Introduction
The progress of combination antiretroviral therapy
(cART) has led to increased life expectancy of patients infected
with human immunodeficiency virus (HIV) (1). However, as life expectancy has
increased, chronic complications have become key challenges
(2). Osteoporosis is among the
chronic complications seen in HIV-infected patients; the prevalence
of osteoporosis is reportedly three times higher among HIV-infected
patients than non-HIV-infected patients (3). The reported prevalence rate of
osteopenia in HIV-infected cohorts ranges from 22 to 71%, and that
of osteoporosis from 3 to 33% (4).
The causes of osteoporosis in HIV-infected patients are multiple,
and include chronic HIV infection, living habits such as smoking
and alcohol consumption, and antiretroviral drug use (5). Among antiretroviral drugs, protease
inhibitors have been reported to be associated with osteoporosis,
and antiretroviral regimens containing protease inhibitors can
accelerate osteopenia and osteoporosis (6).
Osteoporosis is caused by an imbalance of bone
resorption and formation (7).
Osteoclasts have a role in bone formation, and osteoblasts that are
derived from mesenchymal stem cells are responsible for bone
formation (8). Osteoblast
differentiation is regulated by transcription factors including
Runt-related transcription factor 2 (Runx2) (9). Runx2 is a positive regulator of genes
associated with bone matrix proteins including collagen type I α
1/2 chain (9), and triggers the
expression of major bone matrix genes during the early stages of
osteoblast differentiation (9). As
such, Runx2 is considered to serve a central role in skeletal
development and be associated with osteoporosis (9). However, it remains to be determined
specifically how anti-HIV drugs affect osteoblast differentiation.
Thus, in the present study, the influence of anti-HIV drugs on
osteoblast differentiation was examined in vitro.
Materials and methods
Cell line and culture
The clonal mouse osteoblastic cell line, MC3T3-E1
subclone 14, was purchased from American Type Culture Collection
(Manassas, VA, USA). The MC3T3-E1 cells were cultured at 37°C in 5%
CO2 in Dulbecco's modified Eagle's medium (DMEM;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) with 10% fetal
bovine serum (Hyclone; GE Healthcare Life Science, Logan, UT, USA),
100 IU/ml penicillin and 100 µg/ml streptomycin. To induce
osteoblast differentiation, MC3T3-E1 cells were cultured at 37°C in
5% CO2 in DMEM with osteoblast inducer reagent
containing 1% L-ascorbic acid, 2% β-glycerophosphate and 0.2%
hydrocortisone (Takara Bio, Inc., Otsu, Japan). The medium was
changed every other day.
Anti-HIV drugs
The HIV protease inhibitors ritonavir, darunavir,
atazanavir and lopinavir were purchased from Toronto Research
Chemicals, Inc. (Toronto, ON, Canada). These drugs were prepared as
stock solutions in methanol. All prepared anti-HIV drugs were mixed
with osteoblast differentiation medium to adjust to the maximum
serum concentration (Cmax) that these drugs achieve when they are
administered at treatment doses in adult HIV patients. Cmax values
of protease inhibitors were based on those on the University of
Liverpool website, www.hiv-druginteraction.org. The Cmax values were:
Ritonavir, 11.20 µg/ml; lopinavir, 9.60 µg/ml; darunavir, 6.50
µg/ml; and atazanavir 3.15 µg/ml.
Alkaline phosphatase (ALP)
activity
MC3T3-E1 cells were seeded at a density of
1×104 cells/well in a 96-well microplate in normal
culture medium. To induce differentiation, the medium was replaced
by DMEM with osteoblast inducer reagent after 1 day in the presence
or absence of each protease inhibitor, respectively. The cells were
incubated for 7 and 9 days in four types of medium: i) DMEM; ii)
DMEM with osteoblast inducer reagent; iii) DMEM with osteoblast
inducer reagent and an anti-HIV drug; and iv) DMEM with vehicle
(methanol). For subsequent treatments with ritonavir, MC3T3-E1
cells were treated with various concentrations (0.1, 0.5, 1.0, 5.0
and 10.0 µg/ml ritonavir) and for various durations (10.0 µg/ml
ritonavir for 9, 11 and 14 days). All cells were cultured at 37°C
in a 5% CO2 atmosphere. ALP activity was evaluated using
a TRACP & ALP double-staining kit (Takara Bio, Inc.) according
to the manufacturer's protocol. Briefly, the plates were incubated
with kit reagents at 37°C for 15 min, absorbance was measured at
405 nm, and ALP activity was calculated from a standard value (bone
ALP; Takara Bio, Inc.). The total protein concentration of each
cell was determined by a DC™ Protein Assay (Bio-Rad Laboratories,
Inc., Hercules, CA, USA), and the activity data were normalized to
the total protein concentration.
