Introduction
Ankylosing spondylitis (AS) is an inflammatory
rheumatic disease characterized by inflammatory back pain, morning
stiffness of the spine and enthesitis (1,2). It
predominantly affects the axial skeleton, although peripheral
joints may also be affected. Disease onset usually begins in the
second or third decade of life. With the progression of disease, it
may lead to structural and functional impairments, work disability
and even loss of self-sufficiency (3,4).
Non-steroidal anti-inflammatory drugs (NSAIDs) are
strongly recommended for patients with AS (5,6). In
addition, several studies have shown that long-term use of NSAIDs
may delay radiographic progression in patients with AS (7,8). Oral
NSAIDs is the most common route of administration. However, the use
of oral NSAIDs may lead to gastrointestinal, cardiovascular and
renal adverse events (9-13).
Loxoprofen sodium (LX), a non-selective NSAID, has a particularly
high risk of gastrointestinal disorders. Therefore, topically
applied NSAIDs have been developed to decrease the risk of
gastrointestinal adverse events while providing good levels of pain
relief for local acute and chronic painful conditions (14,15).
Loxoprofen in LX hydrogel patches (LX-P) can directly penetrate
into the affected site and provide pain relief. Topical LX-P has
been demonstrated to be non-inferior to oral LX tablet (LX-T) in
efficacy and safety for patients with knee osteoarthritis in a
randomized, double bind, controlled trial (16).
To the best of our knowledge, no randomized
controlled trials of topical LX-P have been conducted in patients
with AS to date. The aim of the present 4 week study was to assess
the efficacy and safety of topical LX-P compared with oral LX-T in
the treatment of patients with active AS in China.
Patients and methods
Patients
Patients aged 18-65 years fulfilling the 1984
modified AS New York criteria (17)
were eligible for inclusion. Patients should present with active
disease, defined by a Bath AS Disease Activity Index (BASDAI)
(18) of ≥4 on a 0-10 cm visual
analog scale (VAS) or an Ankylosing Spondylitis Disease Activity
Score using the C-reactive protein level (ASDAS-CRP) (19,20) of
≥1.3. Other defined inclusion criteria were: i) NSAIDs washout
period of at least 5 days prior to randomization; ii)
disease-modifying antirheumatic drugs washout period of at least 4
weeks prior to randomization; iii) corticosteroids washout period
of at least 4 weeks prior to randomization; and iv) biological
agents washout period of at least 3 months prior to randomization.
Sulfasalazine was permitted if the patient was taking a stable dose
for 3 months prior to study entry. Patients with peripheral joint
involvement were also included. Adequate contraception throughout
the trial was required in women of childbearing age.
Patients were excluded if they had peptic ulcer,
unstable cardiac diseases, abnormal hepatic or renal function,
hematologic diseases, psychosis, or malignancy. Patients who were
allergic to LX were also excluded.
Trial design
The trial was a randomized open-label study
conducted in the Department of Rheumatology of The Third Affiliated
Hospital of Sun Yat-Sen University, (Guangzhou, China). The
protocol was approved by the Ethics Committee of the Third
Affiliated Hospital of Sun Yat-Sen University in accordance with
the Declaration of Helsinki principles and the guidelines for Good
Clinical Practice (21). The study
was registered on 10 January 2019 at www.ClinicalTrials.gov (identifier NCT03800797).
Informed consents were obtained from all patients.
Clinical visits were performed at baseline (week 0),
week 2, and week 4, or the time of discontinuing treatment by the
same investigator. The demographic and disease characteristics were
recorded at baseline. Every visit should record clinical and
laboratory variables, evaluate efficacy outcomes and monitor
adverse events.
