Introduction
Glycation is a non-enzymatic chemical reaction that
occurs between a ketone or aldehyde group of fructose or glucose
and an amino acid residue or the hydroxy-group of a protein or
lipid, and is often referred to as the Maillard reaction. Protein
glycation occurs through a complex series of very slow reactions in
the body, including the formation of the stable Amadori-lysine
products (Schiff bases). These give rise to advanced glycation
end-products (AGEs) (1-4).
It is hypothesized that the production and
accumulation of AGEs have causal roles in the development of the
complications associated with aging and lifestyle-related diseases,
such as diabetes, atherosclerosis and hyperlipidemia (1-4).
Furthermore, the production and accumulation of AGEs are involved
in the development of other diseases, such as cardiovascular
diseases, cerebrovascular disorders, chronic renal failure,
Alzheimer's disease and Parkinson's disease (5-9).
Therefore, the identification of safe treatments that can inhibit
glycation is required, as they may exhibit anti-aging effects, or
serve as a therapeutic option for prevention of diseases associated
with glycation (1,10).
In the present study, sodium 4-phenylbutyrate (PBA)
was assessed as a potential candidate for use as an anti-glycation
agent. PBA is an aromatic fatty acid that acts as a histone
deacetylase inhibitor, ammonia scavenger and chemical chaperone
(11,12). It is currently used as a treatment of
urea cycle disorders, as it can promote the excretion of residual
nitrogen (13), and is the subject
of clinical trials for use as a treatment of several other diseases
(14,15). Recently, PBA has been shown to
possess potent anti-oxidative effects that are achieved via the
suppression of endoplasmic reticulum stress, as well as an
anti-inflammatory effect, which is exerted through nuclear
factor-κB (NF-κB) (16-18).
It was previously reported that PBA may be effective
for the treatment of neurodegenerative diseases, including
Parkinson's disease, and it can suppress the onset of dextran
sulfate sodium-induced colitis (19-21).
Furthermore, previous studies have suggested that PBA is effective
against diabetes mellitus and hyperlipidemia (16,22).
Importantly, treatment with PBA is associated with very few side
effects (13,15,19-21).
There are no reports assessing the anti-glycation
effects of existing drugs, to the best of our knowledge. Therefore,
the aim of the present study was to determine whether PBA inhibited
the glycation of proteins in vitro and in vivo.
Materials and methods
Effect of PBA on the glycation of
albumin
The glycation of albumin was measured in
vitro at the Body Support Institute (ARKRAY Karada Lab; ARKRAY,
Inc.) (23). Briefly, the α-glucose
concentration was adjusted to 0.2 mol/l and the human serum albumin
(cat. no. A-1887, Sigma-Aldrich; Merck KGaA) concentration was
adjusted to 8 mg/ml using Dulbecco's PBS (DPBS; Nacalai Tesqe,
Inc.). Subsequently, PBA (LKT Laboratories, Inc.) and the positive
control, aminoguanidine (FUJIFILM Wako Pure Chemical Corporation)
were added at a range of concentrations. After incubation at 60˚C
for 40 h, the concentrations of the AGEs produced were quantified
by measuring the fluorescence intensities of the solutions
(excitation wavelength, 370 nm; emission wavelength: 440 nm) using
a microplate reader (Infinite F200 PRO, Tecan Group, Ltd.). The
experiment was performed three times, in duplicate (n=6).
Effect of PBA on the glycation of
collagen
The glycation of collagen was measured using a
Collagen Glycation assay kit: Glyceraldehyde (cat. no. AK71; Cosmo
Bio., Co., Ltd.), according to the manufacturer's protocol.
Briefly, the neutralized collagen solution was cooled and 50 µl was
carefully added to each well of a 96-well plate, while maintaining
the temperature at <10˚C. Next, the plate was incubated
overnight at 37˚C in a humidified atmosphere. Then, PBA,
aminoguanidine in DPBS and DPBS alone (as the negative control)
were sterilized by filtering using 0.22-µm filters, and 40 µl of
each solution was added to the collagen gel. Finally, 10 µl 500 mM
glyceraldehyde was added to each well and the contents of the wells
were mixed using a plate mixer (Iwaki; AGC Techno Glass Co., Ltd.).
After incubation for 24 h at 37˚C in a humidified atmosphere, the
concentrations of AGEs was assessed by measuring fluorescence
intensity (excitation wavelength, 370 nm; emission wavelength, 440
nm) using a microplate reader. The experiment was repeated three
times in duplicate (n=6).
