Introduction
Gingivitis is a gingival inflammation triggered by
periodontal pathogens present in oral biofilms (1). Unless some physical treatments, such as
plaque control and scaling are administered, gingivitis becomes
more severe and develops into periodontitis with more severe
inflammation, leading to the destruction of connective tissues, the
absorption of alveolar bones and tooth loss (1,2). It has
been demonstrated that excessive inflammation in the gingival
tissue, as well as an immune reaction triggered by the increased
number of periodontal pathogens, such as Porphyromonas
gingivalis (P.g.), plays an important role in the
severity and progression of periodontal diseases (3,4). Thus, the
control of inflammation may help to delay disease progression or
recover severe symptoms in periodontal diseases.
Aged garlic extract (AGE) is prepared by aging
garlic (Allium sativum L.) for a period of >10 months in
aqueous ethanol and contains pharmacologically active
sulfur-containing amino acids, such as S-allylcysteine
(SAC), S-1-propenylcysteine (S1PC) and
S-allylmercaptocysteine (SAMC). In human and animal studies,
AGE and these sulfur compounds have been shown to exert beneficial
effects, such as the improvement of atherosclerosis (5,6), decrease
of high blood pressure (7,8) and the modulation of immunity (9,10). These
effects are possibly ascribed, at least in part, to the
anti-inflammatory (9,11) and/or antioxidant (12,13)
activities of sulfur compounds in AGE.
It has been reported that several naturally
occurring substances, such as curcumin and theaflavin alleviate the
absorption of alveolar bones in animal periodontitis models
(4,14). It has also been demonstrated that one
of the sulfur-containing compounds present in garlic,
diallylsulfide (DAS), reduces inflammatory reactions elicited by
P.g.-derived lipopolysaccharide (LPS) in human gingival
fibroblast cells (15). Furthermore,
DAS and another garlic sulfur compound, alliin, have been shown to
inhibit the growth of periodontal microbials (16-18).
These findings suggest that sulfur compounds in garlic attenuate
moderate or severe inflammation occurring in gingival tissues of
gingivitis and periodontitis. Recently, a clinical study
demonstrated that the intake of AGE significantly improved
gingivitis (1). However, the
mechanisms through which AGE attenuates gingivitis remain to be
fully elucidated. In this study, the authors examined whether SAC,
S1PC and SAMC in AGE exert anti-inflammatory effects against tumor
necrosis factor-α (TNF-α)-induced inflammatory reactions in the
human gingival epithelial cell line, Ca9-22.
Materials and methods
Aged garlic extract (AGE) and sulfur
compounds
AGE was prepared as previously described (12). Briefly, AGE was manufactured according
to the following steps: Originally grown raw garlic (A
sativum L.) was cut into slices, immersed in aqueous ethanol,
and extracted for >10 months at room temperature (12). The sulfur-containing amino acids,
S1PC, SAC and SAMC, are produced during the aging process of raw
garlic in 50% ethanol solution for >10 months (19). The purification and structure
determination of sulfur compounds in AGE was performed by high
performance liquid chromatography (HPLC) and liquid
chromatography-mass spectrometry (LC-MS) systems as previously
described (9).
Reagents
Dulbecco's modified Eagle's medium (DMEM, D6046) and
WST-1 reagent (501594401) were purchased from Sigma-Aldrich. Fetal
bovine serum (FBS, 10270), 0.05% trypsin/EDTA solution (25300-054),
Halt protease and phosphatase inhibitor single-use cocktail
(1861280) and TRIzol reagent (15596018) were from Thermo Fisher
Scientific. Penicillin/streptomycin (164-25251) and recombinant
human TNF-α (207-15261) were from Wako Pure Chemical Industries.
Human β-defensin-3 (hβD3, 4382-s) was from Peptide Institute Inc.
Anti-intercellular adhesion molecule-1 (ICAM-1) (4915S),
anti-phosphorylated nuclear factor κ-light-chain-enhancer of
activated B cells (NF-κB) p65 (3033) antibodies and a horseradish
peroxidase (HRP)-conjugated rabbit immunoglobulin G (IgG) (7074S)
were from Cell Signaling Technologies. An anti-NF-κB p65 antibody
(sc-109X) was from Santa Cruz Biotechnology. RIPA lysis buffer
(20-188) was purchased from Merck Millipore. An HRP-conjugated
anti-β-actin antibody (PM053-7) was from MBL Life Science.
Cells and cell culture
The human gingival epithelial cells, Ca9-22
(JCRB0625, Lot. 11182016, Biomedical Innovation, Health and
Nutrition Research Institute) were cultured in DMEM containing 10%
fetal bovine serum, 100,000 units/ml penicillin and 100 µg/ml
streptomycin in 5% CO2 at 37˚C. The Ca9-22 cells were
subcultured 2 or 3 times per week. For pharmacological experiments,
the cells were seeded onto a 6- or 12-well plate at the density of
1 to 2x105 cells per well and cultured for 36 to 48 h.
When they reached full confluency, the cells were treated with
TNF-α (100 ng/ml) in the absence or presence of AGE (0.01-1 mg/ml),
S1PC, SAC and SAMC (each at 1 to 100 µM) for the 3, 6, 12 or 24 h.
In the experiment with cycloheximide (CHX, Fig. 4), the cells were pre-treated with
TNF-α (100 ng/ml) for 12 h, and then treated with S1PC (10 µM) in
the absence or presence of CHX (10 µM) for 6 h.