Treatment
Patients were randomized 1:1 to either the LX-P
group or the LX-T group for 4 weeks using a computer-generated
schedule. Treatment assignment was open to the investigators and
patients. Each patient qualifying for treatment was dispensed the
corresponding drug. LX-P (Lead Chemical Co., Ltd.) contained 50 mg
LX per patch, while LX-T (Daiichi Sankyo Co., Ltd.) contained 60 mg
LX. Patients in the LX-P group applied LX-P 100 mg onto the pain
spot of vertebra once daily starting at night. Patients in the LX-T
group took LX-T 60 mg 3 times daily following meals. The doses for
LX-P and LX-T were selected according to a previous clinical trial
involving LX-P in knee osteoarthritis (16). Dose adjustments were not allowed
during the study period. Usually, an optimal effect of an NSAID is
reached no later than after 1-2 weeks (11), so the treatment duration was 4
weeks.
At the screening visit, concomitant therapies with
gastrointestinal protective drugs, including misoprostol or proton
pump inhibitors, were stopped if there was no history of
gastroduodenal ulcers.
Efficacy evaluation
The primary efficacy endpoint was the percentage of
patients reaching Assessment in Ankylosing Spondylitis 20% (ASAS20)
response (22) at weeks 2 and 4,
which was defined as an improvement of ≥20% and net improvement of
≥1 unit (0-10-cm VAS) from baseline in ≥3 of the following 4
domains, and absence of worsening in any domain: Patient’s global
assessment of disease activity (PTGA), pain assessment, Bath
Ankylosing Spondylitis Functional Index (BASFI) (23) and clinical inflammation, determined by
2 morning stiffness-associated scores on the BASDAI. The secondary
efficacy endpoint was the percentage of patients achieving ASAS5/6
response at weeks 2 and 4, defined as 20% improvement in 5 of the
following 6 domains: PTGA, pain assessment, BASFI, inflammation,
spinal mobility and CRP. Other efficacy measures included mean
changes from baseline to week 4 for ASDAS-CRP, PTGA, total and
nocturnal pain scores, clinical inflammation, BASDAI and BASFI.
Safety evaluation
Safety evaluations, including adverse events,
physical examination and clinical laboratory tests, were monitored
by the investigators throughout the study.
Statistical analysis
Statistical analysis was performed using SPSS 20.0
software (SPSS, Chicago, IL, USA). Baseline demographic and disease
characteristic variables were summarized using descriptive
statistics. All efficacy endpoint analyses were conducted in the
intention-to-treat (ITT) population, which consisted of all
randomized patients who took at least 1 dose of treatment. We used
the last observation carried forward technique for patients who did
not adhere to the study protocol.
Using the ITT population, the ASAS20 and ASAS5/6
response rates were analyzed by Pearson χ2 test. Patients were also
stratified based on the presence or absence of peripheral arthritis
to determine the effect of peripheral joint involvement on
treatment response. Changes of efficacy endpoints from baseline to
week 4 were analyzed using the Student’s t-test or
Wilcoxon-Mann-Whitney test depending on the Shapiro-Wilk normality
test in the ITT population. The safety assessment were also
conducted on all randomized patients. The incidence of adverse
events was compared between the LX-P and LX-T groups using Fisher’s
exact test. For all analyses, statistical analysis was performed at
α=0.05. P<0.05 was considered to indicate a statistically
significant difference.
Results
Enrollment and characteristics of
patients
From May 2015 to December 2015, 82 patients were
screened, and 70 eligible patients were randomized into the 4-week
trial, with 35 in the LX-P group and 35 in the LX-T group (Fig. 1). In total, 6 of 70 patients withdrew
from the study following randomization, 3 from the LX-P group and 3
from the LX-T group, resulting in an overall dropout rate of 8.6%.
The reasons for dropping out included adverse events, insufficient
efficacy and loss to follow up.
At baseline, 48 (68.6%) patients were male, and 22
(31.4%) had peripheral arthritis. The baseline demographic and
disease characteristics were similar between LX-P and LX-T groups
(Table I).
 | Table IBaseline demographic and disease
characteristics of the patients with ankylosing spondylitis. |
Table I
Baseline demographic and disease
characteristics of the patients with ankylosing spondylitis.
| | Groups | |
|---|
| Characteristics | LX-P (n=35) | LX-T (n=35) | P-value |
|---|
| Demographic
characteristics | | | |
|
Age,
years | 28 (24-36) | 30 (23-36) | 0.642 |
|
Male, no.
