Introduction
As part of the normal aging process, neuroanatomical
and neurophysiological changes occur in the central nervous system
(CNS) (1,2), and our group previously reported
age-dependent increase in antioxidant-like protein-1 expression in
the gerbil hippocampus (3). In the
brain, normal aging affects the functions of N-methyl-D-aspartate
receptors, which may serve important roles in the initiation of
long-term potentiation and be associated with age-related decline
in memory (4,5). Critical for learning and memory, the
hippocampus is considered to be one of the brain regions most
sensitive to changes induced by the normal aging process (6,7).
Nuclear receptor related-1 protein (Nurr1), also
known as nuclear receptor subfamily 4 group a member 2, is a member
of the inducible nuclear receptor superfamily of transcription
factors (8). Nurr1 mRNA expression
has been reported in several regions of the CNS, including parts of
the cortex, hippocampal formation and substantia nigra, in
developing and adult mice and rats (9). It has been documented that Nurr1 is
associated with differentiation, maturation, function and survival
of midbrain dopaminergic neurons and that reduction of Nurr1
expression or polymorphisms in the Nurr1 gene may be associated
with Parkinson's disease etiology (10–15). Nurr1
may protect against inflammation-induced dopaminergic neuronal
death by inhibiting expression of pro-inflammatory mediators in
microglia and astrocytes (16).
Furthermore, a recent study demonstrated that Nurr1 participated in
the regulation of adult hippocampal neurogenesis, and that
activation of Nurr1 using amodiaquine increased hippocampal
neurogenesis by stimulating neural stem cells (17). In addition, Nurr1 has been observed to
be under the regulation of neural activity in cultured hippocampal
neurons, in that basal expression of Nurr1 was reduced when
neuronal activities were blocked (18).
A number of studies on Nurr1 have been performed in
dopaminergic neurons, and significant decrease in Nurr1 expression
has been reported in dopaminergic neurons of the substantia nigra
in aged humans and rats (19,20). However, whether there is age-related
change of Nurr1 expression in the hippocampus, a region which has
been associated with neurogenesis and neural activity (21), is yet to be fully elucidated.
Therefore, the objective of the present study was to investigate
age-dependent change of Nurr1 protein expression in the hippocampi
of young, adult and aged gerbils, as a suitable model for research
on aging (22), using western blot
analysis and immunohistochemistry. Overall this aimed to provide
novel insight into the association of Nurr1 with the decline of
hippocampus-dependent cognitive function during the normal aging
process.
Materials and methods
Experimental animals
The present study used male Mongolian gerbils
(Meriones unguiculatus) at postnatal month 3 (PM 3; 40–50 g)
as a young-group, PM 12 (65–75 g) as an adult-group and PM 24
(85–95 g) as an aged-group (total, n=42; n=14/group). This model
was selected due to its suitability for research on aging (22). The gerbils were obtained from the
Experimental Animal Center of Kangwon National University
(Chuncheon, Republic of Korea). The animals were housed under
conventional conditions at an ambient temperature (23±3°C) and
relative humidity (55±5%) under a 12-h light/dark cycle and were
allowed free access to food and water. The study was conducted to
minimize the number of gerbils. The procedures for animal handling
and care adhered to guidelines in compliance with the current
international laws and policies (Guide for the Care and Use of
Laboratory Animals, The National Academies Press, 8th ed., 2011)
(23). All experimental procedures
involving animals were approved by the Institutional Animal Care
and Use Committee of Kangwon National University (approval no.
KW-160802-2).
Western blot analysis
Changes in Nurr1 protein levels during the normal
aging process were examined in the hippocampi of gerbils
(n=7/group). Western blot analysis was performed according to the
method described in our previous studies (24,25). In
brief, following euthanasia of the animals, hippocampi were
removed. The hippocampi were homogenized and centrifuged, and the
supernatants were subjected to western blot analysis. The tissues
were homogenized in 50 mM phosphate-buffered saline (PBS; pH 7.4)
containing 0.1 mM ethylene
glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid (pH 8.0),
0.2% Nonidet P-40, 10 mM ethylendiamine tetraacetic acid (pH 8.0),
15 mM sodium pyrophosphate, 100 mM β-glycerophosphate, 50 mM NaF,
150 mM NaCl, 2 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl
floride and 1 mM dithiothreitol (DTT; all from Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany). Following centrifugation at 16,000 × g
for 20 min at 4°C, the protein level of Nurr1 in the supernatants
was determined using a micro bicinchoninic acid protein assay kit
(Sigma-Aldrich; Merck KGaA), with bovine serum albumin as the
standard (Pierce; Thermo Fisher Scientific, Inc., Waltham, MA,
USA). Aliquots containing 20 µg total protein were boiled in
loading buffer containing 150 mM Tris (pH 6.8), 3 mM DTT, 6% SDS,
0.3% bromophenol blue and 30% glycerol. The aliquots were then
loaded onto a 10% polyacrylamide gel. Following electrophoresis,
the gels were transferred onto nitrocellulose transfer membranes.
