Introduction
Castleman disease (CD) is a rare atypical
lymphoproliferation disorder, also known as angiofollicular
hyperplasia (1). CD was first
reported by Benjamin Castleman in 1954 and defined 1956(2). Clinically, CD is classified as
unicentric or multicentric CD based on the anatomical distribution
(3). Unicentric CD primarily affects
the mediastinum, and rarely affects the retroperitoneal or pelvic
locations (4). The standard
treatment for unicentric CD is complete surgical resection
(5). However, in some cases, it may
not be possible to resect the problematic mass due to a high degree
of attachment with other organs or hypervascularity (6). Preoperative angiography and
embolization of the arteries that feed the problematic mass can
reduce intraoperative bleeding in cases of CD with hypervascularity
(7).
In the present case report, a rare case of
unicentric CD presented as a pelvic retroperitoneal mass. Due to
the hypervascularity of the mass, preoperative embolization was
performed. The mass was completely resected without any
complications. Additionally, a review of the literature on pelvic
CD and preoperative embolization of CD was performed to provide an
up-to-date reference on the management and outcomes of patients
with CD.
Case report
A 44-year-old man presented with a history of
diarrhea at another hospital. He was diagnosed with acute enteritis
with computed tomography (CT), and the diarrhea was relieved after
a few days. The CT scan incidentally revealed a pelvic
retroperitoneal mass with calcification, and he was referred to
Osaka University Hospital. The patient underwent appendectomy for
appendicitis 30 years ago, and had no viral infection or history of
any other diseases. The pelvic calcification was previously
identified in previous abdominal X-rays, but further examination
was not performed. Physical examination revealed no abnormal
symptoms. Laboratory blood tests, including for tumor makers
(CA19-9 and carcinoembryonic antigen) were normal. Any abnormal
finding was not detected by colonoscopy. The abdominal
contrast-enhanced CT scan revealed a well-defined 50x30 mm mass
behind the sigmoid mesenteric, under the bifurcation of the aorta
in the pelvic retroperitoneal. Non-enhanced phase imaging revealed
coarse calcification inside the mass, and evident contrast
enhancement was observed in the mass during the arterial phase
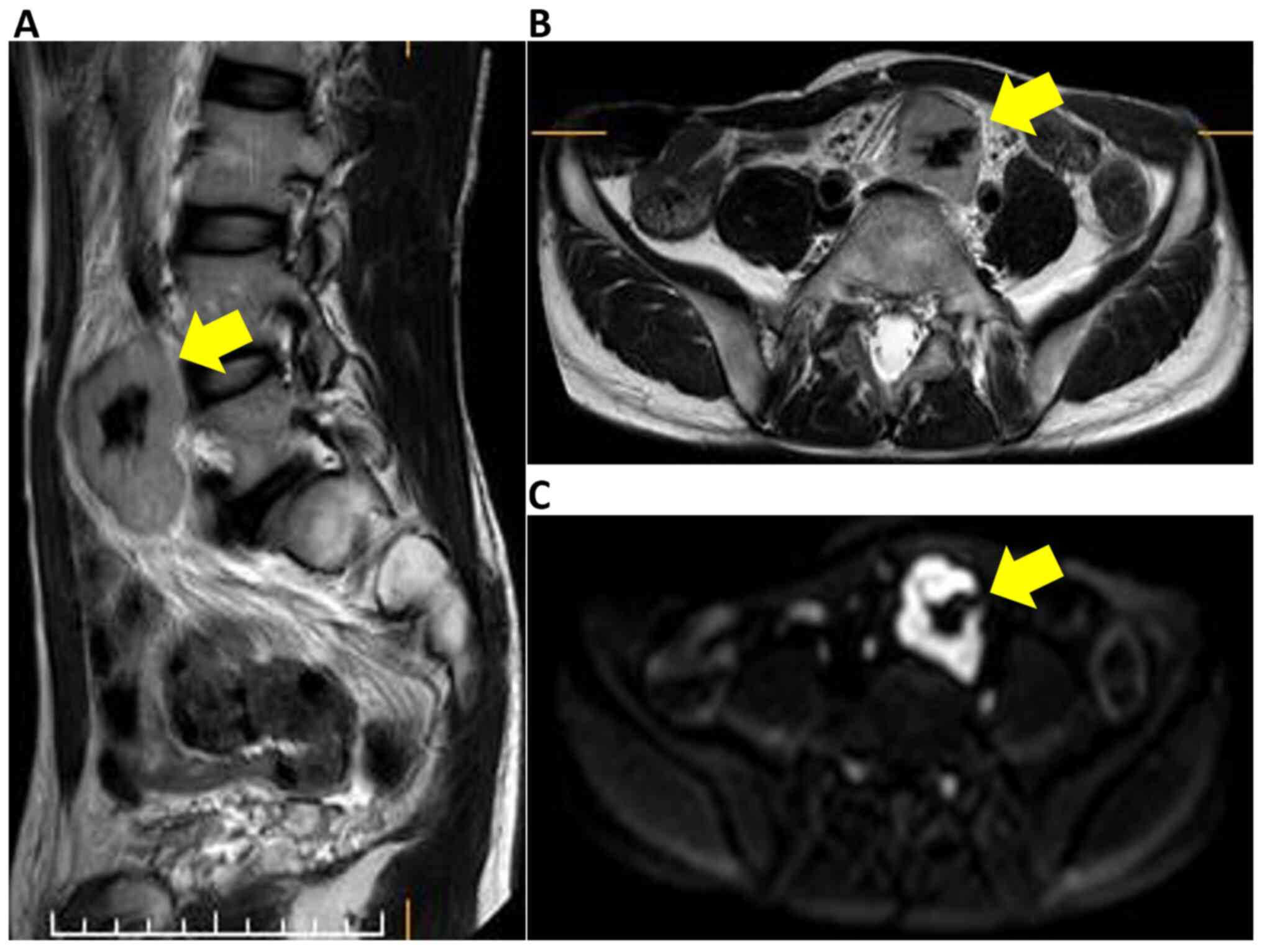
(Fig. 1). Magnetic resonance imaging
(MRI) also revealed a well-defined 50x30 mm solid mass situated in
the pelvic retroperitoneal. The mass demonstrated heterogeneous and
moderately hyperintense signal intensity, and the low signal
intensity corresponded to calcification in the T2-weighed images
and diffusion-weighted images (Fig.
2). A positive emission tomography/CT scan was performed to
exclude the possibility of paraneoplastic manifestations of a
primary tumor, and it revealed a 50x30 mm space-occupying lesion
with hypermetabolic activity (SUVmax at 4.1) (Fig. 3). Possible differential diagnosis
based on the images were CD, primary mesenteric gastrointestinal
