Introduction
Bone tissue is consistently regenerated through a
sequential process of bone resorption and bone formation termed
bone remodeling (1,2). Bone resorption is conducted by
osteoclasts and bone formation is performed by osteoblasts
(1,2).
The process of bone remodeling is initiated with osteoclastic bone
resorption, following bone formation executed by osteoblasts which
migrate to the resorbed sites (1,2).
Appropriate bone quality and quantity, which is strictly regulated
by various hormones, cytokines and growth factors, is maintained by
the orchestrated cooperation of osteoclasts and osteoblasts
(2). Thus, it is considered that
impairment of bone remodeling causes metabolic bone disease mostly
represented by osteoporosis (2).
Furthermore, accumulating data indicates that osteoblast migration
serves crucial roles not only in many processes of physiological
bone metabolism but also in pathological bone processes including
bone-fracture healing (1,3–5). However,
the exact mechanism for this involvement of osteoblast migration
remains unclear.
It is generally recognized that insulin-like growth
factor-I (IGF-I) serves pivotal roles in the regulation of growth
and bone metabolism (6,7). In our previous studies (8,9), it was
demonstrated that IGF-I stimulates the activity of alkaline
phosphatase, a biomarker of bone formation, through the activation
of p44/p42 mitogen-activated protein (MAP) kinase and
phosphatidylinositol 3-kinase/Akt in osteoblast-like MC3T3-E1
cells. Regarding the effect of IGF-I on osteoblast migration, it
has been reported that IGF-I secreted from osteoblast-like MC3T3-E1
cells acts as a chemotactic factor for these cells through Akt
activation (10). However, the
mechanism for how IGF-I induces the migration of osteoblasts
remains to be fully elucidated.
Natural polyphenolic compounds contained in
beverages possess various beneficial properties including
anti-oxidative and anti-inflammatory effects (11,12). Among
these compounds, it is established that chlorogenic acid is a main
phenolic compound in coffee while (−)-epigallocatechin gallate
(EGCG) is a major polyphenol in green tea (13–15).
Regarding the effects of chlorogenic acid on bone, it has been
documented that chlorogenic acid may strengthen the femoral
diaphysis against mechanical stress due to upregulation of
mineralization in the tibia (16). In
addition, chlorogenic acid reportedly inhibits osteoclast
functions, resulting in the suppression of bone resorption
(17). Accumulating data indicates
that green tea consumption in elderly subjects decreases the risk
of bone fracture by increasing bone mass and mineral density
(18). It has further been reported
that EGCG may inhibit osteoclastic bone resorption and stimulate
osteoblastic bone formation (18,19). In
our previous study (20), it was
identified that EGCG enhanced osteoprotegerin synthesis in
osteoblast-like MC3T3-E1 cells. However, the exact roles of
chlorogenic acid and EGCG in bone metabolism are yet to be fully
clarified.
In the present study, using Transwell migration and
wound-healing assays, the effects of chlorogenic acid and EGCG on
the IGF-I-induced migration of osteoblast-like MC3T3-E1 cells were
investigated. Additionally the mechanism underlying IGF-I-induced
migration was examined by western blot analysis, and the effects of
corresponding inhibitors (PD98059, rapamycin and deguelin) on
migration were evaluated.
Materials and methods
Materials
IGF-I was obtained from R&D Systems, Inc.
(Minneapolis, MN, USA). Chlorogenic acid and EGCG were purchased
from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). PD98059,
rapamycin and deguelin were obtained from Calbiochem-Novabiochem
Corporation (San Diego, CA, USA). Phospho-specific p44/p42 MAP
kinase antibody (cat. no. 9101), p44/p42 MAP kinase antibody (cat.
no. 9102), phospho-specific p38 MAP kinase antibody (cat. no.
4511), phospho-specific stress-activated protein kinases
(SAPK)/c-Jun N-terminal kinase (JNK) antibody (cat. no. 9251),
phospho-specific p70 S6 kinase antibody (cat. no. 9205),
phospho-specific Akt antibody (cat. no. 9275) and Akt antibody
(cat. no. 9272) were purchased from Cell Signaling Technology
(Danvers, MA, USA). GAPDH antibody (cat. no. SC-47724) was obtained
from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). An
electrochemiluminescence (ECL) western blotting detection system
was purchased from GE Healthcare (Amersham, UK). Other materials
and chemicals were obtained from commercial sources. Chlorogenic
acid was dissolved in ethanol. EGCG was dissolved in dimethyl
sulfoxide. The maximum concentration of ethanol or dimethyl
sulfoxide was 0.1%, which did not affect the assay for cell
migration or western blot analysis in preliminary experiments.