To reduce background staining, the membranes were incubated with 5%
non-fat dry milk in PBS containing 0.1% Tween-20 for 45 min at room
temperature. Following three washes with PBS with Tween-20 (PBST;
each for 5 min), the membranes were incubated with rabbit
anti-Nurr1 (PA5-13416; 1:500; Invitrogen; Thermo Fisher Scientific,
Inc.) overnight at 4°C. Following another three washes with PBST
(each for 10 min), the membranes were incubated with
peroxidase-conjugated donkey anti-rabbit immunoglobulin G (IgG;
sc-2305; 1:1,000; Santa Cruz Biotechnology, Inc., Dallas, TX, USA)
for 1 h at room temperature, followed by ECL reagents (Pierce;
Thermo Fisher Scientific, Inc.). The resulting protein bands were
scanned, and densitometric analysis for quantification of the bands
was performed using Image J 1.59 software (National Institutes of
Health, Bethesda, MD, USA), which was used to calculate relative
optical density (ROD). The protein level of Nurr1 was normalized to
that of β-actin (A5316; 1:5,000; Sigma-Aldrich; Merck KGaA). A
ratio of the ROD was calibrated as a percentage, with the
young-group designated as 100%.
Immunohistochemistry
The gerbils (n=7/group) were anesthetized with
pentobarbital sodium (40 mg/kg, intraperitoneal injection; JW
Pharmaceutical Corporation, Seoul, Republic of Korea) and perfused
transcardially with 0.1 M PBS (pH 7.4) followed by 4%
paraformaldehyde in 0.1 M PBS (pH 7.4). The brains were removed and
postfixed with the same fixative for 7 h at room temperature. The
brain tissue including the hippocampi was sectioned at 30-µm
thickness with a cryostat.
To examine age-related changes in Nurr1
immunoreactivity in young, adult and aged hippocampi,
immunohistochemical staining was performed according to the method
described in our previous studies (24,25).
Immunohistochemical staining for Nurr1 was performed using the
rabbit anti-Nurr1 antibody (1:100) as the primary antibody
overnight at 4°C. Following three washes with PBS (each for 10
min), the brain tissues were incubated with biotinylated goat
anti-rabbit IgG (BA-1000; 1:200; Vector Laboratories, Burlingame,
CA, USA) for 2 h at room temperature, and then streptavidin
peroxidase complex (SA-5004; 1:200; Vector Laboratories) for 45 min
at room temperature. To establish the specificity of the
immunostaining, a negative control test without primary antibody
was performed, which resulted in the absence of immunoreactivity in
all structures.
A total of six sections at 120-µm intervals per
animal were selected to quantitatively analyze Nurr1
immunoreactivity. Digital images of Nurr1 immunoreactive structures
of the hippocampal regions were observed and captured with an Axio
Imager 2 light microscope (Carl Zeiss AG, Oberkochen, Germany)
equipped with a digital camera (Axiocam; Carl Zeiss). According to
the method of previous studies (24,26),
semi-quantification of the immunostaining intensity of Nurr1 was
evaluated and analyzed using Image J 1.59. The mean intensity of
Nurr1 immunoreactivity in the immunoreactive structures was
measured using a 0–255 gray scale system (white to dark signal
corresponding to 255 to 0). Based on this approach, the background
density was subtracted, and the level of immunoreactivity was
scaled as -, ±, +, ++ or +++, representing no staining (gray scale
value, ≥200), weakly positive (gray scale value, 150–199), moderate
(gray scale value, 100–149), strong (gray scale value, 50–99) or
very strong (gray scale value, ≤49), respectively.