(%) | 22 (62.9) | 26 (74.3) | 0.303 |
|
Height,
cm | 165.3±7.9 | 166.3±6.5 | 0.575 |
|
Weight,
kg | 58.5±11.6 | 60.6±12.0 | 0.465 |
|
Smoking, no.
(%) | 9 (25.7) | 12 (34.3) | 0.434 |
| Disease
characteristics | | | |
|
HLA-B27
positive, no. (%) | 35 (100.0) | 34 (97.1) | 1.000 |
|
Disease
duration, years | 5.0 (2.0-9.0) | 4.0 (1.0-9.0) | 0.773 |
|
Peripheral
arthritis, no. (%) | 9 (25.7) | 13 (37.1) | 0.303 |
| Disease activity | | | |
|
PTGA,
0-10-cm VAS | 5.0 (4.0-7.0) | 6.0 (5.0-7.0) | 0.273 |
|
Total pain
score, 0-10-cm VAS | 5.0 (3.0-7.0) | 5.0 (4.0-7.0) | 0.472 |
|
Nocturnal
pain score, 0-10-cm VAS | 6.0 (3.0-7.0) | 5.0 (3.0-7.0) | 0.387 |
|
Clinical
inflammation, 0-10-cm VAS | 3.8 (2.8-6.5) | 3.8 (2.8-5.0) | 0.557 |
|
BASDAI,
0-10-cm VAS | 4.3±1.4 | 4.2±1.4 | 0.735 |
|
BASFI,
0-10-cm VAS | 1.0 (0.2-1.8) | 0.7 (0.2-1.9) | 0.986 |
|
BASMI,
0-10-cm VAS | 1.0 (0-2.0) | 1.0 (0-3.0) | 0.514 |
|
CRP,
mg/l | 5.9 (1.9-16.8) | 8.5 (5.3-17.6) | 0.109 |
|
ASDAS-CRP | 2.8±0.9 | 3.0±0.7 | 0.223 |
| Prior
medications | | | |
|
NSAIDs, no.
(%) | 30 (85.7) | 29 (82.9) | 0.743 |
|
DMARDs, no.
(%) | 12 (34.3) | 8 (22.9) | 0.290 |
|
Biological
agents, no. (%) | 3 (8.6) | 4 (11.4) | 1.000 |
Efficacy
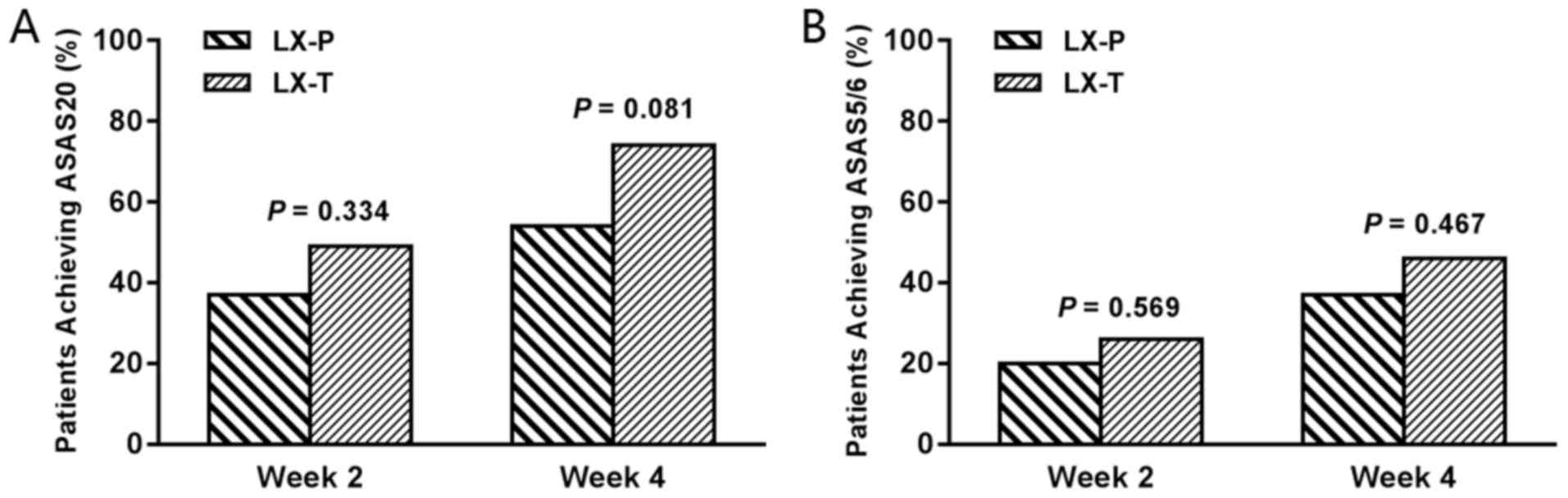
The primary efficacy endpoints of ASAS20 response at
weeks 2 and 4 in the ITT population are shown in Fig. 2A. No significant differences between
the LX-P and LX-T groups in the proportion of patients achieving
ASAS20 response at week 2 [13/35 (37.1%) for LX-P group vs. 17/35
(48.6%) for LX-T group; P=0.334] and week 4 [19/35 (54.3%) for LX-P
group vs. 26/35 (74.3%) for LX-T group; P=0.081] were observed. In
