Introduction
The choroid plexus, located within the brain
ventricles, is a specialized structure consisting of epithelial
cells and underlying vascular-rich connective tissues (1). Choroid plexus epithelial cells are
involved in the production of cerebrospinal fluid, and secrete a
number of neurotrophic factors including brain-derived neurotrophic
factor (BDNF), glial cell-derived neurotrophic factor (GDNF), nerve
growth factor (NGF) and ciliary neurotrophic factor (CNTF)
(2–4).
The neurotrophic factors are known to stimulate neuronal growth and
promote neurite outgrowth (5).
Depletion of these neurotrophic factors has been associated with
pathologies and symptoms of Parkinson's, Alzheimer's and
Huntington's diseases and spinal cord injury, and replacement
strategies are considered as potential therapeutics for these
neural degenerative diseases (3).
In recent years, cell transplantation therapy has
emerged as a promising therapeutic option for neurorepair (6). Transplantation of choroid plexus
epithelial cells from primary culture has been tested in several
animal models, including rat models of Parkinson's disease, spinal
cord injury and cerebral ischemia (7–9). However,
cells are difficult to obtain in primary culture, and therefore the
source of cells is limited. This problem may be overcome if passage
culture and cryopreserved-thawed cells can be used for
transplantation. This may depend on whether passage culture and
cryopreservation impair the secretion of neurotrophic factors from
choroid plexus epithelial cells.
To the best of our knowledge, there has been no
investigation into the effects of passage culture and
cryopreservation on neurotrophic factor secretion from choroid
plexus epithelial cells. The present study was conducted to compare
the levels of BDNF, GDNF, NGF and CNTF secreted by neonatal rat
choroid plexus epithelial cells among primary, first passage and
second passage cultures and cryopreserved-thawed cells.
Materials and methods
Animals and reagents
Neonatal male Sprague-Dawley rats (1-day-old,
weighing 5–6 g) and their mothers were supplied by the Center of
Experimental Animals, Xi'an Jiaotong University (Xi'an, China). All
rat mothers were housed individually with their offspring in
polypropylene cages in a standard animal room maintained at 22±3°C
and 50±20% humidity, and allowed access to food and water ad
libitum under a natural day/night cycle. The experiments were
performed with 24 rat offspring. The protocols for animal care and
experimental management were approved by the Xi'an Jiaotong
University Animal Experimentation Committee. Ethical approval for
the study was obtained from the Ethics Committee of the Second
Affiliated Hospital of Xi'an Jiaotong University.
Dulbecco's modified Eagle's medium (DMEM, low
glucose) and fetal bovine serum (FBS) were purchased from Thermo
Fisher Scientific, Inc. (Waltham, MA, USA), and 6-well plates were
obtained from Corning Inc. (Corning, NY, USA). Recombinant rat
epidermal growth factor (EGF) was obtained from PeproTech, Inc.,
(Rocky Hill, NJ, USA). ELISA kits for BDNF (cat. no. F15100), GDNF
(cat. no. F15600), NGF (cat. no. F16310) and CNTF (cat. no. F15220)
and normal goat serum were obtained from Shanghai Xitang Biological
Technology Co., Ltd. (Shanghai, China). Dimethyl sulfoxide (DMSO)
and trypan blue were obtained from Sigma-Aldrich (Merck KGaA,
Darmstadt, Germany).
Primary culture
Primary culture of choroid plexus epithelial cells
was prepared via the procedures described in our previous studies
(10,11). Briefly, following euthanasia with an
overdose of pentobarbital (150 mg/kg, intraperitoneal injection)
and disinfecting with 75% ethanol, the rat brains were isolated and
the choroid plexus was removed from the lateral ventricles. The
tissues were segregated into small cell aggregates by a mechanical
method, and cultured in DMEM supplemented with 10% FBS and 10 ng/ml
EGF on 6-well plates at 37°C in a humidified incubator at 5%
CO2. The culture medium was replaced every 2 days.
Passage culture and cell
cryopreservation
When primary culture cells reached ~90% confluence,
cells were detached with a 0.25% Trypsin-EDTA solution. Cell
numbers were determined with a hemocytometer. The viability of
cells was assessed by trypan blue exclusion test (12). Then, cells were divided into two equal
groups: One group was used for cryopreservation, and the other
group was seeded into 6-well plates at a density of
1.5×103 cells/ml with DMEM containing 10% FBS, and
incubated in a 37°C, 5% CO2 incubator. The culture
medium was replaced as before every 2 days. When first passage
culture cells reached ~90% confluence, the second passage was
initiated and performed as above.