Cresyl violet (CV) staining
CV staining was performed to investigate cellular
distribution and morphology. In brief, according to the method of
our previous study (27), the
sections of the hippocampal regions were mounted on gelatin-coated
microscopy slides. Cresyl violet acetate (Sigma-Aldrich; Merck
KGaA) was dissolved at 1.0% (w/v) in distilled water, and glacial
acetic acid (0.25% v/v) was added to this solution. The sections
were stained with CV and dehydrated by immersing in serial ethanol
baths. Finally, the stained sections were mounted with Canada
balsam (Kanto Chemical, Co., Inc., Tokyo, Japan). A total of six
sections at 120-µm intervals per animal were selected to identify
the distribution of Nurr1 immunoreactivity in the hippocampus.
Digital images of CV stained structures were observed and captured
with the Axio Imager 2 microscope and digital camera.
Statistical analysis
Data are expressed as the mean ± standard error of
the mean. Differences in the mean ROD among the groups were
statistically analyzed using one-way analysis of variance followed
by post hoc Bonferroni's multiple comparison tests using GraphPad
InStat (version 3.05; GraphPad Software, Inc., La Jolla, CA, USA).
Statistical significance was considered at P<0.05.
Results
Age-related changes in Nurr1 protein
level
Results from western blot analysis indicated an
age-related change of Nurr1 protein level in the gerbil hippocampus
(Fig. 1). The protein expression of
Nurr1 in the adult hippocampus was significantly decreased compared
with that in the young hippocampus (P<0.05). In addition, the
expression of Nurr1 in the aged hippocampus was significantly
decreased compared with that in the adult and young hippocampi
(P<0.05). Notably, the protein level of Nurr1 in the aged
hippocampus was reduced by 73.6% compared with that in the young
hippocampus.
Age-related change in Nurr1
immunoreactivity
Age-related changes in Nurr1 immunoreactivity
(Table I and Fig. 2) were identified in the hippocampus
proper (CA1-3 regions). In the young group, very strong Nurr1
immunoreactivity was primarily observed in pyramidal neurons of the
stratum pyramidale (SP; Fig. 2A and
D). In the adult group, Nurr1 immunoreactivity in pyramidal
neurons was decreased compared with that in the young group
(Table I and Fig. 2B and E). Furthermore, a marked
reduction of Nurr1 immunoreactivity in the SP was identified in the
aged group, compared with that in the adult group (Table I and Fig. 2C
and F).
 | Figure 2.Nurr1 immunohistochemistry in
hippocampal regions of young, adult and aged gerbils. Nurr1
expression was detected in the (A-C) CA1 and (D-F) CA3 regions and
(G-I) DG. In the young group, strong Nurr1 immunoreactivity was
detected in pyramidal neurons of the SP in the CA1 and 3 regions
(arrows) and in granule cells of the GCL in the DG (arrowheads).
Nurr1 immunoreactivity in the SP and GCL gradually decreased in the
adult and aged groups. Magnification, ×20; scale bar, 100 µm.
Nurr1, nuclear receptor related-1 protein; DG, dentate gyrus; SO,
stratum oriens; SP, stratum pyramidale; SR, stratum radiatum; ML,
molecular layer; GCL, granule cell layer; PL, polymorphic
layer. |
 | Table I.Mean intensity of Nurr1
immunoreactivity in principal cells of the gerbil hippocampus
during normal aging. |
Table I.
Mean intensity of Nurr1
immunoreactivity in principal cells of the gerbil hippocampus
during normal aging.
|
| Postnatal
month |
|---|
|
|
|
|---|
|
| Young | Adult | Aged |
|---|
| Pyramidal neurons
of | +++ | ++ | + |
| hippocampus
proper |
|
|
|
| Granule cells
of | +++ | ++ | + |
| dentate gyrus |
|
|
|
In the dentate gyrus (DG), Nurr1 immunoreactivity
was primarily observed in granule cells of the granule cell layer
(GCL; Fig. 2G, H and I). Similar to
the change of Nurr1 immunoreactivity in the hippocampus proper,
Nurr1 immunoreactivity in the DG gradually and markedly decreased
during normal aging (Table I and
Fig. 2G, H and I). Therefore, Nurr1
immunoreactivity decreased in the hippocampal regions in an
age-dependent manner.
CV positive cells
In the young, adult and aged groups, CV positive
cells were well distributed, mainly in the SP of the hippocampal
CA1-3 regions, and the granular cell layer (GCL) and polymorphic
layer (PL) of the DG in the hippocampus (Fig. 3A-I). The distribution of Nurr1
immunoreactivity was concentrated to pyramidal neurons of the SP in
the hippocampus proper and to granule cells of the GCL in the DG
when comparing with the results of CV staining (Fig. 3).