Cell culture
Cloned osteoblast-like MC3T3-E1 cells derived from
newborn mouse calvaria (21) were
provided by Dr Masayoshi Kumegawa (Meikai University, Sakado,
Japan) and maintained as previously described (22). Briefly, MC3T3-E1 cells were cultured
in α-minimum essential medium (α-MEM) containing 10% fetal bovine
serum (FBS) at 37°C in a humidified atmosphere of 5%
CO2/95% air. The cells were seeded into 90-mm diameter
dishes (2×105 cells/dish) in the α-MEM containing 10%
FBS for 5 days. The medium was then exchanged for α-MEM containing
0.3% FBS, and the cells were subsequently used for western blot
analysis after 48 h culture at 37°C. For the cell migration assay,
MC3T3-E1 cells in α-MEM containing 10% FBS cultured for 3 days were
sub-cultured in α-MEM containing 0.3% FBS for 6 h (all at 37°C),
and then were used for the migration experiments.
Cell migration assay
A Transwell cell migration assay was performed using
a Boyden chamber (polycarbonate membrane with 8-µm pores; Corning
Inc., Corning, NY, USA) as described previously (23). In brief, MC3T3-E1 cells were
trypsinized and seeded (1.0×105 cells/well) onto the
upper chamber in α-MEM containing 0.3% FBS. IGF-I (0 or 10 nM) was
added to the lower chamber in α-MEM containing 0.3% FBS and
incubated for 16 h at 37°C, and the cells on the upper surface of
the membrane were then mechanically removed. The migrated cells
adherent to the underside of the membrane were fixed with 4%
paraformaldehyde and stained with DAPI solution. The migrated cells
were photographed and quantified using fluorescent microscopy at a
magnification of ×20 by counting the stained cells in three
randomly selected fields. Prior to assays, subsets of cells were
pretreated with chlorogenic acid (0, 10, 30 or 50 µM), EGCG (0,
0.1, 0.3 or 1.0 µM), PD98059 (0 or 30 µM), rapamycin (0 or 50
ng/ml) or deguelin (0 or 0.5 µM) for 60 min at 37°C.
For a wound-healing assay, MC3T3-E1 cells were
seeded in α-MEM containing 10% FBS at 1.0×105 cells/well
into an ibidi Culture-Insert 2 Well (ibidi GmbH, Martinsried,
Germany) with a 500-µm margin from the side of the well and grown
for 24 h at 37°C. Following removal of the insert, the cells were
stimulated with 70 nM IGF-I or vehicle (phosphate-buffered saline
supplemented with 0.01% bovine serum albumin) for 8 h at 37°C.
Based on results of the Transwell assays, subsets of control and
stimulated cells were pretreated with 1 µM EGCG or vehicle for 60
min. The cells were photographed using an EOS Kiss X4 digital
camera (Canon Inc., Tokyo, Japan) connected to a CK40 culture
microscope (Olympus Co., Ltd., Tokyo, Japan) prior to the
stimulation with IGF-I and after 8 h. The area comprised of
migrated cells was measured by ImageJ software (version 1.48;
National Institutes of Health, Bethesda, MD, USA).
Western blot analysis
MC3T3-E1 cells pretreated with the stated doses of
EGCG, PD98059, rapamycin, deguelin or vehicle, then stimulated by
10 nM IGF-I or vehicle in 1 ml α-MEM containing 0.3% FBS for
different time periods (1, 3, 5, 10, 15, 20 and 30 min) at 37°C.
The cells were then lysed, homogenized and sonicated in a lysis
buffer containing 62.5 mM Tris/HCl, pH 6.8, 2% sodium dodecyl
sulfate (SDS), 50 mM dithiothreitol and 10% glycerol.
SDS-polyacrylamide gel electrophoresis (PAGE) was performed
according to the method of Laemmli (24) on 10% polyacrylamide gels. The protein
was fractionated and transferred onto an Immun-Blot polyvinylidene
difluoride (PVDF) membrane (Bio-Rad Laboratories, Inc., Hercules,
CA, USA). The membranes were blocked at 4°C with 5% fat-free dry
milk in Tris-buffered saline-Tween (TBS-T; 20 mM Tris/HCl, pH 7.6,
137 mM NaCl, 0.1% Tween-20) for 1 h prior to incubation with
primary antibodies. Western blot analysis was performed as
described previously (25) using the
phospho-specific p44/p42 MAP kinase, p44/p42 MAP kinase,
phospho-specific p38 MAP kinase, phospho-specific SAPK/JNK,
phospho-specific p70 S6 kinase, phospho-specific Akt, Akt and GAPDH
(loading control) antibodies as primary antibodies, and
peroxidase-labeled antibodies raised in goat against rabbit
immunoglobulin G (cat. no. 5110-0336; KPL, Inc., Gaithersburg, MD,
USA) used as secondary antibodies. The primary and secondary
antibodies were diluted at 1:1,000 with 5% fat-free dry milk in
TBS-T and incubated at room temperature. The peroxidase activity on
the PVDF membrane was visualized on X-ray film by means of the ECL
western blotting detection system.