Cryopreservation and thawing of
cells
Choroid plexus epithelial cells collected from the
previous steps were aliquoted at a density of 2×106
cells/cryovial with DMEM containing 90% FBS and 10% DMSO. Cryovials
with primary cultures were incubated sequentially at 4°C for 0.5 h,
−20°C for 2 h, and −80°C for 16 h, and then transferred to and
stored in a liquid nitrogen tank (−196°C) until use. After 14 days
of cryopreservation, the cells were thawed in a 37°C water bath (~1
min). Upon thawing of the cells, the cryovial was removed from the
water bath and the cells were transferred to a centrifuge tube
containing 6 ml DMEM containing 10% FBS. Following centrifugation
at 1,000 × g for 5 min at room temperature, cells were resuspended
with 9 ml complete medium and dispensed into a 6-well plate at a
density of 1.5×103 cells/ml. Thereafter, culture medium
was replaced every 2 days.
Light microscopy
All cultured cells were examined daily for growth
under a phase contrast microscope (Olympus Corporation, Tokyo,
Japan).
Monitoring of secretion function
Each culture of cells was maintained in an incubator
at 37°C with 5% humidified CO2. Supernatant was
collected when cells reached 80–90% confluence (within 2 days), and
was conserved at −70°C following centrifugation at 1,000 × g for 5
min at room temperature. The samples were thawed at 4°C for 24 h,
then tested with the rat BDNF, GDNF, NGF and CNTF ELISA kits at
room temperature. The values were expressed in pg/ml. At least four
samples were analyzed for each culture.
Data analysis
Data were expressed as the mean ± standard
deviation. All statistical analyses were performed using GraphPad
Prism 5.1 (GraphPad Software, Inc., La Jolla, CA, USA). One-way
analysis of variance was performed for statistical evaluation,
followed by the Newman-Keuls multiple comparisons test for stepwise
multiple comparisons among the culture groups. P<0.05 was
considered to indicate statistical significance.
Results
Morphology of cultures
There were no obvious differences in morphological
features among the primary cultured, first passage, second passage
and cryopreserved-thawed cells under phase contrast microscopy.
Choroid plexus epithelial cells grew in fusiform or exhibited
polygon shape prior to fusion (Fig.
1).
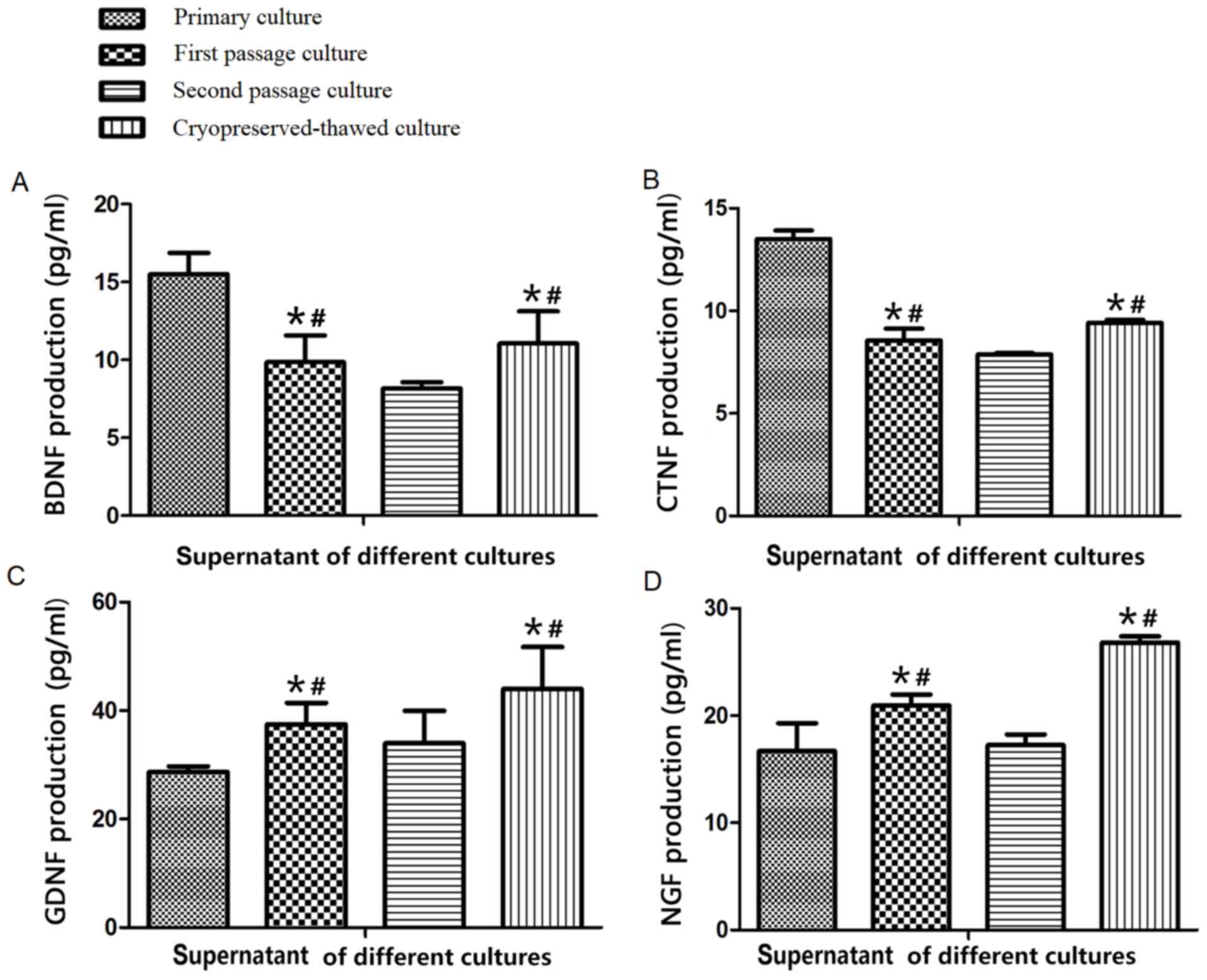
Change of neurotrophic factors
First passage and cryopreserved-thawed cells
secreted less BDNF and CNTF compared with primary cultured cells
and increased levels of these two factors compared with second
passage cells, and increased levels of GDNF and NGF compared with
primary cultured and second passage cells (Fig. 2), with the differences deemed to be
significant (P<0.05). These results suggested that first passage
culture could decrease secretion of the neurotrophic factors BDNF
and CNTF but increase that of NGF and GDNF, while second passage
culture diminished secretion of all four neurotrophic factors.
Cryopreserved-thawed cells secreted the four factors at similar
levels to first passage cells (P>0.05), indicating that
cryopreservation did not weaken the function of choroid plexus
