE
compounds (26). Rapid GDP adsorption
was confirmed in a later study describing the association of
exposure to high- and low-GDP fluids with plasma AGE levels
(16). GDP-dependent systemic
increase of AGE formation has been causally linked with
inflammation, neoangiogenesis and fibrosis via AGE-RAGE
interaction, since RAGE-deficient animals are protected (13).
The aim of the current study was to evaluate the
systemic effect of GDP toxicity, beyond its direct impact on the
peritoneal membrane. For this purpose the study used C.
elegans, a widely acknowledged model organism used for
investigation of the effects of toxic metabolites (36), such as AGEs formed in the presence of
reactive α-oxoaldehydes, mainly carboxymethyllysine (CML),
carboxyethyllysine (CEL) and imidazolones (21), as well as in toxicity studies of the
effects of pharmaceutically used drugs, such as immunosuppressants
(37), on lifespan and neuronal
function (38–43). Despite the lack of a distinct renal
system, basic pathophysiological processes observed in C.
elegans may also be applicable to humans, since this animal
features a 70% homology to human genes (37,44).
Previous studies have demonstrated neurotoxic
effects of AGEs (45) and oxidative
stress (46). Morcos et al
(21) demonstrated in C.
elegans that AGE-induced mitochondrial modifications increased
levels of oxidative stress. The accumulation of AGEs, including CML
and CEL, accounted for modification of various key proteins, thus
inducing age-related damage on a molecular level.
In the current study, it was demonstrated in C.
elegans that reduction of lifespan and neuronal integrity was
not only caused by increased levels of glucose but also by elevated
GDP content in PD fluids. Therefore, both glucose and GDPs
contained in PD fluids may contribute to AGE formation and
accelerate aging via RAGE-mediated pathways.
Notably, protection of lifespan and neuronal
integrity by GLO1 overexpression was restricted to a low-GDP
environment. This suggests that detoxification of MG by GLO1 is not
sufficient to prevent the deleterious effects of PD fluids
containing high levels of various GDPs. The failure of GLO1
overexpression to exert significant beneficial effects in a
high-GDP environment may also be due to the fact that GLO1 activity
is dependent on the presence of glutathione as a co-factor
(47). Previous studies have
demonstrated that the GLO/glutathione system in human peritoneal
mesothelial cells is impaired by conventional high-GDP fluids; a
protective effect could only be established following replenishment
of glutathione (24). Thus,
substitution of the glutathione precursor
L-2-oxothiazolidine-4-carboxylic acid may prevent the loss of GLO1
activity under high-GDP exposure.
Impairment of neuronal function appears to occur
secondary to neurostructural damage. During the exposure time of 7
days, neuronal integrity was affected by PD fluids at 1.5% glucose,
while parameters of animal motility were reduced only by PD fluids
at 4% glucose. GLO1 overexpression protected various parameters of
neuronal function, in line with the observed beneficial effects on
neuronal integrity. Upon exposure to low-GDP PD fluids at 4%
glucose, the parameters of relative head motility, absolute head
motility, relative tail motility and absolute tail motility were
rescued in GLO1-Tg animals.
Overall, the current study demonstrated the
significant impact of both glucose and GDP content in PD fluids on
lifespan, neuronal integrity and neuronal function in C.
elegans. The protective effects induced by GLO1 overexpression
emphasize the relevance of the GLO1 detoxifying pathway as a
potential therapeutic target in the treatment of reactive
metabolite-mediated pathologies. Further studies into the vascular
system of higher organisms are needed to translate these findings
into clinical practice.
Acknowledgements
Not applicable.
References
|
1
|
Davis KE, Prasad C, Vijayagopal P, Juma S
and Imrhan V: Advanced glycation end products, inflammation, and
chronic metabolic diseases: Links in a chain? Crit Rev Food Sci
Nutr. 56:989–998. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vlassara H and Uribarri J: Advanced
glycation end products (AGE) and diabetes: Cause, effect, or both?