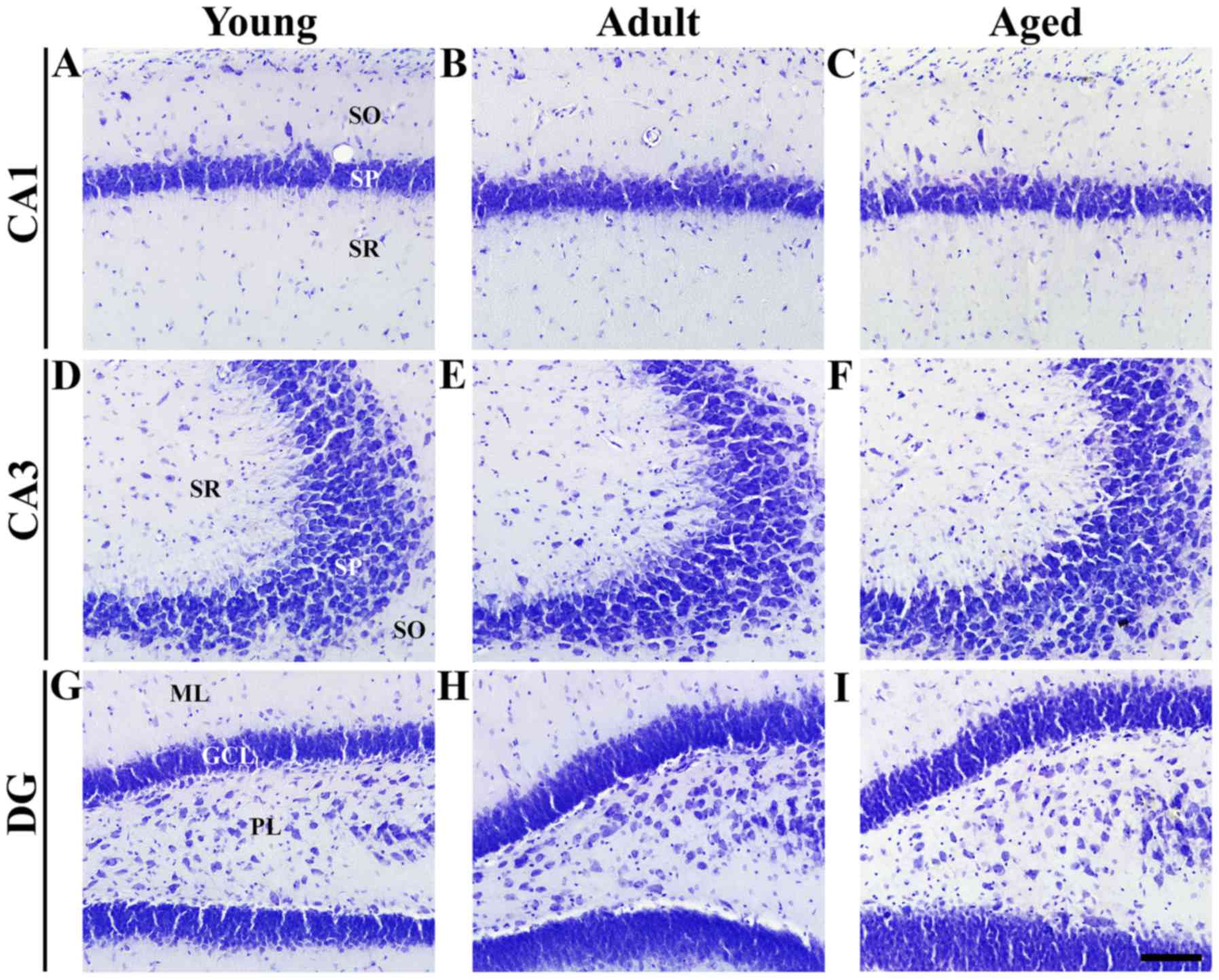 | Figure 3.CV staining in hippocampal regions of
young, adult and aged gerbils. Neuronal morphology and distribution
was observed by CV staining in the (A-C) CA1 and (D-F) CA3 regions
and (G-I) DG. In all groups, pyramidal neurons were identified in
the SP in the CA1 and 3 regions, and granule cells were identified
in the GCL in the DG. Magnification, ×20; scale bar, 100 µm. CV,
cresyl violet; DG, dentate gyrus; SP, stratum pyramidale; SO,
stratum oriens; SR, stratum radiatum; ML, molecular layer; GCL,
granule cell layer; PL, polymorphic layer. |
Discussion
In the present study, Nurr1 immunoreactivity was
primarily observed in pyramidal neurons and granule cells, which
are well established as principal neurons of the hippocampus
(28), in young, adult and aged
gerbil hippocampi. The results of Nurr1 immunoreactivity in the
hippocampi were generally consistent with those of a previous study
in C57BL/6 mice, which demonstrated that clear and specific Nurr1
immunoreactivity occurred in the hippocampus (29).