Densitometric analysis
Densitometric analysis was performed using a scanner
and ImageJ version 1.48. The levels of phosphorylated target
proteins were calculated as follows: the background-subtracted
signal intensity of each phosphorylation signal was respectively
normalized to the total protein signal, and plotted as a fold
increase in comparison to that of the control cells treated without
pretreatment or stimulation.
Statistical analysis
The data were analyzed by one-way analysis of
variance followed by the Bonferroni method for multiple comparisons
between pairs, and P<0.05 was considered to indicate statistical
significance. Microsoft Excel 2010 (Microsoft Corporation, Redmond,
WA, USA) was used for the analysis. All data are presented as the
mean ± standard error of the mean of triplicate determinations from
three independent cell preparations.
Results
Effects of chlorogenic acid and EGCG
on IGF-I-induced migration of MC3T3-E1 cells
It has been reported that IGF-I induces the
migration of osteoblasts including osteoblast-like MC3T3-E1 cells
(10). The present study identified
that IGF-I elicited the migration of MC3T3-E1 cells when assessed
by Transwell and wound healing assays. In the Transwell assay, the
effect of IGF-I on cell migration was dose-dependent over the range
3–30 nM, whereas in the wound healing assay, the effect was
dose-dependent over the range 1–100 nM (data not shown).
Initially, the effect of chlorogenic acid or EGCG on
the IGF-I-induced migration of MC3T3-E1 cells was investigated
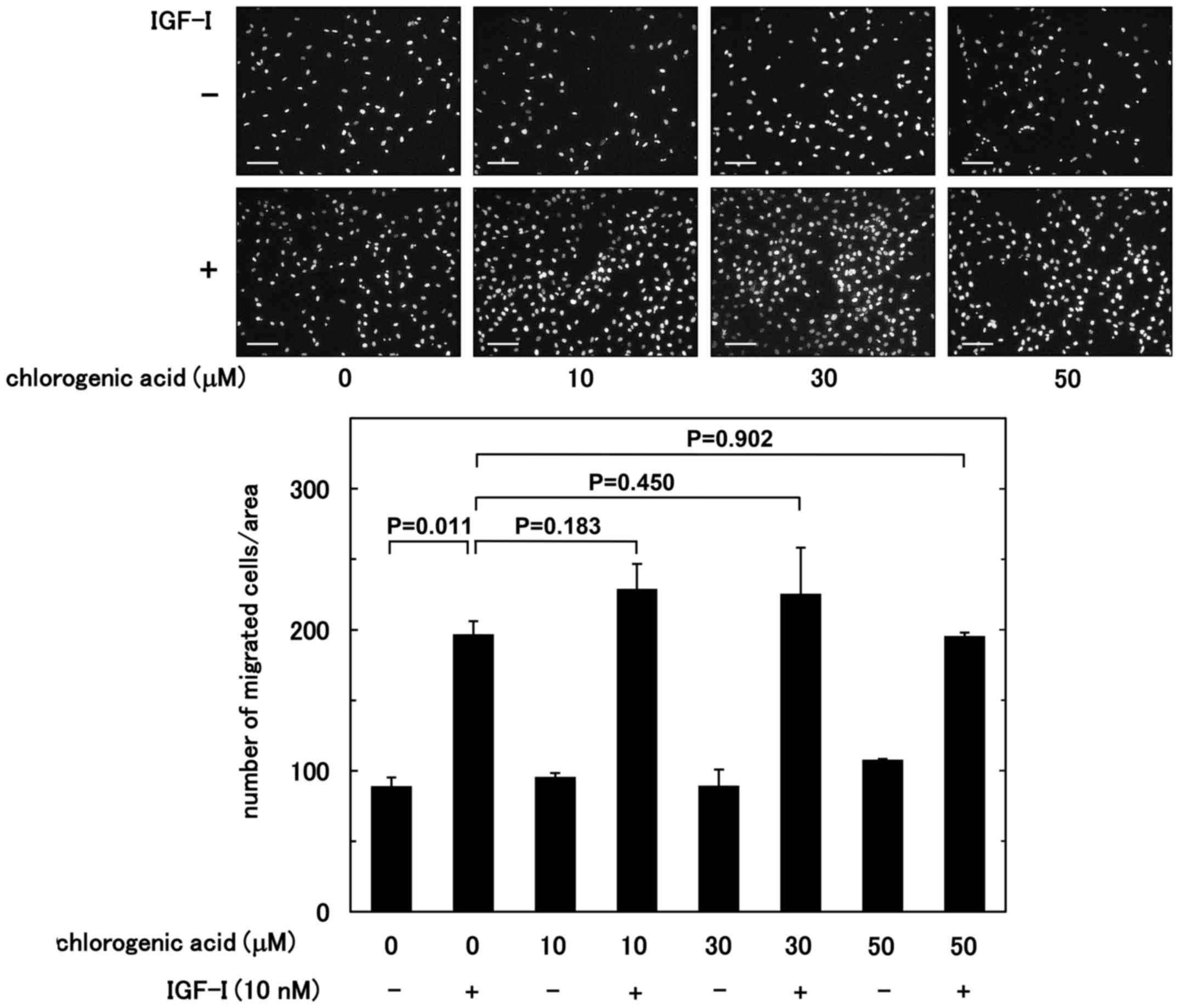
using a Boyden chamber. Chlorogenic acid had no significant effect
on the IGF-I-stimulated migration up to 50 µM (Fig. 1). By contrast, the IGF-I-stimulated
migration was suppressed by EGCG in an apparent dose-dependent
manner in the range 0.1–1.0 µM; with the concentrations 0.3 and 1.0
µM yielding significant effect (P=3.0×10−4 and
2.5×10−4, respectively) (Fig.
2).
The effect of EGCG on the IGF-I-stimulated migration
of osteoblast-like MC3T3-E1 cells was subsequently evaluated by
wound-healing assay. As above, EGCG (1 µM) markedly suppressed the
IGF-I-stimulated MC3T3-E1 cell migration (P=0.020) (Fig. 3).
Effect of IGF-I on the phosphorylation
of p44/p42 MAP kinase, p38 MAP kinase, SAPK/JNK, Akt and p70 S6
kinase in MC3T3-E1 cells
Regarding the intracellular signaling of IGF-I in
osteoblasts, our group previously demonstrated that p44/p42 MAP