Interleukin (IL)-6 secretion
Following treatment of the cells with TNF-α (100
ng/ml) in the absence or presence of AGE (0.01-1 mg/ml), S1PC, SAC
or SAMC (each at 1 to 100 µM) for 6 h, an aliquot of culture medium
was collected and frozen at -80˚C until use. The IL-6 protein
amount in the medium was determined with a human IL-6 Uncoated
enzyme-linked immunosorbent assay (ELISA) kit (88-7066-22, Thermo
Fisher Scientific).
Western blot analysis
Cells were washed with phosphate-buffered saline
(PBS) 5 times and total protein was extracted with RIPA lysis
buffer containing protease inhibitor cocktail. The protein amount
in the extract was determined with the Pierce BCA Protein Assay kit
(23225, Thermo Fisher Scientific) with bovine serum albumin (BSA,
A7030, Sigma) as a standard. The protein extract (10 µg) was
denatured by the addition of sample buffer [5% sodium dodecyl
sulfate, 12.5% glycerol and 50 mM dithiothreitol in 100 mM
Tris-hydrochloric acid (HCl) buffer, pH 6.8], and was heated at
95˚C for 5 min. The samples were separated by electrophoresis and
were blotted onto a nitrocellulose membrane (1620090, Bio-Rad). The
blotted membrane was blocked with 5% non-fat dry milk (999S, Cell
Signaling Technology) in Tris-buffered saline (TBS)-T buffer (0.1%
Tween-20, pH 7.6) for 1 h, and was subsequently incubated in the
presence of the rabbit anti-ICAM-1 (4915S, Cell Signaling
Technology), anti-NF-κB (sc-109X, Santa Cruz Biotechnology) or
anti-phosphorylated NF-κB (3033, Cell Signaling Technology)
antibody at a 1:2,000 dilution overnight at 4˚C. The membrane was
washed 3 times and incubated in the presence of the HRP-conjugated
anti-rabbit IgG antibody (7074S, Cell Signaling Technology) for 1 h
at room temperature. On the other hand, another blotted membrane
was incubated with the HRP-conjugated anti-β-actin antibody
(PM053-7, MBL Life science) at a 1:2,000 dilution for 1 h at room
temperature, in order to quantitatively normalize the
immunoreactive ICAM-1 protein levels. After washing, immunoreactive
proteins on the nitrocellulose membrane were visualized with
Armasham ECL Prime peroxidase solution (RPN2232V, GE Healthcare),
by using a luminoimage analyzer (ChemiDoc™ MP, Bio-Rad).
The density of each immunoreactive band was analyzed with Image
Lab™ software ver. 4.1 (Bio-Rad).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from cells with TRIzol
reagent. Contaminated genomic DNA in the total RNA was removed by
incubation with gDNA eraser (RR047A, Takara) for 5 min at room
temperature, and total RNA was reverse transcribed into
complementary DNA (cDNA) with a PrimeScript RT reagent kit with
genomic DNA Eraser (RR047A, Takara). cDNA was amplified with primer
pairs using a SYBR Premix Ex Taq II (RR820A, Takara). The level of
gene expression relative to the internal control (β-actin) was
calculated by performing a quantitative (real-time) PCR with a
Real-time PCR system (PikoReal 96, Life Technologies; Thermo Fisher
Scientific). The reaction was run using the following program: 30
sec at 95˚C, 40 repeats of 10 sec at 95˚C, 10 sec of 63˚C and 15
sec of 72˚C. The sequences of the specific primers were as follows:
human ICAM-1 forward, 5'-TCGGCACAAAAGCACTATATG-3' and reverse,
5'-ACAGGACAAGAGGACAAGGC-3'; human IL-6 forward,
5'-TCTCCACAAGCGCCTT-3' and reverse, 5'-CTCAGGGCTGAGATGC-3'; and
β-actin forward, 5'-CGCGAGAAGATGACCCAGAT-3' and reverse,
5'-GGTGAGGATCTTCATGAGGTAGTC-3'. The fold change in the relative
mRNA level to β-actin was calculated based on the Cq (ΔΔCq) method
(20).
Cellular IL-6 protein content
Following treatment of the cells with TNF-α (100
ng/ml) in the absence or presence of AGE (1 mg/ml) or SAC (100 µM)
for 3 h, the total protein was extracted with RIPA buffer. The
amount of IL-6 protein in the extract was determined by ELISA, as
described above. The cellular IL-6 protein content is expressed
relative to 1 mg total protein.
Statistical analysis
Data are expressed as the means ± standard deviation
(SD). Statistical significance was evaluated using the Student's
t-test for differences between 2 groups or one-way analysis of
variance (ANOVA), followed by Tukey's post hoc test for differences
between >3 groups. A P-value <0.05 was statistically
significant in comparison to control or the effect of TNF-α
alone.
Results
Effect of S1PC and hβD3 on the level
of ICAM-1 protein induced by TNF-α
Human gingival fibroblasts and Ca9-22 cells have
been shown to express ICAM-1 in response to the periodontal disease
bacteria, P.g.-derived LPS or TNF-α (21-25).
It was also found that ICAM-1 protein expression in the Ca9-22
cells was profoundly induced by treatment with TNF-α in a
concentration-dependent manner (Fig.