Alizarin Red staining
MC3T3-E1 cells were seeded at a density of
4×104 cells/well in a 24-well plate. The cells were
incubated with 10.0 µg/ml ritonavir or control mediums as above.
Mineralization of MC3T3-E1 cells was confirmed by using an Alizarin
Red S staining kit (Cosmo Bio, Co., Ltd., Tokyo, Japan) after 7,
14, 21 and 28 days of culture. Then, cells were washed three times
with phosphate-buffered saline, with 500 µl of 100% methanol added
to each well, and incubated at 4°C for 20 min. Alizarin Red
solution was added to each well and incubated at room temperature
for 5 min. Each well was then washed again three times and directly
observed.
Total RNA extraction and cDNA
synthesis
MC3T3-E1 cells seeded at 1×105 cells/well
in a 12-well plate were incubated with 10.0 µg/ml ritonavir or
control mediums as above for 3, 5 and 7 days. Total RNA was
extracted from the MC3T3-E1 cells by adding 350 µl Tripure (Roche,
Basel, Switzerland) to each well. RNA was isolated according to the
manufacturer's instructions. Total RNA was reverse-transcribed into
cDNA by using ReverTra Ace® qPCR RT Master Mix (Toyobo
Life Science, Osaka, Japan) according to the manufacturer's
instructions.
Quantitative polymerase chain reaction
(qPCR)
Runx2 gene expression in MC3T3-E1 cells was examined
by qPCR. β-actin was used as an internal control gene. The qPCR was
performed with a Roche LightCycler 480 system using a LightCycler
480 Probe Master kit (Roche Diagnostics, Basel, Switzerland).
Reactions were performed in a final total volume of 20 µl
containing 1× LightCycler 480 Probe Master mix, 0.1 µM of each
forward and reverse primer, 3.8 µl distilled H2O and 5
µl of the cDNA template. Specific primers for mouse Runx2 and
β-actin were designed using the Universal ProbeLibrary (Roche
Diagnostics). The primers used in the current study were as
follows: For Runx2, forward, 5′-cgtgtcagcaaagcttctttt-3′ and
reverse, 5′-ggctcacgtcgctcatct-3′; and for β-actin, forward,
5′-tgacaggatgcagaaggaga-3′ and reverse, 5′-cgctcaggaggagcaatg-3′.
The amplification conditions were as follows: Denaturation at 95°C
for 5 min, 45 cycles of amplification (95°C for 10 sec, 60°C for 30
sec and 72°C for 1 sec), and cooling at 50°C for 10 sec. Relative
quantification of target gene expression was determined using the
E-Method (Efficiency Method) from the LightCycler 480 software
(10).
Lactate dehydrogenase (LDH) activity
assay
LDH activity was measured to determine MC3T3-E1 cell
viability following treatment with ritonavir. MC3T3-E1 cells were
plated in 12-well plates (1×105cells/well) and incubated
in DMEM with osteoblast inducer reagent in the presence or absence
of ritonavir (10 µg/ml). On day 8, culture medium was replenished
and cells were cultured again for 24 h. Cell medium and lysate
extracted with radioimmunoprecipitation assay buffer (Wako Pure
Chemical Industries, Ltd., Osaka, Japan) were collected on day 9,
and cell viability was evaluated using Cytotoxicity detection
KitPLUS (Roche Diagnostics) according to the manufacturer's
protocol. Cells in DMEM only were defined as a control. The
absorbance was measured at 490 nm, and cell viability was
calculated using the following formula: Cell viability (%) =
{1-[(absorbance of medium - absorbance of control
medium)/(absorbance of lysate - absorbance of control lysate)]}
×100.
Statistical analysis
Significant differences among samples were
determined by one-way analysis of variance with post hoc Tukey's
honest significant difference test, with P<0.05 considered to
indicate statistical significance. At least three samples were
tested in three independent experiments. All results are presented
as means ± standard error of the mean. The data analysis was
performed using JMP software version 12.2 (SAS Institute, Inc.,
Cary, NC, USA).
Results
Osteoblast differentiation is
inhibited by ritonavir
As documented, osteoblasts are differentiated by a
medium that contains L-ascorbic acid, β-glycerophosphate and
steroid (11). Since ALP activity is
enhanced during osteoblast differentiation in vitro, ALP may
be used as an osteogenic differentiation marker (12). First, the present study observed that
ALP activity was enhanced in MC3T3-E1 cells at 7 days after the
addition of DMEM with osteoblast inducer reagent compared with in
cells cultured in DMEM only (P<0.05; data not shown).