Chu et al (19)
reported that Nurr1 protein was significantly reduced in the
substantia nigra of elderly human subjects. Furthermore, they
identified a significant age-dependent decline in the number of
Nurr1-immunoreactive neurons in the substantia nigra of middle-aged
and aged individuals compared with in young subjects (19). In aged rats, Nurr1 gene expression has
been reported to be significantly decreased by 33% in dopaminergic
neurons of the substantia nigra (20); as such, it was suggested that
age-dependent decrease of Nurr1 in dopaminergic neurons may be
associated with impairment of nigrostriatal signaling and
compromised motor function with age (20). In addition, it has been reported that
heterozygous Nurr1 knockout mice exhibit accelerated age-dependent
reduction in the number of dopaminergic neurons and impaired
dopamine signaling compared with wild-type littermate controls
(30).
In the current study, the protein level of Nurr1 in
the hippocampus was significantly decreased during the normal aging
process. In addition, Nurr1 immunoreactivity, predominantly
identified in the principal neurons (pyramidal and granule cells)
of the hippocampus, was also decreased during the normal aging
process. To the best of our knowledge, this is the first study to
demonstrate age-dependent decrease of Nurr1 protein expression in
the hippocampus. However, it is difficult to conclude the
implications of this marked reduction in hippocampal Nurr1 due to
aging. Nurr1 has been considered to be among the key target genes
controlled by acetylation during long-term memory formation
(31), and has also been implicated
to serve critical roles in the formation of long-term memory, as
Nurr1 expression is increased during memory acquisition and
consolidation (31–34). In addition, knockdown of Nurr1 or
blocking Nurr1 activity in the hippocampus may lead to impairment
of long-lasting cognitive function (35). Principal neurons are serially or
multi-directionally connected within trisynaptic hippocampal
circuits for information processing (36). Pyramidal cells are involved in
encoding spatial, contextual and emotional information to form
cognitive memory (37), and granule
cells form major structures involved in pattern separation (the
ability to discriminate among similar events) (38). Therefore, it has been suggested that
Nurr1, expressed in principal cells, serves critical roles in
hippocampal-dependent cognitive processes (35). In addition, it has been identified
that cognitive impairment begins around PM 12 and major cognitive
decline occurs around PM 24 in mice (39–41). This
is somewhat consistent with the present results demonstrating that
Nurr1 protein level and immunoreactivity in the hippocampus
gradually decreased with age. Therefore, it may be postulated that
a decrease of Nurr1 protein expression in the hippocampus with
increasing age may be associated with age-dependent cognitive
impairment. However, limitations of the study include the lack of
data on age-related changes in Nurr1 mRNA expression, which should
be obtained in future studies.
In conclusion, the present results indicate that
Nurr1 protein expression age-dependently decreases in the
hippocampus. Overall, the findings suggest that age-dependent
decrease in Nurr1 expression may be associated with a decline of
cognitive function in the hippocampus.
Acknowledgements
Not applicable.
References
|
1
|
Bustamante J, Czerniczyniec A, Cymeryng C
and Lores-Arnaiz S: Age related changes from youth to adulthood in
rat brain cortex: Nitric oxide synthase and mitochondrial
respiratory function. Neurochem Res. 33:1216–1223. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
He WB, Zhang JL, Hu JF, Zhang Y, Machida T
and Chen NH: Effects of glucocorticoids on age-related impairments
of hippocampal structure and function in mice. Cell Mol Neurobiol.
28:277–291. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Park JA, Park JH, Ahn JH, Kim JD, Won MH
and Lee CH: Age dependent increase in the expression of antioxidant
like protein 1 in the gerbil hippocampus. Mol Med Rep.