1A). In addition, the ICAM-1 protein level began to increase at
3 h, reached peak levels at 12 h and remained elevated until 24 h
(Fig. 1B). As shown in Fig. 1C, the presence of S1PC for 24 h
inhibited the increased level of TNF-α-induced ICAM-1 protein in a
concentration-dependent manner, whereas treatment with AGE exerted
minimal inhibitory effects even at the highest concentration (1
mg/ml) (Fig. 1D). Both SAC and SAMC
also had no significant effect (Fig.
1E and F).
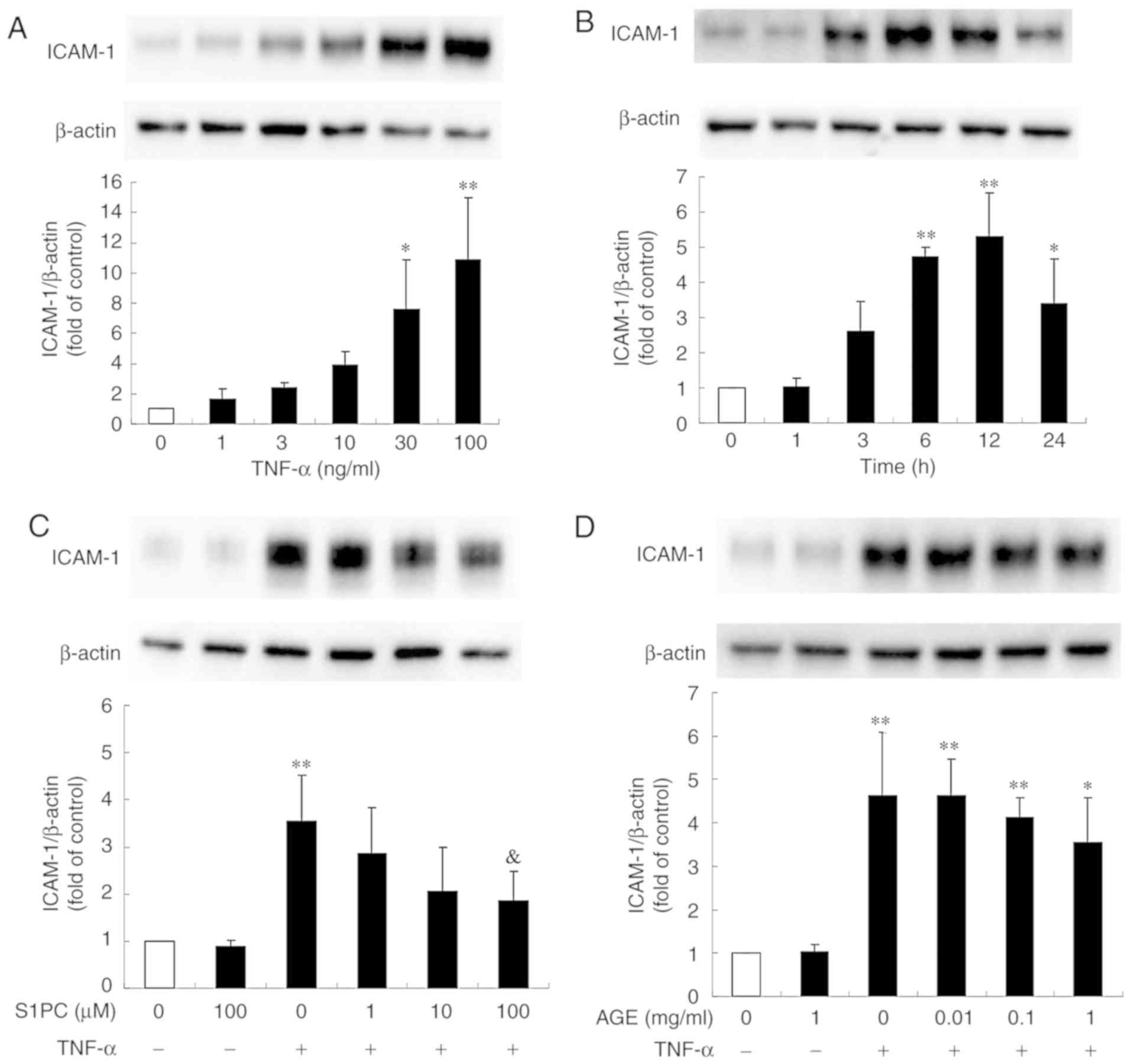 | Figure 1.Effect of S1PC and hβD3 on the level
of ICAM-1 protein induced by TNF-α. (A) The concentration (1 to 100
ng/ml, 12 h)- and (B) time (1 to 24 h, 100 ng/ml)-dependent
induction of ICAM-1 protein stimulated by TNF-α. The cells were
left untreated (control) or treated with TNF-α in the absence or
presence of (C) S1PC (1 to 100 µM), (D) AGE (0.01 to 1 mg/ml), (E)
SAC (10, 100 µM), (F) SAMC (10, 100 µM) or (G) hβD3 (1 to 100 nM)
for 24 h and total protein was extracted. ICAM-1 proteins in the
extract were detected by western blot analysis. Each bar in the
graphs represents the mean ± SD of the band intensity relative to
β-actin (n=3). *P<0.05, **P<0.01 in
comparison to the control (Tukey's multiple comparison test).