Subsequently, the effect of protease inhibitors on osteoblast
differentiation was examined. MC3T3-E1 cells were cultured in
osteogenic differentiation medium in the presence or absence of the
protease inhibitors ritonavir, lopinavir, darunavir and atazanavir
3.15 µg/ml at their Cmax for 9 days, and then ALP activity was
examined. The ALP activity of MC3T3-E1 cells cultured with
ritonavir was significantly reduced compared with that of the
control cells in osteoblast induction medium alone (P<0.05).
However, ALP activity was not reduced in cells cultured with the
other anti-HIV drugs (Fig. 1A). These
results indicated that the protease inhibitor ritonavir inhibited
osteoblast differentiation.
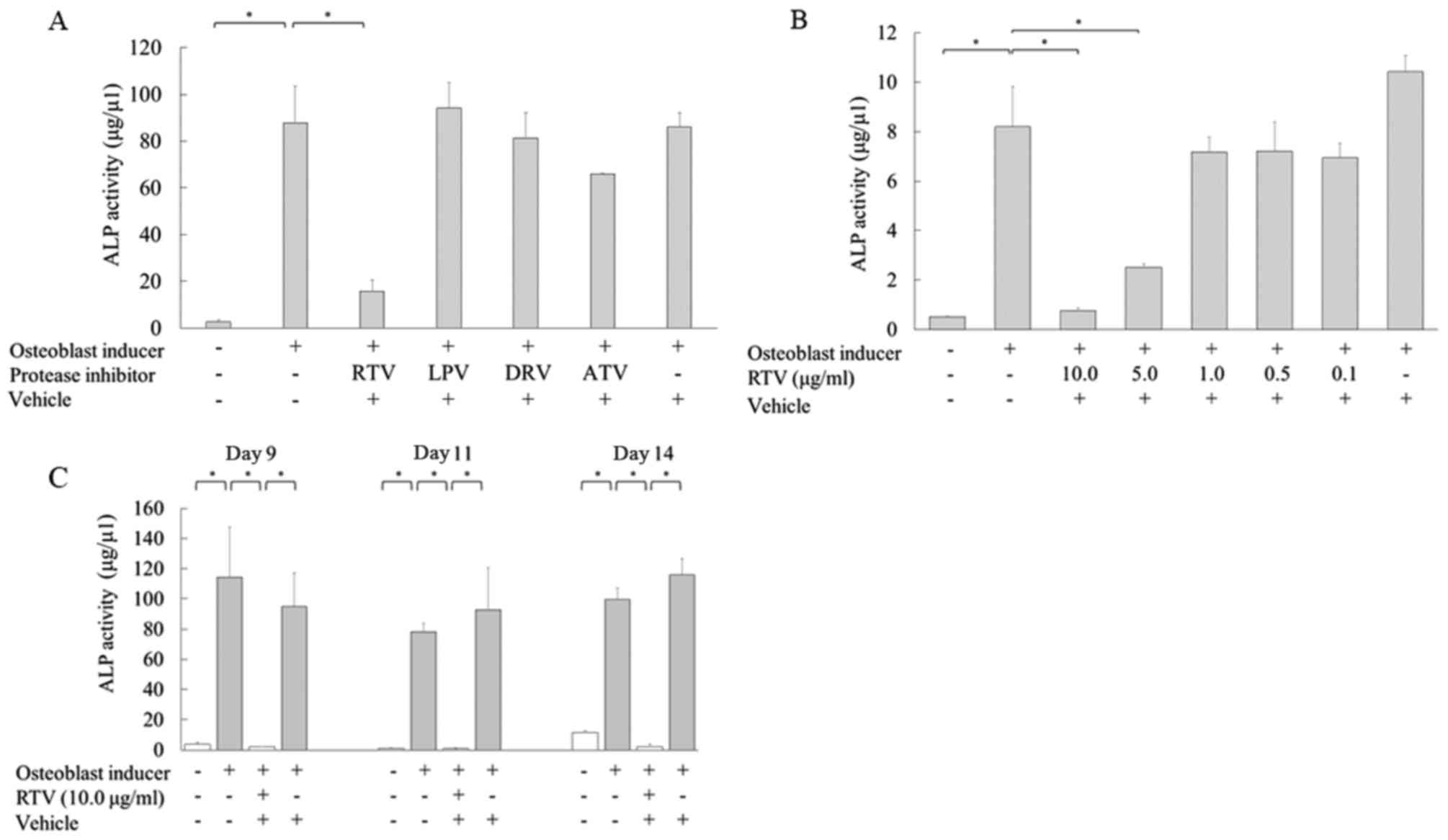 | Figure 1.Ritonavir reduces the ALP activity in
MC3T3-E1 cells. (A) MC3T3-E1 cells were cultured in osteoblast
differentiation medium with or without Cmax doses of protease
inhibitors for 9 days, and ALP activity was analyzed. (B) Cells
were cultured in osteoblast differentiation medium with various
concentrations of ritonavir (0, 0.1, 0.5, 1.0, 5.0 and 10.0 µg/ml)
for 9 days, and ALP activity was measured quantitatively. (C) Cells
were cultured in osteoblast differentiation medium with or without
10.0 µg/ml ritonavir for 9, 11 and 14 days, and ALP activity was
analyzed. All data are presented as means ± standard deviation.