14:3215–3219. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brim BL, Haskell R, Awedikian R, Ellinwood
NM, Jin L, Kumar A, Foster TC and Magnusson KR: Memory in aged mice
is rescued by enhanced expression of the GluN2B subunit of the NMDA
receptor. Behav Brain Res. 238:211–226. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Loza Márquez A, Elias V, Wong CP, Ho E,
Bermudez M and Magnusson KR: Effects of ibuprofen on cognition and
NMDA receptor subunit expression across aging. Neuroscience.
344:276–292. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Geinisman Y, Detoledo-Morrell L, Morrell F
and Heller RE: Hippocampal markers of age-related memory
dysfunction: Behavioral, electrophysiological and morphological
perspectives. Prog Neurobiol. 45:223–252. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jacobson L, Zhang R, Elliffe D, Chen KF,
Mathai S, McCarthy D, Waldvogel H and Guan J: Correlation of
cellular changes and spatial memory during aging in rats. Exp
Gerontol. 43:929–938. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Law SW, Conneely OM, DeMayo FJ and
O'Malley BW: Identification of a new brain-specific transcription
factor, NURR1. Mol Endocrinol. 6:2129–2135. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zetterström RH, Williams R, Perlmann T and
Olson L: Cellular expression of the immediate early transcription
factors Nurr1 and NGFI-B suggests a gene regulatory role in several
brain regions including the nigrostriatal dopamine system. Brain
Res Mol Brain Res. 41:111–120. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jiang C, Wan X, He Y, Pan T, Jankovic J
and Le W: Age-dependent dopaminergic dysfunction in Nurr1 knockout
mice. Exp Neurol. 191:154–162. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Johnson MM, Michelhaugh SK, Bouhamdan M,
Schmidt CJ and Bannon MJ: The transcription factor NURR1 exerts
concentration-dependent effects on target genes mediating distinct
biological processes. Front Neurosci. 5:1352011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kadkhodaei B, Ito T, Joodmardi E, Mattsson
B, Rouillard C, Carta M, Muramatsu S, Sumi-Ichinose C, Nomura T,
Metzger D, et al: Nurr1 is required for maintenance of maturing and
adult midbrain dopamine neurons. J Neurosci. 29:15923–15932. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu H, Wei L, Tao Q, Deng H, Ming M, Xu P
and Le W: Decreased NURR1 and PITX3 gene expression in Chinese
patients with Parkinson's disease. Eur J Neurol. 19:870–875. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zetterström RH, Solomin L, Jansson L,
Hoffer BJ, Olson L and Perlmann T: Dopamine neuron agenesis in
Nurr1-deficient mice. Science. 276:248–250. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zheng K, Heydari B and Simon DK: A common
NURR1 polymorphism associated with Parkinson disease and diffuse
Lewy body disease. Arch Neurol. 60:722–725. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Saijo K, Winner B, Carson CT, Collier JG,
Boyer L, Rosenfeld MG, Gage FH and Glass CK: A Nurr1/CoREST pathway
in microglia and astrocytes protects dopaminergic neurons from
inflammation-induced death. Cell. 137:47–59. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim JI, Jeon SG, Kim KA, Kim YJ, Song EJ,
Choi J, Ahn KJ, Kim CJ, Chung HY, Moon M, et al: The
pharmacological stimulation of Nurr1 improves cognitive functions
via enhancement of adult hippocampal neurogenesis. Stem Cell Res
(Amst). 17:534–543. 2016. View Article : Google Scholar
|
|
18
|
Tokuoka H, Hatanaka T, Metzger D and
Ichinose H: Nurr1 expression is regulated by voltage-dependent
calcium channels and calcineurin in cultured hippocampal neurons.