addition, no statistically significant differences for ASAS5/6
response at weeks 2 and 4 between the two groups were observed
[week 2, 7/35 (20.0%) vs. 9/35 (25.7%); P=0.569; week 4, 13/35
(37.1%) vs. 16/35 (45.7%); P=0.467; Fig. 2B]. Changes in efficacy
endpoints, including ASDAS-CRP, PTGA, total and nocturnal pain
score, clinical inflammation, BASDAI and BASFI, from baseline to
week 4 were compared between the LX-P and LX-T groups. There were
also no significant differences between the two groups observed
(all P>0.05; Table II).
 | Table IIChanges in efficacy outcomes from
baseline to week 4 in the LX-P and LX-T groups. |
Table II
Changes in efficacy outcomes from
baseline to week 4 in the LX-P and LX-T groups.
| | Groups | |
|---|
| Efficacy
outcomes | LX-P (n=35) | LX-T (n=35) | P-value |
|---|
| ASDAS-CRP | -0.6±0.7 | -0.8±0.6 | 0.301 |
| PTGA, 0-10-cm
VAS | -1.0 (-3.0-0) | -2.0 (-2.0-0) | 0.724 |
| Total pain score,
0-10-cm VAS | -1.0 (-2.0-0) | -2.0 (-3.0-
-1.0) | 0.082 |
| Nocturnal pain
score, 0-10-cm VAS | -2.0 (-3.0-0) | -1.0 (-3.0-0) | 0.214 |
| Clinical
inflammation, 0-10-cm VAS | -1.5 (-2.8-0) | -1.5 (-3.0-
-0.5) | 0.415 |
| BASDAI, 0-10-cm
VAS | -1.5±1.2 | -1.7±1.2 | 0.442 |
| BASFI, 0-10-cm
VAS | -0.1 (-0.7-0) | -0.2 (-1.0-0) | 0.435 |
In a subgroup analysis of patients without
peripheral arthritis, an increase in the proportion of patients
achieving ASAS20 response was observed in the LX-P group (Fig. 3). This suggested that patients without
peripheral arthritis in the LX-P group were more likely to achieve
ASAS20 response compared with those with peripheral joints
involvement.