*P<0.05. ALP, alkaline phosphatase; Cmax, maximum serum
concentration; RTV, ritonavir; LPV, lopinavir; DRV, darunavir; ATV,
atazanavir. |
Next, the relationship between ritonavir
concentration and osteoblast differentiation was examined. MC3T3-E1
cells were treated with various concentrations of ritonavir (0.1,
0.5, 1.0, 5.0 and 10.0 µg/ml). ALP activity was significantly
reduced in MC3T3-E1 cells cultured with 5.0 or 10.0 µg/ml ritonavir
compared with the controls in osteoblast induction medium alone
(P<0.05); however, lower concentrations of ritonavir did not
inhibit ALP activity (Fig. 1B).
The time course of ALP activity in MC3T3-E1 cells
cultured with ritonavir was also examined. MC3T3-E1 cells were
cultured in osteogenic differentiation medium with or without 10.0
µg/ml ritonavir for 9, 11 and 14 days. ALP activity was
significantly reduced following ritonavir treatment at each time
point (P<0.05; Fig. 1C).
Effect of ritonavir on the expression
of Runx2 in MC3T3-E1 cells
RUNX2 is the key gene responsible for the
differentiation of human mesenchymal cells into osteoblasts; it
upregulates bone matrix proteins including ALP in the early phase
of osteoblast differentiation (13).
MC3T3-1 cells were cultured in osteogenic differentiation medium
with or without 10.0 µg/ml ritonavir for 3, 5 and 7 days, and the
mRNA expression of Runx2 was measured by reverse
transcription-qPCR. On days 3 and 5, the mRNA levels of Runx2 were
significantly reduced in differentiating cells cultured with
ritonavir compared with in the differentiating controls
(P<0.05); however, no significant differences were observed on
day 7 (Fig. 2). These results
indicated that ritonavir inhibited expression of Runx2 in the early
phase of osteoblast differentiation.
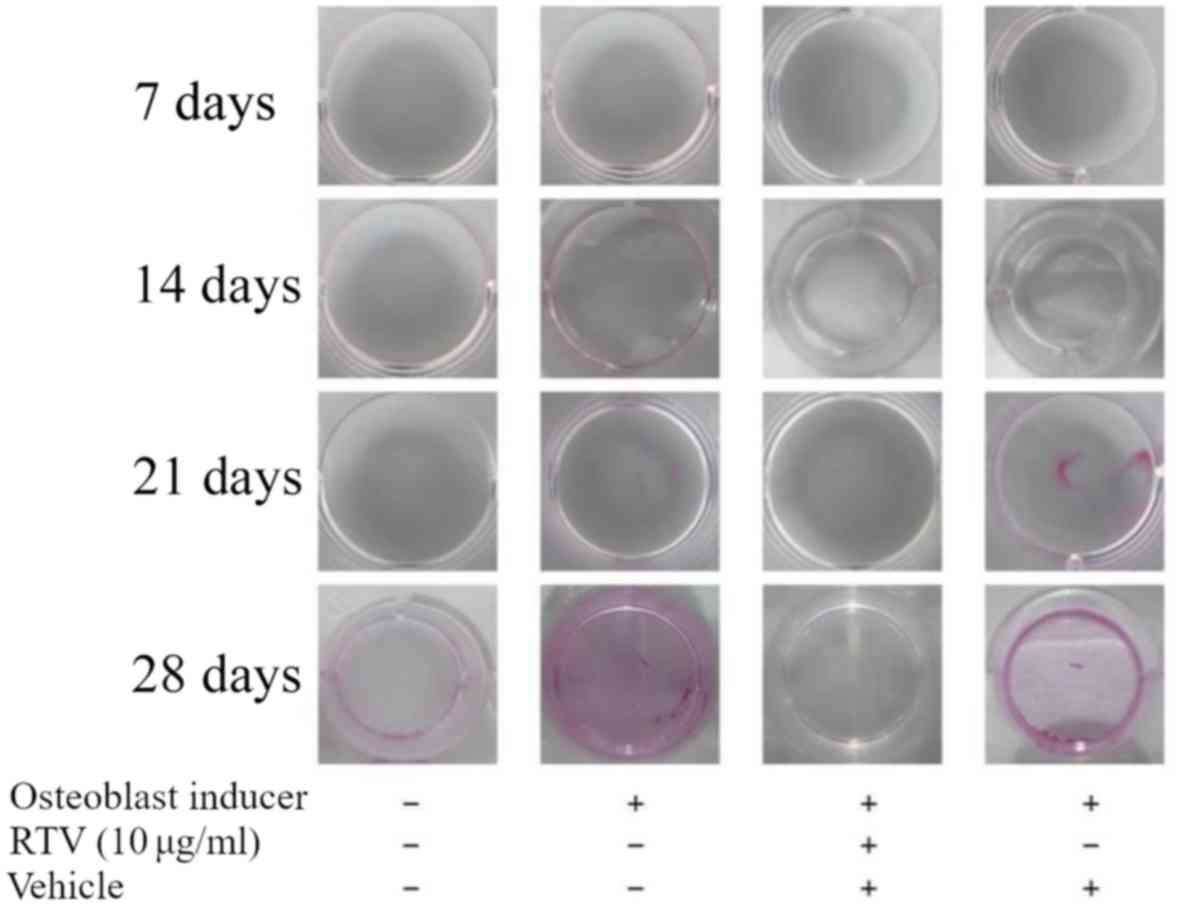
Osteoblast mineralization is inhibited
by ritonavir
In the process of bone formation, osteoblasts need
to differentiate and mineralize. Thus, whether ritonavir inhibited
osteoblast mineralization was examined. MC3T3-E1 cells were
cultured in DMEM with osteoblast inducer reagent with or without 10
µg/ml ritonavir for 7, 14, 21 and 28 days. Then, cells were stained