epithelial cells in secreting neurotrophic factors.
Discussion
The present in vitro study demonstrated, to the best
of our knowledge for the first time, the effects of passage culture
and cryopreservation on the function of choroid plexus epithelial
cells in secreting the neurotrophic factors BDNF, GDNF, NGF and
CNTF. The levels of NGF and GDNF released by first passage and
cryopreserved-thawed cells were higher compared with those from
primary cultured cells, indicating that the function of the cells
in secreting certain neurotrophic factors may be enhanced by first
passage culture and cryopreservation. In the present study,
supernatant was collected when cultured cells reached 80–90%
confluence. At this time point, secretion of neurotrophic factors
from choroid plexus epithelial cells was expected to be maximal, as
the quantity of cells was sufficient and there was no contact
inhibition, and the data should therefore reflect the total
secretion function.
Primary cultures have been used in transplantation
studies for spinal cord injury therapy (8,13). It has
been revealed that neurotrophins are key to the therapeutic effects
(14). BDNF, GDNF and NGF are
important for the survival, maintenance and regeneration of
specific neuronal populations in the adult brain and spinal cord
(15,16). CNTF, which is widely distributed in
the neuronal system, has nutritional function (17,18).
Transplantation of primary cultures of choroid plexus epithelial
cells has been examined in rats with spinal cord injury, as a
therapy to accelerate locomotor improvement and tissue repair
including axonal extension in spinal cord lesions (8,19). Studies
suggest that the therapeutic effect may be linked with the cultured
cells' function of secreting neurotrophic factors including BDNF,
GDNF, NGF and CNTF (4,20). However, cells are difficult to obtain
in primary culture and the cell source for transplantation is thus
limited. If passage cultured and cryopreserved cells can be
demonstrated to secrete neurotrophic factors in high quantities,
they maybe used for transplantation and the shortage of choroid
plexus epithelial cells can be overcome.
The cells in the current study were passage cultured
up to second passage and neurotrophic factors were quantified for
only three generations. Therefore, the data do not offer a trend to
elucidate the effect of continuous passage culture. In future
study, the passage cultured cells and cryopreserved choroids plexus
epithelial cells should be applied in spinal cord injury models, to
determine the efficacy of both cell transplantation and purified
neurotrophic factors.
In conclusion, first passage cultured and
cryopreserved-thawed choroid plexus epithelial cells may secrete
high levels of neurotrophic factors, among which the levels of NGF
and GDNF may be higher than those secreted by primary cultured
cells. Second passage cells secrete comparatively less neurotrophic
factors. Based on the present results, first passage and
cryopreserved-thawed cells may have therapeutic potency in the
treatment of neural degenerative diseases and could be adopted in
the future.
Acknowledgements
Not applicable.