kinase and Akt may serve as positive regulators in the
IGF-I-stimulated activity of alkaline phosphatase, a biochemical
marker of bone formation, in osteoblast-like MC3T3-E1 cells
(8,9).
It is established that the MAP kinase superfamily including p44/p42
MAP kinase, p38 MAP kinase and SAPK/JNK are essential molecules in
the transduction of various messages from a range of stimulators
(26). As for osteoblast migration,
it has been reported that the pathway of PI3K/Akt is involved in
the IGF-I-stimulated migration (10).
In addition, p70 S6 kinase is reportedly implicated in ovarian
cancer cell migration (27). In the
current study, the phosphorylation of p44/p42 MAP kinase, p70 S6
kinase and Akt was markedly induced by IGF-I (10 nM), while IGF-I
had little effect on the phosphorylation of p38 MAP kinase and
SAPK/JNK (Fig. 4), suggesting that
IGF-I stimulates the activation of p44/p42 MAP kinase, Akt and p70
S6 kinase but not of p38 MAP kinase and SAPK/JNK in osteoblast-like
MC3T3-E1 cells. GAPDH was adopted as the control instead of p70 S6
kinase, since reliable antibodies against non-phosphorylated p70 S6
kinase were not obtained.
 | Figure 4.Effects of IGF-I on the
phosphorylation of p44/p42 MAP kinase, p38 MAP kinase, SAPK/JNK,
Akt and p70 S6 kinase in MC3T3-E1 cells. The cells were stimulated
by 10 nM IGF-I for the indicated periods. Western blot analysis was
performed using antibodies against phospho-p44/p42 MAP kinase,
phospho-p38 MAP kinase, phospho-SAPK/JNK, phospho-p70 S6 kinase,
phospho-Akt and GAPDH. IGF-I, insulin-like growth factor-I; MAP,
mitogen-activated protein; SAPK/JNK, stress-activated protein
kinase/c-Jun N-terminal kinase. |
Effects of PD98059, rapamycin and
deguelin on IGF-I-induced migration of MC3T3-E1 cells
To investigate whether p44/p42 MAP kinase, p70 S6
kinase or Akt is involved in the IGF-I-stimulated migration of
osteoblast-like MC3T3-E1 cells, the effects of PD98059, an
inhibitor of the upstream kinase that activates p44/p42 MAP kinase
[MAP kinase kinase (MEK)1/2] (28),
rapamycin, an inhibitor of mammalian target of rapamycin (mTOR)
that activates p70 S6 kinase (29),
and deguelin, an inhibitor of Akt (30) on the induced migration were assessed
(Figs. 5–7). Treatment with PD98059 or deguelin
significantly reduced the IGF-I-induced migration (P=0.002 and
0.014, respectively) (Figs. 5A and
7A), whereas rapamycin did not affect
the migration induced by IGF-I (Fig.