Effect of PBA on glycation in KK
mice
For the in vivo experiments, 10-week-old male
KK/Ta Jcl mice (KK mice) weighing ~30 g were purchased from CLEA
Japan (CLEA Japan, Inc.). Mice were housed individually in cages in
an animal holding room with a 12 h dark/light cycle at 20±5˚C. The
mice were divided randomly into two groups: Untreated control group
(n=5) and a 20 mg/day PBA-treated group (n=5). PBA was administered
orally at a concentration of 20 mg/200 µl H2O once
daily, and 200 µl H2O was administered to the control
mice. The doses used were based on a previous study (21), and equivalent to the doses
administered to humans in existing drug preparations, such as
Buphenyl (14,15,19-21).
The mice were treated for 8 weeks from 10 weeks of age. Their body
mass and urine glucose levels were measured every 7 days, and their
HbA1c levels were measured every 14 days by obtaining blood from
the tail vein (~1 µl) using a HbA1c measuring device (DCA Vantage;
Siemens Healthineers). Glycosuria was identified in the urine using
a dipstick (cat. no. UA-P1G5; Terumo Corporation). Blood glucose
was measured in ~1 µl blood obtained from the tail vein using a
blood glucose meter (Glutest ai; Sanwa Kagaku Kenkyusho Co., Ltd.).
The urinary albumin concentration was analyzed using an
Lbis® Albumin Mouse ELISA kit (FUJIFILM Wako Pure
Chemical Corporation), according to the manufacturer's protocol.
The mice were fed standard laboratory chow and provided with water
ad libitum. Their food intake was measured every 7 days by
measuring the mass of food remaining in each cage after 24 h. At
the end of the experiment, the mice were euthanized by cervical
dislocation after anesthesia by isoflurane inhalation. The animal
experiments were performed in accordance with Fukuoka University
guidelines and were approved by the Ethics Committee for Animal
Care and Use of Fukuoka University (approval no. 1909069).
Statistical analysis
Statistical analysis was performed using GraphPad
Prism version 6 (GraphPad Software, Inc.). Data are presented as
the mean ± standard error of mean. Data were compared using a one
or two-way ANOVA followed by a post-hoc Dunnett's test for multiple
comparisons. P<0.05 was considered to indicate a statistically
significant difference.
Results
Effect of PBA on the glycation of
albumin
When the fluorescence intensity of the control
samples was defined as 100%, the fluorescence intensities measured
when treated with 0.156, 0.625, 2.5, 10 and 40 mM PBA were 92.6,
91.1, 86.6, 73.4 and 57.9%, respectively. The fluorescence
intensities measured when treated with 0.156, 0.625, 2.5, 10 and 40
mM aminoguanidine, a known anti-glycation agent, were 85.9, 64.5,
46.0, 18.7 and 2.2%, respectively (Fig.
1).
Effect of PBA on the glycation of
collagen
When the fluorescence intensity of the control
samples was defined as 100%, the fluorescence intensities when
treated with 0.156, 0.625, 2.5, 10 and 40 mM PBA were 88.0, 92.4,
91.2, 79.2 and 63.1%, respectively. The fluorescence intensities
associated with aminoguanidines were 74.5, 71.5, 54.9, 21.7 and
3.9%, respectively (Fig. 2).