Glossary
Abbreviations
Abbreviations:
|
BDNF
|
brain-derived neurotrophic factor
|
|
CNTF
|
ciliary neurotrophic factor
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
DMSO
|
dimethyl sulfoxide
|
|
EGF
|
epidermal growth factor
|
|
FBS
|
fetal bovine serum
|
|
GDNF
|
glial cell-derived neurotrophic
factor
|
|
NGF
|
nerve growth factor
|
References
|
1
|
Santos CR, Duarte AC, Quintela T, Tomás J,
Albuquerque T, Marques F, Palha JA and Gonçalves I: The choroid
plexus as a sex hormone target: Functional implications. Front
Neuroendocrinol. 44:103–121. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chodobski A and Szmydynger-Chodobska J:
Choroid plexus: Target for polypeptides and site of their
synthesis. Microsc Res Tech. 52:65–82. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Damkier HH, Brown PD and Praetorius J:
Cerebrospinal fluid secretion by the choroid plexus. Physiol Rev.
93:1847–1892. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Huang SL, Wang J, He XJ, Li ZF, Pu JN and
Shi W: Secretion of BDNF and GDNF from free and encapsulated
choroid plexus epithelial cells. Neurosci Lett. 566:42–45. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Scheepens A, Sirimanne ES, Breier BH,
Clark RG, Gluckman PD and Williams CE: Growth hormone as a neuronal
rescue factor during recovery from CNS injury. Neuroscience.
104:677–687. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Feng YP, Sun TS, Chen L, Xie JX, Zhang ZC,
Huang HY and He XJ: Clinical therapeutic guideline for
neurorestoration in spinal cord injury (Chinese version 2016). J
Neurorestoratology. 5:73–83. 2017. View Article : Google Scholar
|
|
7
|
Zhang HL, Wu JJ, Ren HM, Wang J, Su YR and
Jiang YP: Therapeutic effect of microencapsulated porcine retinal
pigmented epithelial cells transplantation on rat model of
Parkinson's disease. Neurosci Bull. 23:137–144. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kanekiyo K, Nakano N, Noda T, Yamada Y,
Suzuki Y, Ohta M, Yokota A, Fukushima M and Ide C: Transplantation
of choroid plexus epithelial cells into contusion-injured spinal
cord of rats. Restor Neurol Neurosci. 34:347–366. 2016.PubMed/NCBI
|
|
9
|
Borlongan CV, Skinner SJ, Geaney M,
Vasconcellos AV, Elliott RB and Emerich DF: CNS grafts of rat
choroid plexus protect against cerebral ischemia in adult rats.
Neuroreport. 15:1543–1547. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang SL, He XJ, Li ZF, Yao L and Shi W: A
novel primary culture method for rat choroidal epithelial cells.
Neurosciences (Riyadh). 18:27–32. 2013.PubMed/NCBI
|
|
11
|
Huang SL, He XJ, Li ZF, Yao L, Yuan GL and
Shi W: Primary culture of choroid plexuses from neonate rats
containing progenitor cells capable of differentiation. Balkan Med
J. 30:350–354. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu JJ, Ding XY, Xiang L, Zhao F and Huang
SL: A novel method for oxygen glucose deprivation model in
organotypic spinal cord slices. Brain Res Bull. 135:163–169. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ide C and Kanekiyo K: Points regarding
cell transplantation for the treatment of spinal cord injury.
Neural Regen Res. 11:1046–1049. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Thanos CG, Bintz BE, Goddard M,
Boekelheide K, Hall S and Emerich DF: Functional modulation of
choroid plexus epithelial clusters in vitro for tissue repair
applications. Cell Transplant. 20:1659–1672. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Allen SJ, Watson JJ, Shoemark DK, Barua NU
and Patel NK: GDNF, NGF and BDNF as therapeutic options for
neurodegeneration. Pharmacol Ther. 138:155–175. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao YZ, Jiang X, Xiao J, Lin Q, Yu WZ,
Tian FR, Mao KL, Yang W, Wong HL and Lu CT: Using NGF
heparin-poloxamer thermosensitive hydrogels to enhance the nerve
regeneration for spinal cord injury. Acta Biomater. 29:71–80. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Louis JC, Magal E, Takayama S and Varon S:
CNTF protection of oligodendrocytes against natural and tumor
necrosis factor-induced death. Science. 259:689–692. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gu YL, Gao GQ, Ma N, Ye LL, Zhang LW, Gao
X and Zhang ZB: CNTF protects neurons from hypoxic injury through
the activation of STAT3pTyr705. Int J Mol Med. 38:1915–1921. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Emerich DF, Vasconcellos AV, Elliott RB,
Skinner SJ and Borlongan CV: The choroid plexus: Function,
pathology and therapeutic potential of its transplantation. Expert
Opin Biol Ther. 4:1191–1201. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Han Y and Kim KT: Neural Growth Factor
Stimulates Proliferation of Spinal Cord Derived-Neural
Precursor/Stem Cells. J Korean Neurosurg Soc. 59:437–441. 2016.
View Article : Google Scholar : PubMed/NCBI
|