&P<0.05 in comparison to TNF-α alone (Student's
t-test). S1PC, S-1-propenylcysteine; hβD3, human
β-defensin-3; TNF-α, tumor necrosis factor-α; ICAM-1, intercellular
adhesion molecule-1; AGE, aged garlic extract; SAC,
S-allylcysteine; SAMC, S-allylmercaptocysteine. |
It has been previously demonstrated that the
anti-microbial peptide hβD3 reduces the level of TNF-α-elicited
ICAM-1 protein in human vein endothelial cells (HUVECs) (26). Thus, this study examined whether the
peptide inhibits the level of ICAM-1 protein in Ca9-22 cells. As
shown in Fig. 1G, the increased level
of TNF-α-induced ICAM-1 protein was moderately but significantly
suppressed by hβD3 at 10 nM, indicating that this peptide is also
effective. Furthermore, it was found that the inhibitory effect of
hβD3 was enhanced by simultaneously treating the cells in the
presence of S1PC (Fig. 2), suggesting
the synergistic effect of hβD3 and S1PC.
Effect of hβD3 and S1PC on ICAM-1 gene
expression induced by TNF-α
It is conceivable that the inhibitory effect of S1PC
on the level of ICAM-1 protein was due to the suppression of ICAM-1
gene transcriptions. Thus, this study then examined the effects of
S1PC as well as those of hβD3 over a period of 3 or 24 h treatment
on the TNF-α-induced ICAM-1 gene expression by RT-qPCR. As shown in
Fig. 3A, TNF-α significantly enhanced
the basal ICAM-1 mRNA transcription level with a peak at 3 h.
Treatment with S1PC or hbD3 for either 3 or 24 h had no effect on
the mRNA expression level of ICAM-1 increased by TNF-α (Fig. 3B and C).
These results suggested that the actions of S1PC and hβD3
occurred at the post-transcriptional level, since these compounds
did not affect ICAM-1 expression even at the concentrations at
which induced a decrease in the protein level.
Effect of S1PC on the level of ICAM-1
protein induced by TNF-α in the presence of cycloheximide
(CHX)
Since S1PC did not affect ICAM-1 gene expression, we
then examined their effect at the translational level using CHX, an
inhibitor of protein synthesis. As shown in Fig. 4, the inhibitory effects of CHX on the
protein level of ICAM-1 were enhanced in the presence of S1PC in
cells treated with TNF-α. This result suggested that the effects of
S1PC occurred at the post-translational level, since this compound
induced a further reduction in the ICAM-1 protein level when total
protein synthesis was blocked.
Effect of AGE, SAC and SAMC on IL-6
secretion induced by TNF-α
IL-6 is produced in human gingival epithelial cells
in response to LPS or TNF-α, leading to the activation of
osteoclasts via the upregulation of receptor activator of NF-κB
ligand (RANKL) in osteoblasts (21).
This study thus examined the effects of TNF-α on IL-6 secretion in
Ca9-22 cells. As shown in Fig. 5A,
TNF-α stimulated basal IL-6 secretion during 6 h of culture. The
enhanced IL-6 secretion induced by TNF-α was reduced by
simultaneous treatment with AGE, SAC or SAMC in a
concentration-dependent manner (Fig.
5A-C). On the other hand, S1PC had no effect on IL-6 secretion
even at the concentration of 100 µM (Fig.
5D).
 | Figure 5.Effect of AGE, SAC and SAMC on IL-6
secretion induced by TNF-α. The cells were left untreated (control)
or treated with TNF-α (100 ng/ml) in the absence or presence of (A)
AGE, (B) SAC, (C) SAMC or (D) S1PC (each at 1 to 100 µM) for 6 h
and culture medium was collected. The IL-6 concentration in the
medium was determined by ELISA. Each bar in graphs represents the
mean ± SD (n=3-4). **P<0.01 in comparison to the
control and #P<0.05, ##P<0.01 (Tukey's
multiple comparison test), &P<0.05 in comparison
to TNF-α alone (Student's t-test). S1PC,
S-1-propenylcysteine; hβD3, human β-defensin-3; TNF-α, tumor
necrosis factor-α; IL-6, interleukin-6; AGE, aged garlic extract;
SAC, S-allylcysteine; SAMC,
S-allylmercaptocysteine. |
Effect of AGE, SAC and SAMC on IL-6
gene expression induced by TNF-α
It was possible that SAC and SAMC downregulated IL-6
gene transcription induced by TNF-α. Thus, the effectσ of these
sulfur compounds on the IL-6 gene expression level were then
examined by RT-qPCR. It was found that TNF-α significantly enhanced
the basal IL-6 mRNA transcription level with a peak at 3 h
(Fig. 6A). As shown in Fig. 6B-D, treatment with AGE, SAC or SAMC
for 3 h had a minimal effect on the enhanced IL-6 mRNA expression
level induced by TNF-α.
 | Figure 6.Effect of AGE, SAC and SAMC on the
IL-6 gene expression induced by TNF-α. The cells were untreated
(control) or treated with (A) TNF-α (100 ng/ml) alone for 3, 6 or
24 h or in the presence of (B) AGE (1 mg/ml) or (B) SAC, (C) SAC,
(D) SAMC (each at 100 µM) for 3 h and total RNA was extracted. The
IL-6 mRNA expression level in total RNA was evaluated by RT-qPCR.
Each bar in graphs represents the mean ± SD (n=3-6).