with Alizarin Red S to assess mineralization. At 7 and 14 days,
cells treated with DMEM or with osteoblast induction medium, with
or without ritonavir, exhibited no positive staining indicative of
mineralization. At 21 and 28 days, cells treated with osteoblast
induction medium with or without vehicle exhibited increasing
mineralization, while those treated with DMEM only or osteoblast
induction medium with ritonavir were not (Fig. 3). These results indicated that
ritonavir suppressed or delayed osteoblast mineralization.
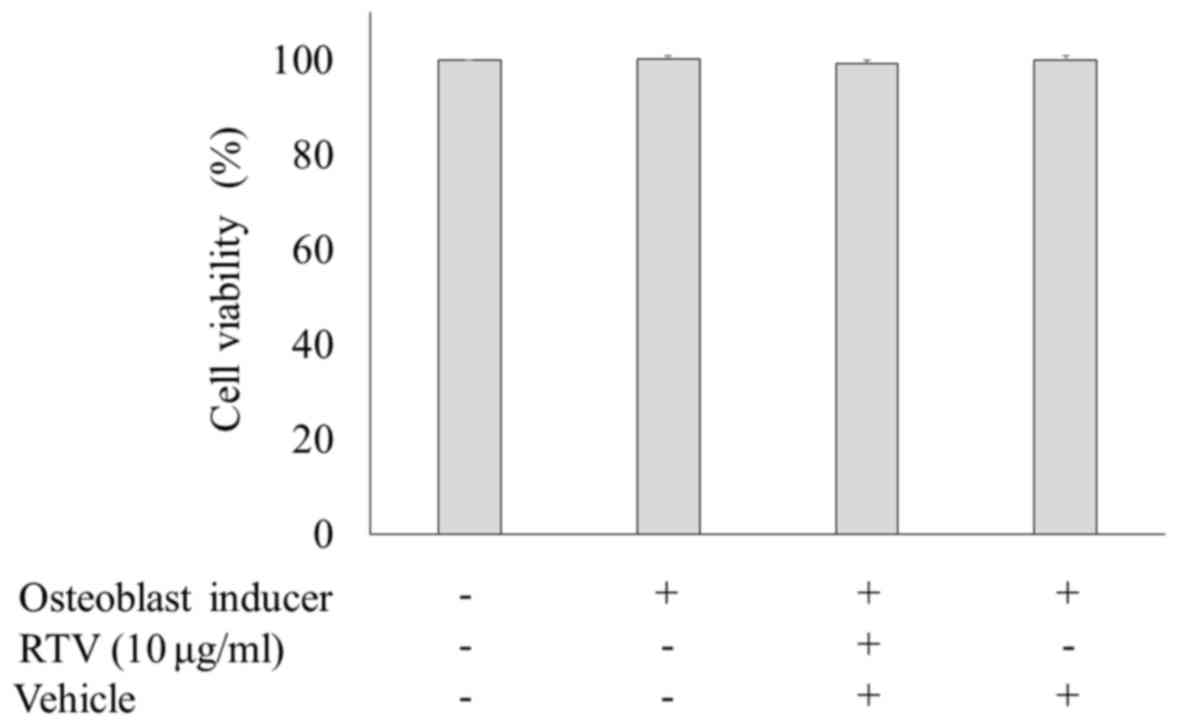
Cell viability of MC3T3-E1 cells
treated with ritonavir
The cytotoxicity of ritonavir was assessed via
measuring LDH activity. MC3T3-E1 cells were cultured in osteogenic
differentiation medium with or without 10.0 µg/ml ritonavir for 9
days, and the levels of LDH activity were measured. There were no
statistically significant differences among the groups (Fig. 4). These results showed that ritonavir
did not exert cytotoxic effects in MC3T3-E1 cells.
Discussion
Osteoporosis is one of the complications seen in
patients with HIV. HIV itself suppresses osteogenesis (14). Several in vivo studies have
reported that osteopenia and osteoporosis are common among patients
treated with antiretroviral drugs (15–18).
Bedimo et al (15) reported
that cumulative exposure to tenofovir and protease inhibitors,
particularly lopinavir/ritonavir, was an independent predictor of
increased risk of osteoporotic fracture in HIV patients on cART.
Further, it was shown in the Data collection on Adverse events of
anti-HIV Drugs study that protease inhibitor use was one of the
risk factors for osteoporosis (16,17).
Duvivier et al (18) reported
that decreased lumbar spine bone mineral density was more
pronounced in patients receiving protease inhibitors
(indinavir/ritonavir or lopinavir/ritonavir) compared with other
antiretroviral drugs. By contrast, a cross-sectional study
identified decreased bone density in HIV-positive patients
irrespective of treatment with or without protease inhibitors
(19). Thus, the association between
osteoporosis and protease inhibitors in vivo is yet to be
confirmed.
The MC3T3-E1 cell line is a pre-osteoblast line