Effect of PBA on glycation in KK
mice
The effect of oral administration of PBA on KK mice
was monitored for 8 weeks. In the PBA-treated group, the
development of glycosuria was delayed, and the weight gained as
well as HbA1c levels were lower when compared with the control
group. No glycosuric PBA-treated mice were identified after 1 week,
whereas 2 mice in the control group were glycosuric after the same
time period. At the end of the experiment, 2 glycosuric PBA-treated
mice were identified, whereas all the mice in the control group
were glycosuric (Fig. 3). The mean
body mass increase in the PBA group was lower than that in the
control group at every week of the study, and the difference in the
mean body mass of the groups was ≤3.1 g during this period. The
results of the two-way ANOVA were as follows: Treatment (PBA or
control): F(1, 4)=26.7, P=0.0066; Time (weeks): F(8, 32)=74.0,
P<0.0001; and Treatment x Time interaction: F(8, 32)=4.6,
P=0.0007 (Fig. 3). In addition, the
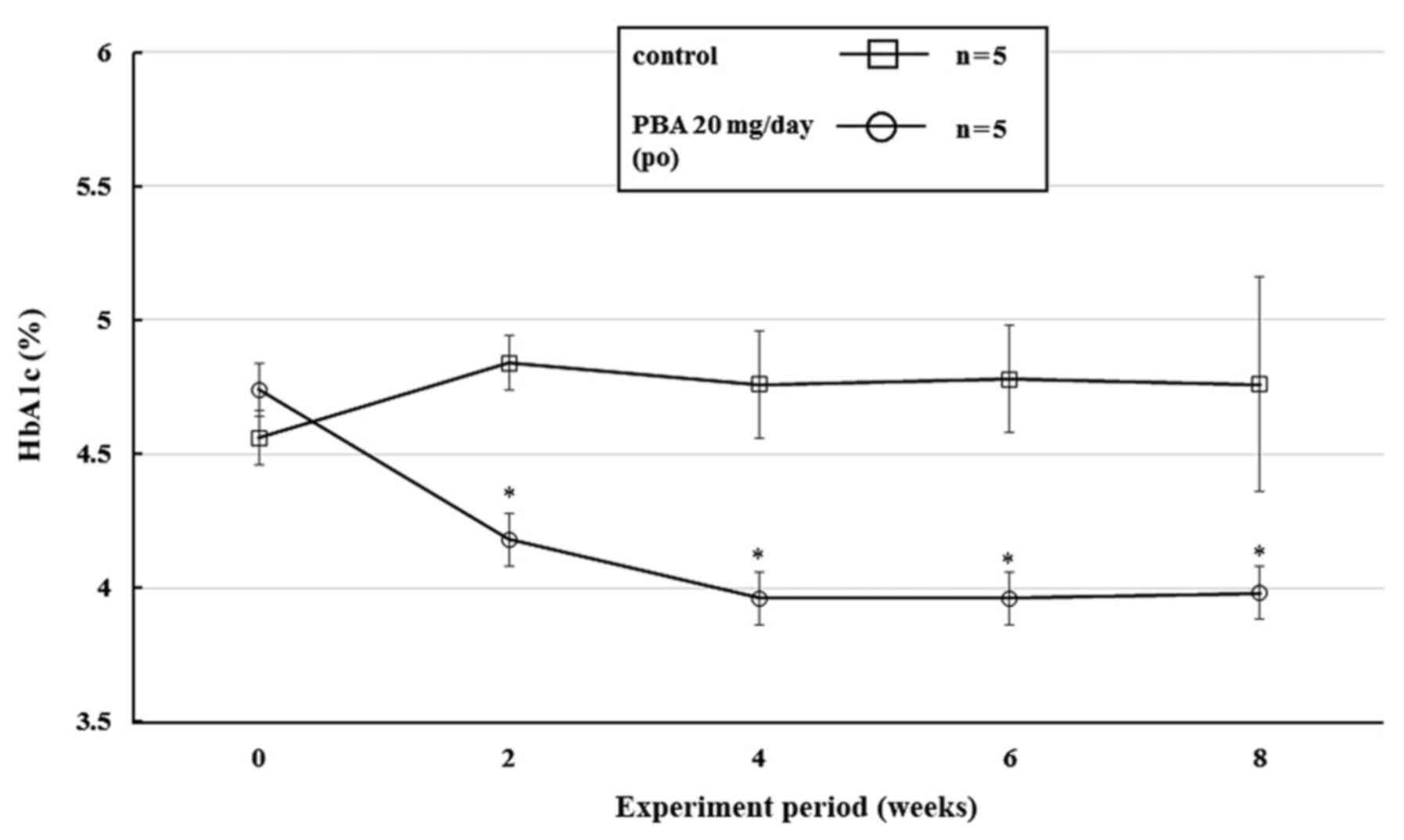
blood HbA1c levels were 3.96% after 4 weeks, and remained ~3.9% in
the PBA-treated group until the end of the study. The results of
the two-way ANOVA were as follows: Treatment (PBA or control): F(1,
4)=18.1, P=0.0131; Time (weeks): F(4, 16)=1.1, P=0.3657; and
Treatment x Time interaction: F(4, 16)=3.4, P=0.0329 (Fig. 4). There were no significant
differences in food intake between the groups during the experiment
(control, 5.3 g/day/mouse; PBA group: 5.4 g/day/mouse).