*P<0.05, **P<0.01 (Tukey's multiple
comparison test) in comparison to the control. S1PC,
S-1-propenylcysteine; hβD3, human β-defensin-3; TNF-α, tumor
necrosis factor-α; IL-6, interleukin-6; AGE, aged garlic extract;
SAC, S-allylcysteine; SAMC,
S-allylmercaptocysteine. |
Effect of AGE, SAC and SAMC on the
intracellular IL-6 protein content
In order to examine whether AGE and sulfur compounds
affect the IL-6 secretion process, the cellular IL-6 protein
content was measured during TNF-α stimulation by ELISA. Treatment
with AGE (1 mg/ml) or SAC (100 µM) for 3 h exhibited a tendency to
enhance the effects of TNF-α on the cellular IL-6 content, although
their effects were not statistically significant (Fig. 7).
Effect of hβD3, S1PC, AGE, SAC and
SAMC on the level of phosphorylated NFkB p65 protein induced by
TNF-α
Stimulation of the cells with TNF-α has been shown
to activate a variety of signaling pathways and transcription
factors, such as NF-κB, which plays an essential role in the
expression of inflammation-related molecules (26-28).
In addition, hβD3 has been shown to alleviate periodontitis
via the suppression of NF-κB (29).
Thus, this study examined the effects of hβD3, S1PC, AGE,
SAC and SAMC on the phosphorylation (activation) of NF-κB induced
by TNF-α. As shown in Fig. 8A and
C-E, hβD3 (100 nM), AGE (1 mg/ml),
SAC and SAMC (each at 100 µM) inhibited NF-κB activation, whereas
S1PC (100 µM) did not have any significant effect (Fig. 8B).
 | Figure 8.Effect of hβD3, S1PC, AGE, SAC
and SAMC on the level of phosphorylated NF-κB p65 protein induced
by TNF-α. The cells were left untreated (control) or treated with
TNF-α (100 ng/ml) in the absence or presence of (A) hβD3 (10, 100
nM) or (B) S1PC (100 µM) for 24 h, (C) AGE (1 mg/ml), (D) SAC or
(E) SAMC (each at 100 µM) for 6 h and total protein was extracted.
Total NF-κB (NF-κB) and phosphorylated NF-κB (pNF-κB) p65 proteins
in the extract were detected by western blot analysis. Each bar in
graphs represents the mean ± SD of the band intensity relative to
β-actin (n=3-4). *P<0.05, **P<0.01 in
comparison to the control and #P<0.05,
##P<0.01 in comparison to TNF-α alone (Tukey's
multiple comparisons test). S1PC, S-1-propenylcysteine;
hβD3, human β-defensin-3; TNF-α, tumor necrosis factor-α; IL-6,
interleukin-6; AGE, aged garlic extract; SAC,
S-allylcysteine; SAMC, S-allylmercaptocysteine. |
Discussion
In gingivitis and periodontitis, TNF-α secreted from
macrophages and neutrophils accumulates in gingival tissues during
the progression of inflammation and induces cytokine production,
such as IL-6 in gingival cells, leading to an increased RANKL
expression in osteoblasts, the activation of osteoclasts and
consequently, to the resorption of alveolar bone (30). Therefore, regulating the excess
inflammation in gingiva is considered to be a key step to treat the
periodontal diseases. In the present study, it was found that
sulfur-containing compounds in AGE differentially inhibited the
TNF-α-elicited ICAM-1 expression and IL-6 secretion, suggesting
that these compounds control inflammation occurring in human
gingival tissues under pathological conditions such as
gingivitis.
ICAM-1 is expressed by several cell types in
response to a variety of stimuli, such as LPS and pro-inflammatory
cytokines, and helps to bind lymphocytes to T cells or vascular
endothelial cells, leading to the activation of immune responses
and the trans-endothelial migration of lymphocytes (31,32). It
has been demonstrated that ICAM-1 is highly expressed in inflamed
tissues, such as atherosclerotic lesions and is associated with
disease progression (32). There are
a number of findings indicating that ICAM-1 is highly expressed by
treating HUVECs with TNF-α or LPS (33-35).
Zhao et al demonstrated that a variety of intracellular
signaling molecules, such as p38 MAPK acting on the MAPK pathway,
nicotinamide adenine dinucleotide phosphate (NADPH) oxidase and the
transcription factor NF-κB, were involved in the ICAM-1 expression
in HUVEC (35). In particular, NF-κB
plays a crucial role in innate immune responses via enhanced
transcription of inflammatory mediators, including interleukins,
chemokines and cytokines (27,28). The
findings of study suggest that S1PC can modulate the level of
ICAM-1 protein in Ca9-22 cells independently of NF-κB. At present,
the S1PC-mediated intracellular pathway remains to be
elucidated.
In human gingival fibroblast cells, ICAM-1 is
expressed in response to pro-inflammatory cytokines, such as TNF-α
and IL-1β (21,22) or periodontal pathogens such as P.
gingivalis (23) via the
activation of NF-κB, p38 MAPK or nucleotide binding oligomerization
domain-containing protein (NOD). On the other hand, to the best of
our knowledge, there are only a few studies available to date
demonstrating the TNF-α-induced expression of ICAM-1 in human
gingival epithelial cells, particularly Ca9-22 cells (22,25), and
the functional mechanisms have not yet been fully clarified.