derived from mouse calvaria (20). It
is established to exhibit a time-dependent and sequential
expression of osteoblast characteristics analogous to in
vivo bone formation, and is used as a bone differentiation and
mineralization model in vitro (21). In the present study, ritonavir was
indicated to affect the pathway of osteoblast differentiation and
the time course of differentiation. There are a number of in
vitro reports describing the relationship between certain
antiretroviral drugs and osteoblast differentiation (21,22). Jain
and Lenhard reported that two protease inhibitors, lopinavir and
nelfinavir, decreased osteoblast ALP activity and gene expression
in human mesenchymal stem cells (21). Another report demonstrated that ALP
activity decreased significantly in human osteoblast cultures
following exposure to nelfinavir and indinavir (22). Santiago et al has previously
reported ritonavir may facilitate osteoclast differentiation
(23); however, other findings have
suggested that ritonavir could inhibit osteoclast formation and
function (24). Thus, the exact
effect of RTV on osteoblast cells remained unknown.
Ritonavir was originally used for its antiviral
action, but is now used as a booster of other protease inhibitors.
The therapeutic adult dose of ritonavir is 600 mg twice a day. When
600 mg ritonavir is taken twice a day, the mean maximum and minimum
serum concentrations (Cmax and Cmin) of ritonavir have been
determined as 11.2±3.6 and 3.7±2.6 µg/ml, respectively (25). In the present study, osteoblast
differentiation was inhibited by 5.0 and 10.0 µg/ml ritonavir.
These results indicated the possibility that administration of
therapeutic doses of ritonavir may inhibit osteoblast
differentiation in vivo. However, as a booster, ritonavir is
used at a dose of 100 to 200 mg a day, and the expected ritonavir
Cmax is ~1.5 µg/ml, and therefore osteoblast differentiation
appears unlikely to be inhibited in vivo. However, to our
knowledge there are no reports of a pharmacokinetic analysis of
ritonavir in bone. Thus, osteoblast differentiation may be
suppressed in bone when the booster dose of ritonavir is
administered.