Discussion
In the present study, the effects of PBA on protein
glycation were assessed. The effects of PBA on non-enzymatic
glycation in vitro were first determined. The effect of PBA
on the glycation of albumin in vitro was assessed as it is
the principal serum protein, and its effect on collagen was
assessed due to the possibility that it may be co-administered with
cosmetics, supplements or pharmaceutical products that also have
effects on collagen. Collagen is the primary structural protein in
the extracellular matrix in various connective tissues. Therefore,
the suppression of collagen glycation can be expected to be applied
as a supplement or cosmetic with a beauty effect (23,24).
Incubation of PBA in a solution of albumin in DPBS and α-glucose
reduced the fluorescence intensity by up to 42.1% compared with the
control, suggesting that PBA reduced the glycation of albumin. The
effect of PBA on the glycation of collagen was then assessed using
a commercially available kit, and found that there was a 36.9%
reduction in collagen glycation, suggesting that PBA also reduced
the glycation of collagen. Thus, through these experiments, it was
confirmed that the addition of PBA reduced the glycation of albumin
and collagen in vitro, and the saccharification of
hemoglobin in vivo. The glycation reaction is complex, and
the in vitro conditions (incubation in 0.2 M glucose at 60˚C
for 40 h) were more extreme than those observed in vivo, in
order to rapidly generate AGEs. The quantity of AGEs produced when
the human serum albumin (HSA) (8 mg/ml) and glucose (0.2 M) are
incubated at 60˚C for 40 h corresponds to ~60 days at 37˚C
(23). However; there are
limitations to this approach. The possible anti-glycation effects
of PBA was evaluated using an established in vitro
anti-glycation evaluation method. The levels of protein glycation
should ideally be evaluated by measuring the residual unreacted
amino/guanidino groups of lysine, arginine, and N-terminal amino
acids, and thus, a modified approach will be used in subsequent
experiments. Furthermore, the in vivo conditions are
complicated by various other factors. Although comparison of in
vitro and in vivo experiments are not easy, it is
possible that PBA administration may inhibit the glycation of
albumin and collagen in vivo. Previous reports have shown
that PBA binds to albumin (25,26);
therefore, it is hypothesized that the binding of PBA to albumin,
the most abundantly present serum protein, may reduce glycation.
The binding of PBA, and its inhibitory effect on the glycation of
albumin and collagen in more detail will be assessed in future
studies.
Having established the effects of PBA on protein
glycation in vitro, KK mice, which develop diabetes, a
disease that involves glycation (27), were administered PBA for 8 weeks.
HbA1c levels were assessed as this is used as a key index of
glucose control in diabetes (28).
The results showed that there was a reduction in HbA1c levels in
PBA-treated mice, suggesting that PBA may have an
anti-saccharification effect as HbA1c is glycated hemoglobin. In
the PBA-treated group, the development of glycosuria was delayed,
and the weight gain and HbA1c levels were lower compared with the
control group, but there was no significant difference in food
intake between the groups during the experiment (~5 g/day/mouse).
These results suggest that it is necessary to evaluate other
markers, such as carboxymethyl lysine (CML), carboxymethyl
arginine, pentosidine and pyraline. However, as HbA1c is a glycated
stress marker, PBA administration is likely to reduce glycation
in vivo.