However, it was previously demonstrated that TNF-α also enhanced
the expression or secretion of IL-6 and IL-8 proteins in another
human gingival epithelial cell line (OBA9) (36) and the oral epithelial cell line,
TR146(37). These findings indicate
that Ca9-22 cells are also available to pharmacologically
investigate the anti-inflammatory function of AGE, although Ca9-22
cells have been often used as an oral cancer model. Thus, this
study first examined whether TNF-α induces the ICAM-1 expression
and found that the levels of both ICAM-1 protein and mRNA were
augmented by TNF-α, but only slightly by P.g.-derived LPS,
indicating that Ca9-22 cells were less sensitive to LPS. It was
also found that S1PC inhibited the level of TNF-α-induced ICAM-1
protein possibly through post-translational modification (Fig. 4), while AGE caused little inhibition,
suggesting that AGE may contain some substances to block the S1PC
action. Furthermore, it was shown that extracellular
signal-regulated kinase-1/2 (ERK1/2), p38 MAPK (data not shown) and
NF-κB (Fig. 8B) were not involved in
the inhibition by S1PC.
The antimicrobial peptide, hβD3, is mainly produced
in gingival epithelial cells and is extracellularly secreted into
the oral cavity, and exhibits antimicrobial activity against
Gram-positive and negative bacteria, fungi and viruses (29,38). hβD3
also plays an important role in the innate immune response via its
immunomodulatory effect, suppressing the periodontal diseases
progression (29,38). It has been reported that the hβD3
expression level in gingival tissues and crevicular fluid is
decreased in patients with periodontitis in comparison to healthy
controls (29). It has been found
that hβD3 reduced the level of ICAM-1 protein and inhibits the
phosphorylation of NF-κB induced by TNF-α, which is consistent with
a finding obtained in HUVECs (26).
Although it was not statistically significant, S1PC at 10 µM
exhibited near the maximal inhibition induced by S1PC at 100 µM on
the elevated ICAM-1 protein by TNF-α (Fig. 1C). Similarly, hβD3 exhibited the
maximal inhibition at 10 nM (Fig.
1G). Since it seemed to be difficult to detect further
inhibition when S1PC and hβD3 at the higher concentrations,
such as 100 µM and 10 nM, respectively, are used, the authors used
S1PC at 10 µM and hβD3 at 1 nM in the synergistic experiment. As
shown in Fig. 2, it was found that
the inhibitory effect of hβD3 was enhanced by simultaneous
treatment with S1PC, suggesting that S1PC synergistically modulates
the action of hβD3 through a signaling pathway different from that
of this peptide.
IL-6 is transiently produced and secreted by several
cell types in response to infections and tissue injuries under
acute inflammation (39). It was well
known that IL-6 exerts beneficial pleiotropic effects, i.e., the
maturation of B cells into antibody-producing cells and development
of effector T-cells (39). On the
other hand, the continuous production of IL-6 in inflamed tissues
results in the progression of chronic inflammation and autoimmunity
(39). Thus, the precise control of
IL-6 secretion is crucial to prevent severe inflammation, which is
often observed under pathophysiological conditions, including
rheumatoid arthritis (39).
In human gingival fibroblasts, the pro-inflammatory
cytokine, IL-1β, secreted from macrophages promotes the production
and secretion of IL-6(40). IL-6
facilitates inflammation at higher concentrations via the
activation of matrix metalloproteases (MMPs) and osteoclasts,
resulting in the gingival tissue destruction and alveolar bone
resorption (40). In human gingival
epithelial OBA9 cells, the IL-6 mRNA level has been shown to be
augmented by stimulation with TNF-α through the phosphorylation of
ERK1/2 and p38 MAPK (36). This study
found that the enhanced IL-6 secretion by Ca9-22 cells stimulated
with TNF-α was significantly inhibited by simultaneously treating
the cells with AGE, SAC or SAMC, suggesting their involvement in
the regulation of IL-6 secretion. However, these substances did not
exhibit any inhibitory effect on the IL-6 mRNA expression levels
(Fig. 6), suggesting the
post-transcriptional mode of control. In addition, it was found
that AGE and SAC tended to augment the content of IL-6 protein in
cells induced by TNF-α, supporting their post-translational
modifications. Based on these findings, it is possible that the
reduced IL-6 release by AGE or SAC may result from the partial
blockade of extracellular secretion, but not from the decreased
production of the cytokine.
In conclusion, this study found that AGE and its
sulfur compounds, S1PC, SAC and SAMC, inhibited inflammatory
reactions induced by TNF-α in human gingival epithelial cells.
Although their mechanisms of action have not been fully clarified,
these data suggest that these sulfur compounds in AGE may directly
reduce inflammation occurring in human gingival epithelial cells by
suppressing the ICAM-1 expression and IL-6 secretion. Moreover, it
is possible that the sulfur compounds in AGE ingested through
systemic circulation reduced gingival inflammation to improve
gingivitis.
Acknowledgements
The authors would like to thank Dr Takami Oka of
Wakunaga Pharmaceutical Co., Ltd. for his helpful advice,
encouragement and critical reading of the manuscript.
Funding
This study was funded by Wakunaga Pharmaceutical
Co., Ltd.
Availability data and materials
All data generated or analyzed during this study are
included in this published article or are available from the
corresponding author on reasonable request.
Authors' contributions
MO drafted the manuscript and performed the
experiments. TN proposed a part of the experimental ideas and
concepts, helped interpret the data and revised the manuscript.