Safety
The incidence of any adverse events described during
the study was similar (P=0.710) between the LX-P and LX-T groups:
3/35 (8.6%) patients in the LX-P group and 5/35 (14.3%) in the LX-T
group (Table III). All adverse
events were mild to moderate in intensity; no serious adverse
events or mortalities were observed in either group. The most
frequent adverse events for patients receiving LX-P were skin
irritation or rashes, while the most common adverse event for
patients receiving LX-T was gastrointestinal disorders.
 | Table IIIAdverse events occurring in each
group during the study. |
Table III
Adverse events occurring in each
group during the study.
| Event
categories | LX-P (n=35)
(%) | LX-T (n=35)
(%) |
|---|
| Any adverse
event | 3 (8.6) | 5 (14.3) |
|
Nausea | 0 (0.0) | 1 (2.9) |
|
Dyspepsia | 0 (0.0) | 1 (2.9) |
|
Skin
pruritus | 2 (5.7) | 1 (2.9) |
|
Dizziness | 0 (0.0) | 1 (2.9) |
|
Upper
respiratory tract infection | 1 (2.9) | 1 (2.9) |
| Serious adverse
event | 0 (0.0) | 0 (0.0) |
| Adverse event
leading to discontinuation | 2 (5.7) | 1 (2.9) |
Discussion
To date, only a few studies of osteoarthritis have
compared the efficacy of topical vs. oral administration routes of
NSAIDs (16,24,25). To
the best of our knowledge, there are no studies comparing topical
vs. oral administration routes of NSAIDs in AS. The results of the
present randomized controlled trial demonstrated that there were no
significant differences in efficacy between topical LX-P and oral
LX-T administration in patients with active AS, which is consistent
with the results from a recent study in patients with knee
osteoarthritis (16). In addition,
topical LX-P was associated with non-significantly fewer
gastrointestinal adverse events compared with oral LX-T.
In the present study, 31.4% of the patients had
peripheral arthritis. However, LX-P treatments were only applied to
the pain area of the vertebra, in order to maintain the same
dosage. Considering that peripheral arthritis may affect the
treatment response of topical LX-P, an exploratory analysis of
patient subgroups with and without peripheral arthritis was
performed. The results suggested that patients without peripheral
arthritis in the LX-P group were more likely to achieve ASAS20
response. Therefore, we hypothesize that LX-P may have an improved
efficacy in patients whose pain is localized in one area. However,
additional investigation is required to explore the efficacy of
topical LX-P in patients with AS, in particular those with
generalized pain.
NSAIDs are recommended as the first-line drug option
for the treatment of AS (5,6), and long-term use of NSAIDs may delay the
radiographic progression for patients with AS (7,8). However,
long-term exposure of NSAIDs may cause gastrointestinal,
cardiovascular, and renal adverse events (9-13).
Topical NSAIDs are considered to be associated with fewer
gastrointestinal adverse events (24,25).
Topical LX-P allows LX to penetrate into the affected site directly
instead of through the gastrointestinal tract. In the present
study, there were no significant differences in safety between the
LX-P and LX-T groups, probably due to the limited number of
patients. Notably, skin disorders were the most frequently reported
treatment-associated adverse events in the LX-P group, but this was
not significant. Furthermore, a decreased incidence of
gastrointestinal disorders was observed in patients receiving LX-P.
These results suggest that LX-P may be a potentially useful
therapeutic drug with the advantages of easy administration and few
gastrointestinal adverse events.
There are several limitations in the present study.
Firstly, the study was a single-center, open-label trial, which may
have introduced a certain level of bias into the analysis.
Secondly, the sample size of the study may have been too small to
draw definite conclusions. Therefore, to strengthen the results,
several disease activity indexes were assessed. Thirdly, patients
with peripheral joint involvement were enrolled. This may have
affected the efficacy of LX-P. The subgroup analysis revealed that
patients without peripheral arthritis in the LX-P group were more
likely to achieve ASAS20 response. Fourthly, only the short-term
efficacy and safety of topical LX-P in the treatment of AS was
investigated. Future studies with adequate follow-up are required
to assess the long-term efficacy and safety of LX-P in AS.
In summary, the results of the present study suggest
that there were no significant differences in efficacy between
topical LX-P and oral LX-T for patients with active AS. In
addition, topical LX-P was well tolerated and associated with few
gastrointestinal adverse events.