In vivo glycation and the formation of AGEs
can also be induced by several other carbonyl molecules; therefore,
the levels of major protein glycosylation markers, such as CML,
glucospine, pentosidine and glucoalbumin (a glycated protein)
should be directly measured in future studies. The safety of PBA at
the administered doses has been shown to be safe and is the
established amount administered to humans in existing drug
preparations (such as Buphenyl: 450-600 mg/kg daily, divided into
3-6 doses and orally administered with or immediately after meals
or nutritional supplementation) (14,15,19-21)
(and Buphenyl interview form). In addition, it is necessary to
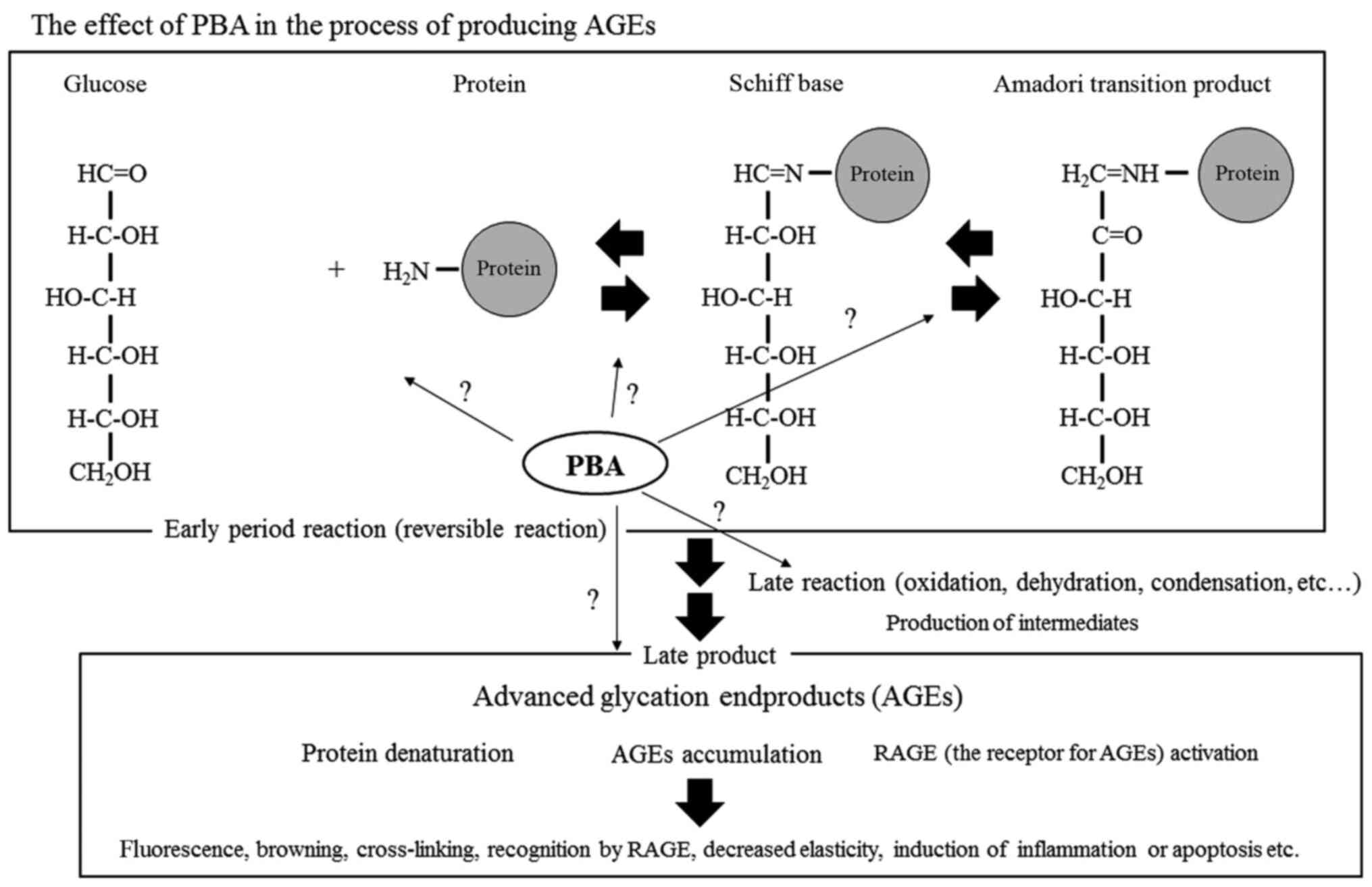
determine in detail at which stage of AGE production PBA exhibits
its effects; for example, the effect of PBA on reversible reactions
(such as Schiff base formation and Amadori transition formation)
should be investigated. Furthermore, the effects of PBA on
oxidation, dehydration, condensation as well as other aspects of
the late reactions, such as oxidative stress, inflammatory reaction
and protein denaturation, should be determined. Figure 5 shows the action of PBA in a
simplified glycation reaction system (Fig. 5).
In vitro results in the present study
confirmed that PBA exhibited an inhibitory effect on albumin and
collagen glycation. Furthermore, it was shown that HbA1c levels
were reduced by PBA when administered to KK mice. The present study
is the first to show the effects of PBA on albumin and collagen
glycation in vitro, as well as its in vivo effects on
HbA1c levels, to the best of our knowledge. However, the reduction
in weight gain in vivo, or the mechanism by which PBA
affects HbA1c levels in the absence of an effect on blood glucose
concentration cannot be explained, and thus requires further study.