Both authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zini A, Mann J, Mazor S and Vered Y: The
efficacy of aged garlic extract on gingivitis-A randomized clinical
trial. J Clin Dentist. 29:52–56. 2018.PubMed/NCBI
|
|
2
|
Sojod B, Chateau D, Mueller CG, Babajko S,
Berdal A, Lézot F and Castaneda B: RANK/RANKL/OPG signalization
implication in periodontitis: New evidence from a RANK transgenic
mouse model. Front Physiol. 8(338)2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Tzach-Nahman R, Nashef R, Fleisigg O,
Palmon A, Shapira L, Wilensky A and Nussbaum G: Oral fibroblasts
modulate the macrophage response to bacterial challenge. Sci Rep.
7(11516)2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wu YH, Kuraji R, Taya Y, Ito H and Numabe
Y: Effects of theaflavins on tissue inflammation and bone
resorption on experimental periodontitis in rats. J Periodontal
Res. 53:1009–1019. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Morihara N, Hino A, Yamaguchi T and Suzuki
JI: Aged garlic extract suppresses the development of
atherosclerosis in apolipoprotein E-knockout mice. J Nutr.
146:460S–463S. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zeb I, Ahmadi N, Nasir K, Kadakia J,
Larijani VN, Flores F, Li D and Budoff MJ: Aged garlic extract and
coenzyme Q10 have favorable effect on inflammatory markers and
coronary atherosclerosis progression: A randomized clinical trial.
J Cardiovasc Dis Res. 3:185–190. 2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Matsutomo T, Ushijima M, Kodera Y,
Nakamoto M, Takashima M, Morihara N and Tamura K: Metabolomic study
on the antihypertensive effect of S-1-propenylcysteine in
spontaneously hypertensive rats using liquid chromatography coupled
with quadrupole-Orbitrap mass spectrometry. J Chromatogr B.
1046:147–155. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ried K, Travica N and Sali A: The effect
of aged garlic extract on blood pressure and other cardiovascular
risk factors in uncontrolled hypertensives: The AGE at heart trial.
Integr Blood Press Control. 9:9–21. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Suzuki JI, Kodera Y, Miki S, Ushijima M,
Takashima M, Matsutomo T and Morihara N: Anti-inflammatory action
of cysteine derivative S-1-propenylcysteine by inducing
MyD88 degradation. Sci Rep. 8(14148)2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Suzuki J, Yamaguchi T, Matsutomo T, Amano
H, Morihara N and Kodera Y: S-1-Propenylcysteine promotes
the differentiation of B cells into IgA-producing cells by the
induction of Erk1/2-dependent Xbp1 expression in Peyer's patches.
Nutrition. 32:884–889. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Anandasadagopan SK, Sundaramoorthy C,
Pandurangan AK, Nagarajan V, Srinivasan K and Ganapasam S:
S-Allyl cysteine alleviates inflammation by modulating the
expression of NF-κB during chromium (VI)-induced hepatotoxicity in
rats. Hum Exp Toxicol. 36:1186–1220. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Hiramatsu K, Tsuneyoshi T, Ogawa T and
Morihara N: Aged garlic extract enhances heme oxygenase-1 and
glutamate-cysteine ligase modifier subunit expression via the
nuclear factor erythroid 2-related factor 2-antioxidant response
element signaling pathway in human endothelial cells. Nutr Res.
36:143–149. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Tsuneyoshi T, Kunimura K and Morihara N:
S-1-Propenylcysteine augments BACH1 degradation and heme
oxygenase 1 expression in a nitric oxide-dependent manner in
endothelial cells. Nitric Oxide. 84:22–29. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wang HH, Lee HM, Raja V, Hou W, Lacono VJ,
Sacduto J, Johnson F, Golub MN and Gu Y: Enhanced efficacy of
chemically modified curcumin in experimental periodontitis:
Systemic implications. J Exp Pharmacol. 11:1–14. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Fu E, Tsai MC, Chin YT, Tu HP, Fu MM,
Chian CY and Chiu HC: The effects of diallyl sulfide upon
Porphyromonas gingivalis lipopolysaccharide stimulated
proinflammatory cytokine expressions and nuclear factor-kappa B
activation in human gingival fibroblasts. J Periodontal Res.
50:380–388. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Bachrach G, Jamil A, Noar R, Tal G, Ludmer
Z and Steinberg D: Garlic allicin as a potential agent for
controlling oral pathogens. J Med Food. 14:1338–1343.
2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Bakri IM and Douglas CW: Inhibitory effect
of garlic extract on oral bacteria. Arch Oral Biol. 50:645–651.
2005.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Velliyagounder K, Ganeshnarayan K,
Velusamy SK and Fine DH: In vitro efficacy of diallyl sulfides
against the periodontopathogen Aggregatibacter
actinomycetemcomitans. Antimicrob Agents Chemother. 56:2397–2407.
2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kodera Y, Ushijima M, Amano H, Suzuki JI
and Matsutomo T: Chemical and biological properties of
S-1-propenyl-L-cysteine in aged garlic extract. Molecules.
22(570)2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2002.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hosokawa Y, Hosokawa I, Ozaki K, Nakae H
and Matsuo T: Cytokines differentially regulate ICAM-1 and VCAM-1
expression on human gingival fibroblasts. Clin Exp immunol.