stromal tumor or leiomyoma. At first, a diagnosis of CD was doubted
as the tumor had calcification, exhibited a strong contrast in
imaging, had an uniform edge and a relatively uniform inside on the
abdominal CT scan; the tumor was generally isointense on T1
weighted images and hyperintense on T2 images (8). Surgical resection following
embolization was suggested. Angiographically, the tumor was
hypervascular with a dense capillary blush, and it was supplied by
the middle sacral artery (Fig. 4).
The vasculature of the mass was embolized by DMSO and the patient
was operated on the following day.
During the laparotomy, the mass was located at the
bifurcation of the aorta behind the sigmoid mesentery. Mobilization
of the sigmoid mesentery revealed that the mass was 50x30 mm in
size, rubbery, rich in vasculature and exhibited a high-degree of
attachment to the left common iliac vein. Following surgical
ligation and dissection of the vasculature to the mass, the mass
was completely resected from the adjacent organs without any
complications. The patient lost 160 ml of blood, but no blood
transfusion was required. The excised mass was round, well
circumscribed and encapsulated. The cut surface was dark red with a
central white zone of fibrosis and calcification, and it had a
granular appearance (Fig. 5).
Histopathological examination revealed the lymphoid tissue had a
hyalinized vasculature, calcification and noticeable hemorrhaging.
Furthermore, a germinal center was not observed, and thus germinal
center atrophy was suspected (Fig.
6). Immunohistochemical analysis showed protein expression of
CD3, CD20 and CD79a. Immunohistochemistry did not show an increase
in IgG4 antibody expression compared with total immunoglobulin
expression. Clonality analysis using genomic DNA extraction from
the surgical specimen showed no clonality and DNA fragmentation.
These histological findings suggested CD of a hyaline vascular (HV)
type. Currently, at 20 months post-operation, the patient has not
experienced a recurrence. A schematic of this case is shown
(Fig. 7).
Discussion and literature review
The classification of CD into unicentric or
multicentric CD is based on the presence of this
lymphoproliferative disorder in one or more regions, respectively
(9). There are three
histopathological types of the disease, HV, plasmacytic (PC) and
mixed type (10). HV type occurs in
80-90% of cases and usually appears more frequently as a unicentric
localization, whereas PC is primarily multicentric and accounts for
10-20% of cases (11). Furthermore,
90% of patients with unicentric CD are usually asymptomatic
(1). The large lymph node due to
unicentric CD is located only at a single site, exhibits slow
progression and is rarely observed in radiographs (1). CD is often overlooked as a possible
diagnosis due to its low incidence rate. The possibility of CD
should be considered following the identification of a homogeneous
vascular mass (8). CD most commonly
affects the mediastinum (63%), followed by the abdomen (11%),
retroperitoneum (7%) and axilla (4%) (12).