It is hypothesized that the glycation of albumin, collagen and
other proteins also occurs in mice. A previous study showed that
human serum albumin and PBA bind to each other, thus PBA may bind
to albumin and inhibits its binding to glucose at an early stage in
the process of glycation (25,26). As
the process of saccharification in vivo is complex, it is
first necessary to identify measurable AGEs and compare the levels
of glycation of each in the control and PBA-administered mice.
Additionally, the strength of the interaction between PBA and
albumin will be assessed using surface plasmon resonance in future
studies. However, it should be noted that the PBA-treated mice did
not exhibit increased urinary albumin concentration levels compared
with the control mice (data not shown).
In conclusion, PBA may limit the aging process and
delay the development of lifestyle-related and other chronic
diseases, such as diabetes, atherosclerosis, hyperlipidemia,
cardiovascular diseases, cerebrovascular disorders, chronic renal
failure and neurodegenerative diseases, which are characterized by
the glycation of proteins. Reducing the prevalence of
lifestyle-related diseases, which are increasing annually
worldwide, may substantially reduce the economic burden on
healthcare systems. Although it is necessary to elucidate the
mechanism by which PBA reduces glycation in more detail, the method
of administration and the side-effects of PBA are well established,
as it is a currently used therapeutic. Therefore, administering PBA
clinically for alleviating aging and lifestyle related disorders
may be an additional use in the relatively near future.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
KO and MN conceived the study and drafted the
manuscript. KO acquired the data. KO and MN analyzed the data and
revised the manuscript. Both authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The animal protocol was approved by the Experimental
Laboratory Animal Committee of Fukuoka University (approval no.
1909069).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sadowska-Bartosz I and Bartosz G: Effect
of glycation inhibitors on aging and age-related diseases. Mech
Ageing Dev. 160:1–18. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Neves D: Advanced glycation end-products:
A common pathway in diabetes and age-related erectile dysfunction.
Free Radic Res. 47 (Suppl 1):S49–S69. 2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Rowan S, Bejarano E and Taylor A:
Mechanistic targeting of advanced glycation end-products in
age-related diseases. Biochim Biophys Acta Mol Basis Dis.
1864:3631–3643. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Gautieri A, Redaelli A, Buehler MJ and
Vesentini S: Age- and diabetes-related nonenzymatic crosslinks in
collagen fibrils: candidate amino acids involved in Advanced
Glycation End-products. Matrix Biol. 34:89–95. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Takata T, Sakasai-Sakai A, Ueda T and
Takeuchi M: Intracellular toxic advanced glycation end-products in
cardiomyocytes may cause cardiovascular disease. Sci Rep.
9(2121)2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Salahuddin P, Rabbani G and Khan RH: The
role of advanced glycation end products in various types of
neurodegenerative disease: A therapeutic approach. Cell Mol Biol
Lett. 19:407–437. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Prasad K and Tiwari S: Therapeutic
interventions for advanced glycation-end products and its receptor-
mediated cardiovascular disease. Curr Pharm. 23:937–943.
2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Gasparotto J, Girardi CS, Somensi N,
Ribeiro CT, Moreira JCF, Michels M, Sonai B, Rocha M, Steckert AV,
Barichello T, et al: Receptor for advanced glycation end products
mediates sepsis-triggered amyloid-β accumulation, Tau
phosphorylation, and cognitive impairment. J Biol Chem.
293:226–244. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
König A, Vicente Miranda H and Outeiro TF:
Alpha-synuclein glycation and the action of anti-diabetic agents in
parkinson's disease. J Parkinsons. 8:33–43. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Abul Qais F, Alam MM, Naseem I and Ahmad
I: Understanding the mechanism of non-enzymatic glycation
inhibition by cinnamic acid: An in vitro interaction and molecular
modelling study. RSC Adv. 6:65322–65337. 2016.
|
|
11
|
Iannitti T and Palmieri B: Clinical and
experimental applications of sodium phenylbutyrate. Drugs R D.