In the present study, ritonavir suppressed the
expression of Runx2 mRNA. To the best of our knowledge, this is the
first study to report a suppressive effect of ritonavir on the
expression of Runx2, and that ritonavir may affect osteoblast
differentiation and mineralization in MC3T3-E1 cells. There are
numerous pathways associated with osteoblast differentiation
including the bone morphogenetic protein BMP and parathyroid
hormone pathways (26). Runx2 is an
essential transcription factor required for osteogenesis;
Runx2-knockout mice exhibit a complete absence of mature
osteoblasts and ossification (26).
In particular, Runx2 is a key regulator of osteoblast
differentiation and regulates the expression of several
osteoblastic genes, including collagen 1, osteopontin, osteocalcin
and bone sialoprotein, to induce differentiation (13). In primary osteoblast cells isolated
from the calvariae of rats, study has found ALP activity to be
significantly decreased by Runx2 small interfering RNA treatment
when cells were treated with osteoblast differentiation medium that
included ascorbic acid and β-glycerophosphate (27), indicating that Runx2 expression is
upstream of ALP. In the present study, the expression of Runx2 mRNA
was suppressed on days 3 and 5, but not on day 7 of treatment with
ritonavir in osteoblast differentiation medium. Prior to day 7, the
ALP activities of cells with or without differentiation medium did
not change (not shown). But ALP activities were suppressed on day 9
or later of treatment with ritonavir in osteoblast differentiation
medium. These results collectively indicate that ritonavir
suppressed Runx2 directly or other regulators of osteoblast
differentiation upstream of Runx2, and, as a result, ALP activity
was suppressed.
The present results also suggested inhibition of
osteoblast mineralization by ritonavir. However, bone formation
takes 3 to 4 months (28), and cells
in the current study were cultured for only 28 days (4 weeks).
Thus, the possibility that ritonavir simply delayed osteoblast
mineralization cannot be excluded.
In conclusion, the present study is seemingly the
first to demonstrate that ritonavir may inhibit osteoblast
differentiation in vitro. Expression of Runx2 was suppressed
by ritonavir in MC3T3-E1 cells. This may explain the mechanism of
osteopenia induced by cART involving protease inhibitors.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YW, YY, KS, IK, TK and YO designed the study. YW
processed the experimental data, performed the analysis, drafted
the manuscript and produced the figures. All authors discussed the
results and contributed to the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lohse N, Hansen AB, Gerstoft J and Obel N:
Improved survival in HIV-infected persons: Consequences and
perspectives. J Antimicrob Chemother. 60:461–463. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Maartens G, Celum C and Lewin SR: HIV
infection: Epidemiology, pathogenesis, treatment, and prevention.
Lancet. 384:258–271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brown TT and Qaqish RB: Antiretroviral
therapy and the prevalence of osteopenia and osteoporosis: A
meta-analytic review. AIDS. 20:2165–2174. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Del C, arpio-Cano FE, Dela Cadena RA and
Sawaya BE: HIV and bone disease: A perspective of the role of
microRNAs in bone biology upon HIV infection. J Osteoporos.
2013:5714182013.PubMed/NCBI
|
|
5
|
McComsey GA, Tebas P, Shane E, Yin MT,
Overton ET, Huang JS, Aldrovandi GM, Cardoso SW, Santana JL and
Brown TT: Bone disease in HIV infection: A practical review and
recommendations for HIV care providers. Clin Infect Dis.
51:937–946. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tebas P, Powderly WG, Claxton S, Marin D,
Tantisiriwat W, Teitelbaum SL and Yarasheski KE: Accelerated bone
mineral loss in HIV-infected patients receiving potent
antiretroviral therapy. AIDS. 14:F63–F67. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kanis JA: Assessment of fracture risk and
its application to screening for postmenopausal osteoporosis:
Synopsis of a WHO report. WHO Study Group. Osteoporos Int.
4:368–381. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Endo I and Mastumoto T: Bone and stem
cells: Regulatory mechanism of mesenchymal stem cell
differentiation to osteoblasts. Clin Calcium. 24:555–564. 2014.(In
Japanese). PubMed/NCBI
|
|
9
|
Komori T: Regulation of bone development
and maintenance by Runx2. Front Biosci. 13:898–903. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tellmann G: The E-Method: A highly
accurate technique for gene-expression analysis. Nat Methods.