144:494–502. 2006.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kato Y, Hagiwara M, Ishihara Y, Isoda R,
Sugiura S, Komatsu T, Ishida N, Noguchi T and Matsushita K: TNF-α
augmented Porphyromonas gingivalis invasion in human
gingival epithelial cells through Rab5 and ICAM-1. BMC Microbiol.
14(229)2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Liu J, Duan J, Wang Y and Ouyang X:
Intracellular adhesion molecule-1 is regulated by Porphyromonas
gingivalis through nucleotide binding oligomerization
domain-containing proteins 1 and 2 molecules in periodontal
fibroblasts. J Periodontol. 85:358–368. 2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Song H, Zhao H, Qu Y, Sun Q, Zhang F, Du
Z, Liang W, Qi Y and Yang P: Carbon monoxide releasing molecule-3
inhibits concurrent tumor necrosis factor-α- and
interleukin-1β-induced expression of adhesion molecules on human
gingival fibroblasts. J Periodontal Res. 46:48–57. 2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Tancharoen S, Matsuyama T, Abeyama K,
Matsushita K, Kawahara K, Sanqalungkam V, Tokuda M, Hashiguchi T,
Maruyama I and Izumi Y: The role of water channel aquaporin 3 in
the mechanism of TNF-alpha-mediated proinflammatory events:
Implication in periodontal inflammation. J Cell Physiol.
217:338–349. 2008.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Bian T, Li H, Zhou Q, Ni C, Zhang Y and
Yan F: Human β-defensin 3 reduces TNF-α-induced inflammation and
monocyte adhesion in human umbilical vein endothelial cells.
Mediators Inflamm. 2017(8529542)2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Nennig SE and Schank JR: The role of NFkB
in drug addiction: Beyond inflammation. Alcohol Alcohol.
52:172–179. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Yamaguchi H, Ishida Y, Hosomichi J, Suzuki
JI, Usumi-Fujita R, Shimizu Y, Kaneko S and Ono T: A new approach
to transfect NF-κB decoy oligodeoxynucleotides into the periodontal
tissue using the ultrasound-microbubble method. Int J Oral Sci.
9:80–86. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Cui D, Lyu J, Li H, Lei L, Bian T, Li L
and Yan F: Human β-defensin 3 inhibits periodontitis development by
suppressing inflammatory responses in macrophages. Mol Immunol.
91:65–74. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Li S, Song Z, Dong J and Shu R:
MicroRNA-142 is upregulated by tumor necrosis factor-alpha and
triggers apoptosis in human gingival epithelial cells by repressing
BACH2 expression. Am J Transl Res. 9:175–183. 2017.PubMed/NCBI
|
|
31
|
Lawson C and Wolf S: ICAM-1 signaling in
endothelial cells. Pharmacol Rep. 61:22–32. 2009. View Article : Google Scholar
|
|
32
|
Lyck R and Enzmann G: The physiological
roles of ICAM-1 and ICAM-2 in neutrophil migration into tissues.
Curr Opin Hepatol. 22:53–59. 2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Liu CW, Sung HC, Lin SR, Wu CW, Lee CW,
Lee IT, Yang YF, Yu IS, Lin SW, Chiang MH, et al: Resveratrol
attenuates ICAM-1 expression and monocyte adhesiveness to
TNF-α-treated endothelial cells: Evidence for an anti-inflammatory
cascade mediated by the miR-221/222/AMPK/p38/NF-κB pathway. Sci
Rep. 7(44689)2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Yan W, Zhao K, Jiang Y, Huang Q, Wang J,
Kan W and Wang S: Role of p38 MAPK in ICAM-1 expression of vascular
endothelial cells induced by lipopolysaccharide. Shock. 17:433–438.
2002.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Zhao W, Feng H, Guo S, Han Y and Chen X:
Danshenol A inhibits TNF-α-induced expression of intercellular
adhesion molecule-1 (ICAM-1) mediated by NOX4 in endothelial cells.
Sci Rep. 7(12953)2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Imai H, Fujita T, Kajiya M, Ouhara K,
Yoshimoto T, Matsuda S, Takeda K and Kurihara H: Mobilization of
TLR4 into lipid rafts by Aggregatibacter actinomycetemcomitans in
gingival epithelial cells. Cell Physiol Biochem. 39:1777–1786.
2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Hosokawa Y, Hosokawa I, Ozaki K and Matsuo
T: IL-27 modulates chemokine production in TNF-α-stimulated human
oral epithelial cells. Cell Physiol Biochem. 43:1198–1206.
2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Güncü GN, Yilmaz D, Könönen E and Gürsoy
U: Salivary antimicrobial peptides in early detection of
periodontitis. Front Cell Infect Microbiol. 5(99)2015.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Tanaka T, Narazaki M and Kishimoto T: IL-6
in inflammation, immunity, and disease. Cold Spring Harb Perspect
Biol. 6(a016295)2014.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Ahn SH, Lee JK, Kim ND, Kim SH, Lee S,
Jung S, Chay KO and Lee TH:
[2-(1,2-diphenyl-1H-indol-3-yl)ethanamine] augments
pro-inflammatory cytokine production in IL-1b-stimulated primary
human oral cells. Int J Mol Sci. 19(1835)2018.PubMed/NCBI View Article : Google Scholar
|






