Curr Diab Rep. 14:4532014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hellwig M and Henle T: Baking, ageing,
diabetes: A short history of the Maillard reaction. Angew Chem Int
Ed Engl. 53:10316–10329. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Uribarri J, del Castillo MD, de la Maza
MP, Filip R, Gugliucci A, Luevano-Contreras C, Macías-Cervantes MH,
Bastos Markowicz DH, Medrano A, Menini T, et al: Dietary advanced
glycation end products and their role in health and disease. Adv
Nutr. 6:461–473. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ott C, Jacobs K, Haucke E, Santos
Navarrete A, Grune T and Simm A: Role of advanced glycation end
products in cellular signaling. Redox Biol. 2:411–429. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Johnson DW, Dent H, Hawley CM, McDonald
SP, Rosman JB, Brown FG, Bannister K and Wiggins KJ: Association of
dialysis modality and cardiovascular mortality in incident dialysis
patients. Clin J Am Soc Nephrol. 4:1620–1628. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Borazan A, Ustün H, Ustundag Y, Aydemir S,
Bayraktaroglu T, Sert M and Yilmaz A: The effects of peritoneal
dialysis and hemodialysis on serum tumor necrosis factor-alpha,
interleukin-6, interleukin-10 and C-reactive-protein levels.
Mediators Inflamm. 13:201–204. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chung SH, Heimbürger O, Stenvinkel P,
Bergström J and Lindholm B: Association between inflammation and
changes in residual renal function and peritoneal transport rate
during the first year of dialysis. Nephrol Dial Transplant.
16:2240–2245. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jörres A, Witowski J and Bender TO: PD and
loss of peritoneal function. Prilozi. 28:275–281. 2007.PubMed/NCBI
|
|
10
|
Erixon M, Wieslander A, Lindén T, Carlsson
O, Forsbäck G, Svensson E, Jönsson JA and Kjellstrand P: How to
avoid glucose degradation products in peritoneal dialysis fluids.
Perit Dial Int. 26:490–497. 2006.PubMed/NCBI
|
|
11
|
Linden T, Forsback G, Deppisch R, Henle T
and Wieslander A: 3-Deoxyglucosone, a promoter of advanced
glycation end products in fluids for peritoneal dialysis. Perit
Dial Int. 18:290–293. 1998.PubMed/NCBI
|
|
12
|
Miyata T, Horie K, Ueda Y, Fujita Y,
Izuhara Y, Hirano H, Uchida K, Saito A, van Ypersele de Strihou C
and Kurokawa K: Advanced glycation and lipidoxidation of the
peritoneal membrane: Respective roles of serum and peritoneal fluid
reactive carbonyl compounds. Kidney Int. 58:425–435. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schwenger V, Morath C, Salava A, Amann K,
Seregin Y, Deppisch R, Ritz E, Bierhaus A, Nawroth PP and Zeier M:
Damage to the peritoneal membrane by glucose degradation products
is mediated by the receptor for advanced glycation end-products. J
Am Soc Nephrol. 17:199–207. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cooker LA, Luneburg P, Holmes CJ, Jones S
and Topley N; Bicarbonate/Lactate Study Group: Interleukin-6 levels
decrease in effluent from patients dialyzed with
bicarbonate/lactate-based peritoneal dialysis solutions, . Perit
Dial Int. 21 Suppl 3:S102–107. 2001.PubMed/NCBI
|
|
15
|
Szeto CC, Chow KM, Lam CW, Leung CB, Kwan
BC, Chung KY, Law MC and Li PK: Clinical biocompatibility of a
neutral peritoneal dialysis solution with minimal
glucose-degradation products - a 1-year randomized control trial.