Acknowledgements
Not applicable.
Funding
The present study was supported by National Natural
Science Foundation of China (grant nos. 81571595 and 81702327), the
President foundation of Nanfang Hospital, Southern Medical
University (grant no. 2016C010), China Postdoctoral Science
Foundation: 2018M640834, and Innovation Program of Shenzhen (grant
no. JCYJ20180508165208399).
Availability of data and materials
The datasets analyzed during the current study are
available from the corresponding author on reasonable request.
Authors' contributions
MF participated in the design of the study, clinical
visits, data collection, design of the statistical analysis plan
and wrote the first draft of the manuscript. SC and LT participated
in clinical visits and critically reviewed the manuscript. QW and
RY participated in data collection and critically reviewed the
manuscript. JG conceived the study and participated in the
interpretation of data. XL critically reviewed the manuscript and
provided final approval of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Third
Affiliated Hospital of Sun Yat-Sen University, (Guangzhou, China);
all patients provided written informed consent.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sieper J and Poddubnyy D: Axial
spondyloarthritis. Lancet. 390:73–84. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Braun J and Sieper J: Ankylosing
spondylitis. Lancet. 369:1379–1390. 2007.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zink A, Braun J, Listing J and Wollenhaupt
J: Disability and handicap in rheumatoid arthritis and ankylosing
spondylitis - results from the German rheumatological database.
German Collaborative Arthritis Centers. J Rheumatol. 27:613–622.
2000.PubMed/NCBI
|
|
4
|
Boonen A, Chorus A, Miedema H, van der
Heijde D, van der Tempel H and van der Linden S: Employment, work
disability, and work days lost in patients with ankylosing
spondylitis: a cross sectional study of Dutch patients. Ann Rheum
Dis. 60:353–358. 2001.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ward MM, Deodhar A, Akl EA, Lui A, Ermann
J, Gensler LS, Smith JA, Borenstein D, Hiratzka J, Weiss PF, Inman
RD, et al: American College of Rheumatology/Spondylitis Association
of America/Spondyloarthritis Research and Treatment Network 2015
Recommendations for the Treatment of Ankylosing Spondylitis and
Nonradiographic Axial Spondyloarthritis. Arthritis Care Res
(Hoboken). 68:151–166. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
van der Heijde D, Ramiro S, Landewe R,
Baraliakos X, Van den Bosch F, Sepriano A, Regel A, Ciurea A,
Dagfinrud H, Dougados M, et al: 2016 update of the ASAS-EULAR
management recommendations for axial spondyloarthritis. Ann Rheum
Dis. 76:978–991. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wanders A, Heijde Dv, Landewé R, Béhier
JM, Calin A, Olivieri I, Zeidler H and Dougados M: Nonsteroidal
antiinflammatory drugs reduce radiographic progression in patients
with ankylosing spondylitis: a randomized clinical trial. Arthritis
Rheum. 52:1756–1765. 2005.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Poddubnyy D, Rudwaleit M, Haibel H,
Listing J, Märker-Hermann E, Zeidler H, Braun J and Sieper J:
Effect of non-steroidal anti-inflammatory drugs on radiographic
spinal progression in patients with axial spondyloarthritis:
results from the German Spondyloarthritis Inception Cohort. Ann
Rheum Dis. 71:1616–1622. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Singh G and Triadafilopoulos G:
Epidemiology of NSAID induced gastrointestinal complications. J
Rheumatol Suppl. 56:18–24. 1999.PubMed/NCBI
|
|
10
|
Fosbøl EL, Køber L, Torp-Pedersen C and
Gislason GH: Cardiovascular safety of non-steroidal
anti-inflammatory drugs among healthy individuals. Expert Opin Drug
Saf. 9:893–903. 2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Song IH, Poddubnyy DA, Rudwaleit M and
Sieper J: Benefits and risks of ankylosing spondylitis treatment
with nonsteroidal antiinflammatory drugs. Arthritis Rheum.