6A). It was confirmed that in MC3T3-E1 cells, PD98059, deguelin
and rapamycin acted as an inhibitor of their respective targets
(Figs. 5B, 6B and 7B).
Effect of EGCG on IGF-I-induced
phosphorylation of p44/p42 MAP kinase and Akt in MC3T3-E1
cells
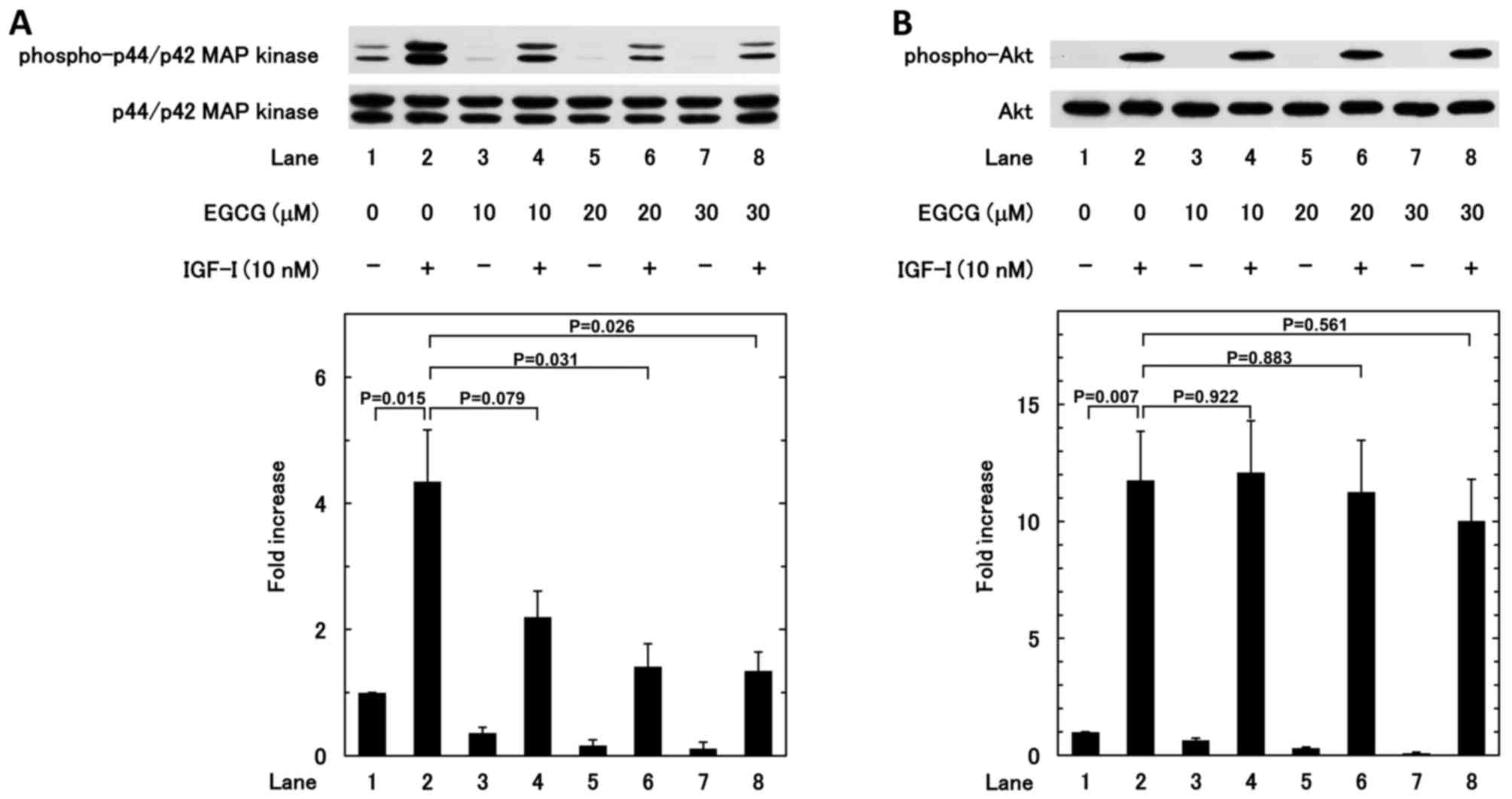
To clarify the mechanism underlying the inhibition
of IGF-I-induced migration by EGCG, the effect of EGCG on the
IGF-I-induced phosphorylation of p44/p42 MAP kinase and Akt was
investigated. EGCG (20 and 30 µM) significantly reduced the
IGF-I-induced phosphorylation of p44/p42 MAP kinase (Fig. 8A); whereas the IGF-I-induced
phosphorylation of Akt was not affected by EGCG (Fig. 8B).