3:i–ii. 2006. View
Article : Google Scholar
|
|
11
|
Jaiswal N, Haynesworth SE, Caplan AI and
Bruder SP: Osteogenic differentiation of purified, culture-expanded
human mesenchymal stem cells in vitro. J Cell Biochem. 64:295–312.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pittenger MF, Mackay AM, Beck SC, Jaiswal
RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S and
Marshak DR: Multilineage potential of adult human mesenchymal stem
cells. Science. 284:143–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xiao ZS, Liu SG, Hinson TK and Quarles LD:
Characterization of the upstream mouse Cbfa1/Runx2 promoter. J Cell
Biochem. 82:647–659. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cotter EJ, Malizia AP, Chew N, Powderly WG
and Doran PP: HIV proteins regulate bone marker secretion and
transcription factor activity in cultured human osteoblasts with
consequent potential implications for osteoblast function and
development. AIDS Res Hum Retroviruses. 23:1521–1530. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bedimo R, Maalouf NM, Zhang S, Drechsler H
and Tebas P: Osteoporotic fracture risk associated with cumulative
exposure to tenofovir and other antiretroviral agents. AIDS.
26:825–831. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Friis-Møller N, Reiss P, Sabin CA, Weber
R, Monforte A, El-Sadr W, Thiébaut R, De Wit S, Kirk O, Fontas E,
et al; DAD Study Group, . Class of antiretroviral drugs and the
risk of myocardial infarction. N Engl J Med. 356:1723–1735. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Flint OP, Noor MA, Hruz PW, Hylemon PB,
Yarasheski K, Kotler DP, Parker RA and Bellamine A: The role of
protease inhibitors in the pathogenesis of HIV-associated
lipodystrophy: Cellular mechanisms and clinical implications.
Toxicol Pathol. 37:65–77. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Duvivier C, Kolta S, Assoumou L, Ghosn J,
Rozenberg S, Murphy RL, Katlama C and Costagliola D; ANRS 121
Hippocampe study group, . Greater decrease in bone mineral density
with protease inhibitor regimens compared with nonnucleoside
reverse transcriptase inhibitor regimens in HIV-1 infected naive
patients. AIDS. 23:817–824. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Amiel C, Ostertag A, Slama L, Baudoin C,
N'Guyen T, Lajeunie E, Neit-Ngeilh L, Rozenbaum W and De Vernejoul
MC: BMD is reduced in HIV-infected men irrespective of treatment. J
Bone Miner Res. 19:402–409. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kodama H, Amagai Y, Sudo H, Kasai S and
Yamamoto S: Establishment of a clonal osteogenic cell linefrom
newborn mouse calvaria. Jap J Oral Biol. 23:899–901. 1981.
View Article : Google Scholar
|
|
21
|
Jain RG and Lenhard JM: Select HIV
protease inhibitors alter bone and fat metabolism ex vivo. J Biol
Chem. 277:19247–19250. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Malizia AP, Cotter E, Chew N, Powderly WG
and Doran PP: HIV protease inhibitors selectively induce gene
expression alterations associated with reduced calcium deposition
in primary human osteoblasts. AIDS Res Hum Retroviruses.
23:243–250. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Santiago F, Oguma J, Brown AM and Laurence
J: Noncanonical Wnt signaling promotes osteoclast differentiation
and is facilitated by the human immunodeficiency virus protease
inhibitor ritonavir. Biochem Biophys Res Commun. 417:223–230. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang MW, Wei S, Faccio R, Takeshita S,
Tebas P, Powderly WG, Teitelbaum SL and Ross FP: The HIV protease
inhibitor ritonavir blocks osteoclastogenesis and function by
impairing RANKL-induced signaling. J Clin Invest. 114:206–213.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ltd AL: Norvir® Tablets Summary
of Product Characteristics. 2010.
|
|
26
|
Komori T, Yagi H, Nomura S, Yamaguchi A,
Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, et al:
Targeted disruption of Cbfa1 results in a complete lack of bone
formation owing to maturational arrest of osteoblasts. Cell.
89:755–764. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lin L, Shen Q, Leng H, Duan X, Fu X and Yu
C: Synergistic inhibition of endochondral bone formation by
silencing Hif1α and Runx2 in trauma-induced heterotopic
ossification. Mol Ther. 19:1426–1432. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sims NA and Martin TJ: Coupling the
activities of bone formation and resorption: A multitude of signals
within the basic multicellular unit. Bonekey Rep. 3:4812014.
View Article : Google Scholar : PubMed/NCBI
|