11:227–49. 2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kusaczuk M, Bartoszewicz M and
Cechowska-Pasko M: Phenylbutyric Acid: Simple structure-multiple
effects. Curr Pharm Des. 21:2147–2166. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
De Las Heras J, Aldámiz-Echevarría L,
Martínez-Chantar ML and Delgado TC: An update on the use of
benzoate, phenylacetate and phenylbutyrate ammonia scavengers for
interrogating and modifying liver nitrogen metabolism and its
implications in urea cycle disorders and liver disease. Expert Opin
Drug Metab Toxicol. 13:439–448. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
El-Kasaby A, Kasture A, Koban F, Hotka M,
Asjad HMM, Kubista H, Freissmuth M and Sucic S: Rescue by
4-phenylbutyrate of several misfolded creatine transporter-1
variants linked to the creatine transporter deficiency syndrome.
Neuropharmacology. 161(107572)2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Matsufuji M, Takeshita E, Nakashima M,
Watanabe Y, Fukui K, Hanai T, Ishibashi H and Takashima S: Sodium
phenylbutyrate improved the clinical state in an adult patient with
arginase 1 deficiency. Brain Dev. 42:231–235. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Khan S, Komarya SK and Jena G:
Phenylbutyrate and β-cell function: Contribution of histone
deacetylases and ER stress inhibition. Epigenomics. 9:711–720.
2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zeng M, Sang W, Chen S, Chen R, Zhang H,
Xue F, Li Z, Liu Y, Gong Y, Zhang H and Kong X: 4-PBA inhibits
LPS-induced inflammation through regulating ER stress and autophagy
in acute lung injury models. Toxicol Lett. 271:26–37.
2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wang X, Zhang M, Jiang N and Zhang A:
Sodium Phenylbutyrate ameliorates inflammatory response induced by
staphylococcus aureus lipoteichoic acid via suppressing
TLR2/NF-κB/NLRP3 Pathways in MAC-T Cells. Molecules.
23(3056)2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ono K, Ikemoto M, Kawarabayashi T, Ikeda
M, Nishinakagawa T, Hosokawa M, Shoji M, Takahashi M and Nakashima
M: A chemical chaperone, sodium 4-phenylbutyric acid, attenuates
the pathogenic potency in human alpha-synuclein A30P + A53T
transgenic mice. Parkinsonism Relat Disord. 15:649–654.
2009.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ono K, Nimura S, Nishinakagawa T,
Hideshima Y, Enjyoji M, Nabeshima K and Nakashima M: Sodium
4-phenylbutyrate suppresses the development of dextran sulfate
sodium-induced colitis in mice. Exp Ther Med. 7:573–578.
2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ono K, Nimura S, Hideshima Y, Nabeshima K
and Nakashima M: Orally administered sodium 4-phenylbutyrate
suppresses the development of dextran sulfate sodium-induced
colitis in mice. Exp Ther Med. 14:5485–5490. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Pomozi V, Brampton C, Szeri F, Dedinszki
D, Kozák E, van de Wetering K, Hopkins H, Martin L, Váradi A and Le
Saux O: Functional rescue of ABCC6 deficiency by 4-phenylbutyrate
therapy reduces dystrophic calcification in Abcc6-/- Mice. J Invest
Dermatol. 137:595–602. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Hori M, Yagi M, Nomoto K, Ichijo Ryo,
Shimode A, Kitano T and Yonei Y: Experimental models for advanced
glycation end product formation using albumin, collagen, elastin,
keratin and proteoglycan. Anti-Aging Med. 9:125–134. 2012.
|
|
24
|
Yagi M and Yonei Y: Glycative stress and
anti-aging 4. The evaluation of glycative Stress: Evaluation for
anti-glycative effect. Glycative Stress Res. 4:87–92. 2017.
|
|
25
|
Enokida T, Yamasaki K, Okamoto Y, Taguchi
K, Ishiguro T, Maruyama T, Seo H and Otagiri M: Tyrosine411 and
Arginine410 of human serum albumin play an important role in the
binding of Sodium 4-Phenylbutyrate to Site II. J Pharm Sci.
105:1987–1994. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Yamasaki K, Enokida T, Taguchi K, Miyamura
S, Kawai A, Miyamoto S, Maruyama T, Seo H and Otagiri M: Species
differences in the binding of sodium 4-phenylbutyrate to serum
albumin. J Pharm Sci. 106:2860–2867. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ikeda H: KK mouse. Diabetes Res Clin
Pract. 24 (Suppl):S313–S316. 1994.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Sato A: Indicators of glycemic
control-hemoglobin A1c (HbA1c), glycated albumin (GA), and
1,5-anhydroglucitol (1,5-AG). Rinsho Byori. 62:45–52.
2014.PubMed/NCBI(In Japanese).
|