Discussion
In the present study, it was demonstrated the
IGF-I-induced migration of osteoblast-like MC3T3-E1 cells was
significantly suppressed by EGCG, a major catechin in green tea
(14,18), whereas chlorogenic acid, a main
polyphenol in coffee (13), did not
affect the induced migration. The intracellular signaling system
behind the IGF-I-induced migration of osteoblast-like MC3T3-E1
cells was also investigated, as well as the exact mechanism
underlying the inhibition of IGF-I-induced migration by EGCG. The
IGF-I-induced migration of MC3T3-E1 cells was suppressed by
PD98059, an MEK inhibitor (28), and
deguelin, an Akt inhibitor (30), in
osteoblast-like MC3T3-E1 cells. Regarding the intracellular
signaling of IGF-I in osteoblast migration, it has been reported
that phosphatidylinositol 3-kinase is involved in the migration
event (10). Since it is generally
known that Akt acts at a point downstream of phosphatidylinositol
3-kinase, the current finding is consistent with that reported
previously. Based on overall findings, it is probable that p44/p42
MAP kinase and Akt serve as positive regulators in the
IGF-I-induced migration of osteoblast-like MC3T3-E1 cells. In
addition, it was identified that EGCG markedly reduced the
IGF-I-induced phosphorylation of p44/p42 MAP kinase without
affecting the induced phosphorylation of Akt in MC3T3-E1 cells.
Collectively, these findings suggest that EGCG repressed the
IGF-I-induced migration of osteoblast-like MC3T3-E1 cells through
inhibition of p44/p42 MAP kinase.
In physiological bone remodeling, the migration of
osteoblasts to the sites resorbed by osteoclasts is an essential
step, as the migrated osteoblasts are activated and initiate bone
formation at the bone resorbed sites, resulting in the maintenance
of moderate bone mass (3–5). In addition, the migration of osteoblasts
is crucial also in the case of pathological bone states including
osteoporosis and bone fracture repair (3–5). Thus,
appropriate migration of osteoblasts is necessary for the
regulation of bone remodeling, and proper osteoblast migration is
considered to be essential for maintaining both the quantity and
quality of bone mass (1,3–5). It has
been proposed that green tea consumption may be associated with
increased bone mass, improved bone density and decreased risk of
bone fracture (18). Green tea
polyphenols including EGCG reportedly protected bone loss in
middle-aged female rats following ovariectomy in vivo
(31). It has also been reported that
lower concentrations (1, 5 and 10 µM) of EGCG did not affect the
migration of alveolar bone cells, whereas higher concentrations (25
and 50 µM) suppressed cell migration analyzed by wound-healing
assay (32). In the present study,
the significant suppressive effect of EGCG on the IGF-I-induced
migration of MC3T3-E1 cells was observed at 1 µM in Transwell and
wound healing assays. Based on these findings, it is possible that
EGCG elicits a modulatory effect in osteoblast migration, leading
to appropriate bone remodeling. It has been documented that the
maximum plasma concentration of EGCG reaches approximately 0.7 µM
following moderate green tea ingestion (3 g decaffeinated green tea
solids) in humans (33). Regarding
chlorogenic acid, it has been reported that when single servings of
coffee beverage containing low (412 µmol), medium (635 µmol) and
high (795 µmol) levels of chlorogenic acids were administered to
healthy subjects, a value of maximum peak plasma concentration of
approximately 1.5 µM was observed in following the medium level
serving (34), which is considerably
lower than the doses used in the present study. Taking these
findings into account, the concentrations of EGCG and chlorogenic
acid used for osteoblast migration in vitro in the present
study appeared physiologically relevant to green tea drinkers but
rather super-physiological to coffee consumers in vivo. In
addition, it has been reported that IGF-I upregulates
osteoclastogenesis from monocytes in vitro (35). Thus, it is possible that IGF-I can
potentiate the osteoclast supply in the process of bone remodeling
in addition to stimulating osteoblast migration. In the present
study, the effects of EGCG and chlorogenic acid were elucidated
only with regard to osteoblast-like MC3T3-E1 cells, and not
monocyte-osteoclast lineage cells. To understand the exact
mechanism underlying the potential favorable effects of natural
compounds including EGCG and chlorogenic acid on human bone health
and protection against osteoporosis, experiments in
monocyte-osteoclast lineage cells in addition to osteoblasts would
be useful. Further investigations are also required to clarify the
molecular mechanism underlying the apparent inhibition of
IGF-I-induced osteoblast migration by EGCG.