Nephrol Dial Transplant. 22:552–559. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schmitt CP, von Heyl D, Rieger S, Arbeiter
K, Bonzel KE, Fischbach M, Misselwitz J, Pieper AK and Schaefer F:
Mid European Pediatric Peritoneal Dialysis Study Group (MEPPS):
Reduced systemic advanced glycation end products in children
receiving peritoneal dialysis with low glucose degradation product
content. Nephrol Dial Transplant. 22:2038–2044. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Thornalley PJ: Dietary AGEs and ALEs and
risk to human health by their interaction with the receptor for
advanced glycation endproducts (RAGE) - an introduction. Mol Nutr
Food Res. 51:1107–1110. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Thornalley PJ: Endogenous
alpha-oxoaldehydes and formation of protein and nucleotide advanced
glycation endproducts in tissue damage. Novartis Found Symp.
285:229–243; discussion 243–246. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Thornalley PJ: Glyoxalase I - structure,
function and a critical role in the enzymatic defence against
glycation. Biochem Soc Trans. 31:1343–1348. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Thornalley PJ: Protein and nucleotide
damage by glyoxal and methylglyoxal in physiological systems - role
in ageing and disease. Drug Metabol Drug Interact. 23:125–150.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Morcos M, Du X, Pfisterer F, Hutter H,
Sayed AA, Thornalley P, Ahmed N, Baynes J, Thorpe S, Kukudov G, et
al: Glyoxalase-1 prevents mitochondrial protein modification and
enhances lifespan in Caenorhabditis elegans. Aging Cell.
7:260–269. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mendler M, Schlotterer A, Morcos M and
Nawroth PP: Understanding diabetic polyneuropathy and longevity:
What can we learn from the nematode Caenorhabditis elegans?
Exp Clin Endocrinol Diabetes. 120:182–183. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schalkwijk CG, Posthuma N, ten Brink HJ,
ter Wee PM and Teerlink T: Induction of 1,2-dicarbonyl compounds,
intermediates in the formation of advanced glycation end-products,
during heat-sterilization of glucose-based peritoneal dialysis
fluids. Perit Dial Int. 19:325–333. 1999.PubMed/NCBI
|
|
24
|
Korybalska K, Wisniewska-Elnur J,
Trómińska J, Jörres A, Breborowicz A and Witowski J: The role of
the glyoxalase pathway in reducing mesothelial toxicity of glucose
degradation products. Perit Dial Int. 26:259–265. 2006.PubMed/NCBI
|
|
25
|
Altun-Gultekin Z, Andachi Y, Tsalik EL,
Pilgrim D, Kohara Y and Hobert O: A regulatory cascade of three
homeobox genes, ceh-10, ttx-3 and ceh-23, controls cell fate
specification of a defined interneuron class in C. elegans.
Development. 128:1951–1969. 2001.PubMed/NCBI
|
|
26
|
Zeier M, Schwenger V, Deppisch R, Haug U,
Weigel K, Bahner U, Wanner C, Schneider H, Henle T and Ritz E:
Glucose degradation products in PD fluids: Do they disappear from
the peritoneal cavity and enter the systemic circulation? Kidney
Int. 63:298–305. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hutter H: Axonale Wegfindung im ventralen
Nervenstrang von C. elegans. Tätigkeitsbericht. 2003.(In
German).
|
|
28
|
Hobert O and Loria P: Uses of GFP in
Caenorhabditis elegansGreen Fluorescent Protein: Properties,
Applications, and Protocols. Chalfie M and Kain SR: 47. 2nd
edition. John Wiley & Sons, Inc.; Hoboken, NJ: pp. 203–226.
2006
|
|
29
|
Negga R, Rudd DA, Davis NS, Justice AN,
Hatfield HE, Valente AL, Fields AS and Fitsanakis VA: Exposure to
Mn/Zn ethylene-bis-dithiocarbamate and glyphosate pesticides leads
to neurodegeneration in Caenorhabditis elegans.