58:929–938. 2008.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Shau WY, Chen HC, Chen ST, Chou HW, Chang
CH, Kuo CW and Lai MS: Risk of new acute myocardial infarction
hospitalization associated with use of oral and parenteral
non-steroidal anti-inflammation drugs (NSAIDs): a case-crossover
study of Taiwan's National Health Insurance claims database and
review of current evidence. BMC Cardiovasc Disord.
12(4)2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Shukla A, Rai MK, Prasad N and Agarwal V:
Short-Term Non-Steroid Anti-Inflammatory Drug Use in
Spondyloarthritis Patients Induces Subclinical Acute Kidney Injury:
Biomarkers Study. Nephron. 135:277–286. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Massey T, Derry S, Moore RA and McQuay HJ:
Topical NSAIDs for acute pain in adults. Cochrane Database Syst
Rev. 6(CD007402)2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Derry S, Moore RA and Rabbie R: Topical
NSAIDs for chronic musculoskeletal pain in adults. Cochrane
Database Syst Rev. 9(CD007400)2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Mu R, Bao CD, Chen ZW, Zheng Y, Wang GC,
Zhao DB, Hu SX, Li YJ, Shao ZW, Zhang ZY, et al: Efficacy and
safety of loxoprofen hydrogel patch versus loxoprofen tablet in
patients with knee osteoarthritis: a randomized controlled
non-inferiority trial. Clin Rheumatol. 35:165–173. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
van der Linden S, Valkenburg HA and Cats
A: Evaluation of diagnostic criteria for ankylosing spondylitis. A
proposal for modification of the New York criteria. Arthritis
Rheum. 27:361–368. 1984.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Garrett S, Jenkinson T, Kennedy LG,
Whitelock H, Gaisford P and Calin A: A new approach to defining
disease status in ankylosing spondylitis: the Bath Ankylosing
Spondylitis Disease Activity Index. J Rheumatol. 21:2286–2291.
1994.PubMed/NCBI
|
|
19
|
van der Heijde D, Lie E, Kvien TK, Sieper
J, Van den Bosch F, Listing J, Braun J and Landewé R; Assessment of
SpondyloArthritis international Society (ASAS): ASDAS, a highly
discriminatory ASAS-endorsed disease activity score in patients
with ankylosing spondylitis. Ann Rheum Dis. 68:1811–1818.
2009.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Machado P, Landewé R, Lie E, Kvien TK,
Braun J, Baker D and van der Heijde D; Assessment of
SpondyloArthritis international Society: Ankylosing Spondylitis
Disease Activity Score (ASDAS): defining cut-off values for disease
activity states and improvement scores. Ann Rheum Dis. 70:47–53.
2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Grimes DA, Hubacher D, Nanda K, Schulz KF,
Moher D and Altman DG: The Good Clinical Practice guideline: a
bronze standard for clinical research. Lancet. 366:172–174.
2005.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Anderson JJ, Baron G, van der Heijde D,
Felson DT and Dougados M: Ankylosing spondylitis assessment group
preliminary definition of short-term improvement in ankylosing
spondylitis. Arthritis Rheum. 44:1876–1886. 2001.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Calin A, Garrett S, Whitelock H, Kennedy
LG, O'Hea J, Mallorie P and Jenkinson T: A new approach to defining
functional ability in ankylosing spondylitis: the development of
the Bath Ankylosing Spondylitis Functional Index. J Rheumatol.
21:2281–2285. 1994.PubMed/NCBI
|
|
24
|
Banning M: The use of topical diclofenac
for pain in osteoarthritis of the knee: a review. Br J Community
Nurs. 11:487–492. 2006.PubMed/NCBI
|
|
25
|
Tugwell PS, Wells GA and Shainhouse JZ:
Equivalence study of a topical diclofenac solution (pennsaid)
compared with oral diclofenac in symptomatic treatment of
osteoarthritis of the knee: a randomized controlled trial. J
Rheumatol. 31:2002–2012. 2004.PubMed/NCBI
|