In conclusion, the present results suggest that EGCG
represses IGF-I-induced migration of osteoblasts, possibly
resulting in the adjustment of bone remodeling, and that the effect
of EGCG is exerted through the suppression of p44/p42 MAP
kinase.
Acknowledgements
The authors are thankful to Mrs. Yumiko Kurokawa
(Department of Pharmacology, Gifu University Graduate School of
Medicine, Gifu, Japan) for her technical assistance.
Funding
The current investigation was supported in part by
Grants-in-Aid for Scientific Research (grant nos. 26462289 and
15K10487) from the Ministry of Education, Culture, Sports, Science
and Technology of Japan, a Grant-in-Aid for Scientific Research
(grant no. H25-Aging-General-004) from the Ministry of Health,
Labour and Welfare of Japan, and Research Funding for Longevity
Sciences (grant nos. 26-12 and 28-9) from the National Center for
Geriatrics and Gerontology, Japan.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TK, TO and OK conceived and designed the
experiments. TK, GS, KF, and RM-N performed the experiments. TK,
GS, KF, RM-N, HT and OK analyzed the data. TK, TO, HT and OK wrote
the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Karsenty G and Wagner EF: Reaching a
genetic and molecular understanding of skeletal development. Dev
Cell. 2:389–406. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kular J, Tickner J, Chim SM and Xu J: An
overview of the regulation of bone remodelling at the cellular
level. Clin Biochem. 45:863–873. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Khan SN, Bostrom MP and Lane JM: Bone
growth factors. Orthop Clin North Am. 31:375–388. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lieberman JR, Daluiski A and Einhorn TA:
The role of growth factors in the repair of bone. Biology and
clinical applications. J Bone Joint Surg Am. 84-A:1–1044.
2002.PubMed/NCBI
|
|
5
|
Reddi AH, Roodman D, Freeman C and Mohla
S: Mechanisms of tumor metastasis to the bone: Challenges and
opportunities. J Bone Miner Res. 18:190–194. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Clemens TL and Chernausek SD: Genetic
strategies for elucidating insulin-like growth factor action in
bone. Growth Horm IGF Res. 14:195–199. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Niu T and Rosen CJ: The insulin-like
growth factor-I gene and osteoporosis: A critical appraisal. Gene.
361:38–56. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Noda T, Tokuda H, Yoshida M, Yasuda E,
Hanai Y, Takai S and Kozawa O: Possible involvement of
phosphatidylinositol 3-kinase/Akt pathway in insulin-like growth
factor-I-induced alkaline phosphatase activity in osteoblasts. Horm
Metab Res. 37:270–274. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hanai Y, Tokuda H, Ishisaki A,
Matsushima-Nishiwaki R, Nakamura N, Yoshida M, Takai S, Ohta T and
Kozawa O: Involvement of p44/p42 MAP kinase in insulin-like growth
factor-I-induced alkaline phosphatase activity in
osteoblast-like-MC3T3-E1 cells. Mol Cell Endocrinol. 251:42–48.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nakasaki M, Yoshioka K, Miyamoto Y, Sasaki
T, Yoshikawa H and Itoh K: IGF-I secreted by osteoblasts acts as a
potent chemotactic factor for osteoblasts. Bone. 43:869–879. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jankun J, Selman SH, Swiercz R and
Skrzypczak-Jankun E: Why drinking green tea could prevent cancer.
Nature. 387:5611997. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Koo SH and Montminy M: In vino veritas: A
tale of two sirt1s? Cell. 127:1091–1093. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
George SE, Ramalakshmi K and Mohan Rao LJ:
A perception on health benefits of coffee. Crit Rev Food Sci Nutr.
48:464–486. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Thielecke F and Boschmann M: The potential
role of green tea catechins in the prevention of the metabolic
syndrome - a review. Phytochemistry. 70:11–24. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shimizu M, Adachi S, Masuda M, Kozawa O
and Moriwaki H: Cancer chemoprevention with green tea catechins by
targeting receptor tyrosine kinases. Mol Nutr Food Res. 55:832–843.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Folwarczna J, Pytlik M, Zych M, Cegieła U,
Nowinska B, Kaczmarczyk-Sedlak I, Sliwinski L, Trzeciak H and
Trzeciak HI: Effects of caffeic and chlorogenic acids on the rat
skeletal system. Eur Rev Med Pharmacol Sci. 19:682–693.