Unicentric CD in the retroperitoneum is commonly
found in the retroperitoneal space (53%), followed by the pararenal
(15%), peripancreatic (9.7%) and pelvic regions (6.7%) (4). The most common presentation is
abdominal pain (42%) (13). Due to
its rarity and lack of disease-specific makers and indications,
preoperative diagnosis is difficult. The differential diagnosis
includes lymphoma, sarcoma, lymph node metastasis, gastrointestinal
stromal tumor, lipomas, leiomyomas, neurofibromas, paraganglioma
and infectious/inflammatory diseases (14). The imaging findings of unicentric CD
are commonly seen on contrast-enhanced CT as a well-defined,
solitary soft tissue tumor with evident contrast enhancement during
the arterial phase (12). Most
unicentric CD lesions are isointense or slightly hyperintense
relative to skeletal muscle on T1-weighted images, and hyperintense
on T2-weighted images, reflecting the vascularity of the mass
(15). The first choice of treatment
for unicentric CD is surgical resection if it is curatively
resectable; the 10-year overall survival rate is 95% and the 5-year
disease-free survival rate is over 90%, suggesting a good prognosis
following complete resection (16).
All previously reported cases of abdominal,
retroperitoneal and pelvic unicentric CD were searched in PubMed,
focusing on studies published in English with images to support the
location of the masses identified. A total of 152 cases of
abdominal, retroperitoneal and pelvic unicentric CD were found (as
of July 2020). A summary of the areas of the abdomen where
unicentric CD has been reported is shown in Fig. 8. The most frequently reported site
was the superior mesenteric artery feeding mesentery (25%; 38/152).
In the retroperitoneal, the paraaortic and left peri-renal areas
were found to be the most common: 13.8% (21/152) and 11.2%
(17/152), respectively. A small number of cases have also been
reported in solid organs such as the liver, pancreas and kidneys
(17-19).
Pelvic unicentric CD occurred less frequently than intra-abdominal
or extra pelvic retroperitoneal unicentric CD, accounting for 15.1%
(25/152) cases of abdominal unicentric CD.
Intraabdominal presentations of CD were the second
most common location, and pelvic presentations were rare. The
present case report was compared with other reported cases in which
unicentric CD was present as a pelvic mass. There were 10 cases,
and the clinical data and surgical outcomes of these patients are
reviewed and listed in Table I. The
mean age of the patients was 35.4 years, and the mean greatest
diameter of the lesion was 5.88 cm. HV type was observed in 10 out
of 11 cases. Furthermore, 2 cases were treated using a laparoscopic
approach. All cases in Table I were
treated with complete resection and there were no cases of
recurrence. Unicentric CD with calcification was found in 2 cases
in Table I. The case reported in the
present study was the only case in which calcifications were
present, and was resected after embolization for pelvic CD.
 | Table ISummary of the clinical data and
outcomes of patients with pelvic unicentric Castleman's disease who
underwent surgical resection. |
Table I
Summary of the clinical data and
outcomes of patients with pelvic unicentric Castleman's disease who
underwent surgical resection.
| First author,
year | Case | Age, years | Sex | Greatest diameter,
cm | Histological
subtype | Calcification on US,
CT or MRI | Preoperative
diagnosis | Preoperative
embolization | Treatment | Follow up period | (Refs.) |
|---|
| Menenakos et
al, 2007 | 1 | 63 | Male | 10.3 | HV | Exist on CT | Castleman's
disease | - | Laparotomy, complete
resection | No recurrence in 2
months | (26) |
| Sato et al,
2013 | 2 | 22 | Female | 9.5 | HV | No on US and
MRI | Could not be
made | - | Laparotomy,
complete resection | No recurrence in
108 months | (27) |
| Al-Natour et
al, 2010 | 3 | 41 | Male | 8 | HV | No on CT | Extra-adrenal
pheochromocytoma | - | Laparotomy,
complete resection | No recurrence in 6
months | (28) |
| Yu et al,
2019 | 4 | 23 | Male | 6.2 | Mixed | No on US and
MRI | Castleman's
disease | + | Laparotomy,
anterior resection | N/A | (29) |
| Benjamin et
al, 2015 | 5 | 29 | Female | 6 | HV | No on MRI | Ovarian
torsion | - | Laparotomy, low
anterior resection | No recurrence in 23
months | (30) |
| Hwang et al,
2011 | 6 | 34 | Female | 6 | HV | No on MRI | Neurogenic
tumor | - | Laparoscopy,
complete resection | N/A | (31) |
| Watson et
al, 2000 | 7 | 46 | Female | 4 | HV | No on MRI | Vascular tumor,
AVM | - | Laparotomy,
complete resection | N/A | (32) |
| Zhang and Jia,
2008 | 8 | 10 | Female | 4 | HV | No on CT | N/A | - | Laparotomy,
complete resection | No recurrence in 6
months | (33) |
| Schelble and
Merritt, 2019 | 9 | 13 | Female | 4 | HV | No on US and
MRI | Biopsy: HV type
Castleman's disease | - | Open
retroperitoneal approach, complete resection | N/A | (34) |
| Guthrie et
al, 2016 | 10 | 64 | Male | 1.7 | HV | No on MRI | Genitourinary or
hematologic malignancy | - | Robotic-assisted
laparoscopy, bilateral pelvic lymph node dissection | N/A | (35) |
| Present case | 11 | 44 | Male | 5 | HV | Exist on CT | Castleman's
disease | + | Laparotomy,
complete resection | No recurrence in 21
months | Present case
report |
Several previous cases were diagnosed with abdominal
unicentric CD following post-surgical histological examination. The
optimal therapy for unicentric CD is surgical resection, which is
usually curative if the disease is amenable to complete resection
(5). Surgical resection is a useful
approach for the diagnosis and treatment of the disease (8).