Neurotoxicology. 32:331–341. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mendler M, Schlotterer A, Ibrahim Y,
Kukudov G, Fleming T, Bierhaus A, Riedinger C, Schwenger V, Herzig
S, Hecker M, et al: daf-16/FOXO and glod-4/glyoxalase-1 are
required for the life-prolonging effect of human insulin under high
glucose conditions in Caenorhabditis elegans. Diabetologia.
58:393–401. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schlotterer A, Greten HJ, Remppis BA,
Kukudov G, Efferth T, Machado J, Humpert P, Hammes HP and Morcos M:
Neuroprotection and antioxidative effects of Sijunzi Tang Decoction
in the nematode Caenorhabditis elegans. Eur J Integr Med.
8:526–532. 2016. View Article : Google Scholar
|
|
32
|
Ortiz A, Wieslander A, Linden T,
Santamaria B, Sanz A, Justo P, Sanchez-Niño MD, Benito A and
Kjellstrand P: 3,4-DGE is important for side effects in peritoneal
dialysis what about its role in diabetes. Curr Med Chem.
13:2695–2702. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Choi HY, Kim DK, Lee TH, Moon SJ, Han SH,
Lee JE, Kim BS, Park HC, Choi KH, Ha SK, et al: The clinical
usefulness of peritoneal dialysis fluids with neutral pH and low
glucose degradation product concentration: An open randomized
prospective trial. Perit Dial Int. 28:174–182. 2008.PubMed/NCBI
|
|
34
|
Schwenger V: GDP and AGE receptors:
Mechanisms of peritoneal damage. Contrib Nephrol. 150:77–83. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Müller-Krebs S, Kihm LP, Zeier B, Gross
ML, Deppisch R, Wieslander A, Henle T, Penndorf I, Oh J, Reiser J,
et al: Renal toxicity mediated by glucose degradation products in a
rat model of advanced renal failure. Eur J Clin Invest. 38:296–305.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dengg M and van Meel JC: Caenorhabditis
elegans as model system for rapid toxicity assessment of
pharmaceutical compounds. J Pharmacol Toxicol Methods. 50:209–214.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Artal-Sanz M, de Jong L and Tavernarakis
N: Caenorhabditis elegans: A versatile platform for drug
discovery. Biotechnol J. 1:1405–1418. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ailion M, Inoue T, Weaver CI, Holdcraft RW
and Thomas JH: Neurosecretory control of aging in Caenorhabditis
elegans. Proc Natl Acad Sci USA. 96:7394–7397. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Boulianne GL: Neuronal regulation of
lifespan: Clues from flies and worms. Mech Ageing Dev. 122:883–894.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Geanacopoulos M: The determinants of
lifespan in the nematode Caenorhabditis elegans: A short
primer. Sci Prog. 87:227–247. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Luo Y: Long-lived worms and aging. Redox
Rep. 9:65–69. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Paik YK, Jeong SK, Lee EY, Jeong PY and
Shim YH: C. elegans: An invaluable model organism for the
proteomics studies of the cholesterol-mediated signaling pathway.
Expert Rev Proteomics. 3:439–453. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shen LL, Wang Y and Wang DY: Involvement
of genes required for synaptic function in aging control in C.
elegans. Neurosci Bull. 23:21–29. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Murakami S: Caenorhabditis elegans
as a model system to study aging of learning and memory. Mol
Neurobiol. 35:85–94. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Choei H, Sasaki N, Takeuchi M, Yoshida T,
Ukai W, Yamagishi S, Kikuchi S and Saito T: Glyceraldehyde-derived
advanced glycation end products in Alzheimer's disease. Acta
Neuropathol. 108:189–193. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Fatokun AA, Stone TW and Smith RA:
Oxidative stress in neurodegeneration and available means of
protection. Front Biosci. 13:3288–3311. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Deponte M: Glutathione catalysis and the
reaction mechanisms of glutathione-dependent enzymes. Biochim
Biophys Acta. 1830:3217–3266. 2013. View Article : Google Scholar : PubMed/NCBI
|