2015.PubMed/NCBI
|
|
17
|
Kwak SC, Lee C, Kim JY, Oh HM, So HS, Lee
MS, Rho MC and Oh J: Chlorogenic acid inhibits osteoclast
differentiation and bone resorption by down-regulation of receptor
activator of nuclear factor κ-B ligand-induced nuclear factor of
activated T cells c1 expression. Biol Pharm Bull. 36:1779–1786.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shen CL, Yeh JK, Cao JJ and Wang JS: Green
tea and bone metabolism. Nutr Res. 29:437–456. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Singh R, Akhtar N and Haqqi TM: Green tea
polyphenol epigallocatechin-3-gallate: Inflammation and arthritis.
[corrected]. Life Sci. 86:907–918. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fujita K, Otsuka T, Yamamoto N, Kainuma S,
Ohguchi R, Kawabata T, Sakai G, Kuroyanagi G, Matsushima-Nishiwaki
R, Kozawa O, et al: (−)-Epigallocatechin gallate but not
chlorogenic acid upregulates osteoprotegerin synthesis through
regulation of bone morphogenetic protein-4 in osteoblasts. Exp Ther
Med. 14:417–423. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sudo H, Kodama HA, Amagai Y, Yamamoto S
and Kasai S: In vitro differentiation and calcification in a new
clonal osteogenic cell line derived from newborn mouse calvaria. J
Cell Biol. 96:191–198. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kozawa O, Tokuda H, Miwa M, Kotoyori J and
Oiso Y: Cross-talk regulation between cyclic AMP production and
phosphoinositide hydrolysis induced by prostaglandin E2 in
osteoblast-like cells. Exp Cell Res. 198:130–134. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Karagiosis SA, Chrisler WB, Bollinger N
and Karin NJ: Lysophosphatidic acid-induced ERK activation and
chemotaxis in MC3T3-E1 preosteoblasts are independent of EGF
receptor transactivation. J Cell Physiol. 219:716–723. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Laemmli UK: Cleavage of structural
proteins during the assembly of the head of bacteriophage T4.
Nature. 227:680–685. 1970. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kato K, Ito H, Hasegawa K, Inaguma Y,
Kozawa O and Asano T: Modulation of the stress-induced synthesis of
hsp27 and alpha B-crystallin by cyclic AMP in C6 rat glioma cells.
J Neurochem. 66:946–950. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kyriakis JM and Avruch J: Mammalian MAPK
signal transduction pathways activated by stress and inflammation:
A 10-year update. Physiol Rev. 92:689–737. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ip CK, Cheung AN, Ngan HY and Wong AS: p70
S6 kinase in the control of actin cytoskeleton dynamics and
directed migration of ovarian cancer cells. Oncogene. 30:2420–2432.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Alessi DR, Cuenda A, Cohen P, Dudley DT
and Saltiel AR: PD 098059 is a specific inhibitor of the activation
of mitogen-activated protein kinase kinase in vitro and in vivo. J
Biol Chem. 270:27489–27494. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li Y, Corradetti MN, Inoki K and Guan KL:
TSC2: Filling the GAP in the mTOR signaling pathway. Trends Biochem
Sci. 29:32–38. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Toker A and Newton AC: Cellular signaling:
Pivoting around PDK-1. Cell. 103:185–188. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shen CL, Wang P, Guerrieri J, Yeh JK and
Wang JS: Protective effect of green tea polyphenols on bone loss in
middle-aged female rats. Osteoporos Int. 19:979–990. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mah YJ, Song JS, Kim SO, Lee JH, Jeon M,
Jung UW, Moon SJ, Kim JH and Choi HJ: The effect of
epigallocatechin-3-gallate (EGCG) on human alveolar bone cells both
in vitro and in vivo. Arch Oral Biol. 59:539–549. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang CS, Chen L, Lee MJ, Balentine D, Kuo
MC and Schantz SP: Blood and urine levels of tea catechins after
ingestion of different amounts of green tea by human volunteers.
Cancer Epidemiol Biomarkers Prev. 7:351–354. 1998.PubMed/NCBI
|
|
34
|
Stalmach A, Williamson G and Crozier A:
Impact of dose on the bioavailability of coffee chlorogenic acids
in humans. Food Funct. 5:1727–1737. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhu Z, Huang P, Chong Y, George SK, Wen B,
Han N, Liu Z, Kang L and Lin N: Nucleus pulposus cells derived
IGF-1 and MCP-1 enhance osteoclastogenesis and vertebrae disruption
in lumbar disc herniation. Int J Clin Exp Pathol. 7:8520–8531.
2014.PubMed/NCBI
|