Neurosci Lett. 559:50–55. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chu Y, Kompoliti K, Cochran EJ, Mufson EJ
and Kordower JH: Age-related decreases in Nurr1 immunoreactivity in
the human substantia nigra. J Comp Neurol. 450:203–214. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Parkinson GM, Dayas CV and Smith DW:
Age-related gene expression changes in substantia nigra dopamine
neurons of the rat. Mech Ageing Dev. 149:41–49. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kitabatake Y, Sailor KA, Ming GL and Song
H: Adult neurogenesis and hippocampal memory function: New cells,
more plasticity, new memories? Neurosurg Clin N Am. 18:105–113.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cheal ML: The gerbil: A unique model for
research on aging. Exp Aging Res. 12:3–21. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
National Research Council: Guide for the
Care and Use of Laboratory Animals. 8th edition. National Acadamies
Press; Washington, DC: 2011
|
|
24
|
Choi HS, Ahn JH, Park JH, Won MH and Lee
CH: Age-dependent changes in the protein expression levels of Redd1
and mTOR in the gerbil hippocampus during normal aging. Mol Med
Rep. 13:2409–2414. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee CH and Won MH: Increased dynamin-1 and
−2 protein expression in the aged gerbil hippocampus. Cell Mol
Neurobiol. 34:791–796. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Himeda T, Mizuno K, Kato H and Araki T:
Effects of age on immunohistochemical changes in the mouse
hippocampus. Mech Ageing Dev. 126:673–677. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ahn JH, Shin BN, Park JH, Kim IH, Cho JH,
Chen B, Lee TK, Tae HJ, Lee JC, Cho JH, et al: Long-term
observation of neuronal degeneration and microgliosis in the gerbil
dentate gyrus after transient cerebral ischemia. J Neurol Sci.
363:21–26. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jinno S and Kosaka T: Stereological
estimation of numerical densities of glutamatergic principal
neurons in the mouse hippocampus. Hippocampus. 20:829–840.
2010.PubMed/NCBI
|
|
29
|
Moon M, Jeong I, Kim CH, Kim J, Lee PK,
Mook-Jung I, Leblanc P and Kim KS: Correlation between orphan
nuclear receptor Nurr1 expression and amyloid deposition in 5XFAD
mice, an animal model of Alzheimer's disease. J Neurochem.
132:254–262. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang L, Le W, Xie W and Dani JA:
Age-related changes in dopamine signaling in Nurr1 deficient mice
as a model of Parkinson's disease. Neurobiol Aging. 33(1001):
e7–16. 2012.
|
|
31
|
McNulty SE, Barrett RM, Vogel-Ciernia A,
Malvaez M, Hernandez N, Davatolhagh MF, Matheos DP, Schiffman A and
Wood MA: Differential roles for Nr4a1 and Nr4a2 in object location
vs. object recognition long-term memory. Learn Mem. 19:588–592.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
McQuown SC, Barrett RM, Matheos DP, Post
RJ, Rogge GA, Alenghat T, Mullican SE, Jones S, Rusche JR, Lazar MA
and Wood MA: HDAC3 is a critical negative regulator of long-term
memory formation. J Neurosci. 31:764–774. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
de Ortiz Peña S, Maldonado-Vlaar CS and
Carrasquillo Y: Hippocampal expression of the orphan nuclear
receptor gene hzf-3/nurr1 during spatial discrimination learning.
Neurobiol Learn Mem. 74:161–178. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
von Hertzen LS and Giese KP: Memory
reconsolidation engages only a subset of immediate-early genes
induced during consolidation. J Neurosci. 25:1935–1942. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Colón-Cesario WI, Martínez-Montemayor MM,
Morales S, Félix J, Cruz J, Adorno M, Pereira L, Colón N,
Maldonado-Vlaar CS and de Ortiz Peña S: Knockdown of Nurr1 in the
rat hippocampus: Implications to spatial discrimination learning
and memory. Learn Mem. 13:734–744. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Neves G, Cooke SF and Bliss TV: Synaptic
plasticity, memory and the hippocampus: A neural network approach
to causality. Nat Rev Neurosci. 9:65–75. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Graves AR, Moore SJ, Bloss EB, Mensh BD,
Kath WL and Spruston N: Hippocampal pyramidal neurons comprise two
distinct cell types that are countermodulated by metabotropic
receptors. Neuron. 76:776–789. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lopez-Rojas J and Kreutz MR: Mature
granule cells of the dentate gyrus - Passive bystanders or
principal performers in hippocampal function? Neurosci Biobehav
Rev. 64:167–174. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Das SR and Magnusson KR: Changes in
expression of splice cassettes of NMDA receptor GluN1 subunits
within the frontal lobe and memory in mice during aging. Behav
Brain Res. 222:122–133. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yanai S, Ito H and Endo S: Long-term
cilostazol administration prevents age-related decline of
hippocampus-dependent memory in mice. Neuropharmacology. 129:57–68.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zamzow DR, Elias V, Shumaker M, Larson C
and Magnusson KR: An increase in the association of GluN2B
containing NMDA receptors with membrane scaffolding proteins was
related to memory declines during aging. J Neurosci.
33:12300–12305. 2013. View Article : Google Scholar : PubMed/NCBI
|

