The masses found in patients with CD often exhibit a
moderate to high degree of attachment contiguous with surrounding
anatomical structures (6). A high
degree of attachment to the contiguous anatomical structures is
often observed in lesions >5 cm in diameter (6). Furthermore, significant bleeding may
obstruct surgical procedures (4).
In cases of HV-type CD where there is a notably
higher risk of massive bleeding due to the hypervascularity,
preoperative angiography and embolization of the arteries that
supply the tumor should be considered to reduce intraoperative
bleeding (7). Preoperative
embolization has also been suggested where there is encasement or
invasion of the adjacent structures (20-22).
The present case was compared with the other
reported cases in which patients with unicentric CD were treated
using complete surgical resection after angiography and
embolization of the feeding artery. There were 10 such cases, and
the clinical data and surgical outcomes of these patients were
reviewed and are listed in Table
II. The mean age of the patients was 28.6 years and the mean
greatest diameter of the lesion was 8.58 cm. HV type was observed
in 10 of 11 cases (aforementioned 10 cases and the present case;
Table II) The mean blood loss
during operation ranged from minimal to 940 ml, and the clinical
course was uneventful in all cases (Table II). Preoperative embolization may
affect the histological findings on the resected specimens. In
relation to the histological findings after embolization, fibrosis
and marked hemorrhage were reported.
 | Table IISummary of clinical data and outcomes
in patients with unicentric Castleman's disease who underwent
preoperative embolization. |
Table II
Summary of clinical data and outcomes
in patients with unicentric Castleman's disease who underwent
preoperative embolization.
| First author,
year | Case | Age | Sex | Greatest diameter,
cm | Location | Feeding artery | Time to operation,
day | Preoperative
diagnosis | Procedure | Blood loss, ml | Follow up
period | (Refs.) |
|---|
| Robert et
al, 2008 | 1 | 31 | Female | 12 | Posterior
mediastinum | Bronchial and
extrabronchial arteries | 7 | N/A | Right anterior
thoracotomy | 200 | N/A | (23) |
| Nagano et
al, 2013 | 2 | 33 | Male | 11 | Next to the right
kidney | Right lumbar
arteries | 1 | Schwannoma,
inflammatory myofibroblastic tumor, and liposarcoma | Complete
resection | 940 | No recurrence in 12
months | (36) |
| Gorospe et
al, 2017 | 3 | 31 | Male | 10 | Mediastinum | Right bronchial
artery | N/A | Biopsy: CD | Right
posterolateral thoracotomy | Little blood
loss | No recurrence in 18
months | (37) |
| Sanchez-Ros-Sanchez
et al, 2012 | 4 | 34 | Female | 9 | Cervical
region | Left transverse
cervical artery and dorsal scapular artery | 1 | CD | Complete
resection | N/A | No recurrence in 30
months | (38) |
| Aydemir et
al, 2010 | 5 | 32 | Female | 9 | Under the azygous
vein | Right bronchial
artery | 14 | N/A | Complete
resection | N/A | No recurrence in 12
months | (39) |
| Safford et
al, 2003 | 6 | 11 | Male | 8 | Middle mediastinal
masses | Right intercostal
artery and right internal mammary artery | 1 | Open biopsy:
CD | Complete
resection | 50 (Open biopsy:
Lots of blood loss) | No recurrence in 1
month | (40) |
| Swee et al,
2009 | 7 | 15 | Female | 7 | Right paratracheal
lesion | Bronchial
artery | N/A | Biopsy: CD | Complete
resection | 50 | N/A | (22) |
| Yu et al,
2019 | 8 | 23 | Male | 6.2 | Pelvic | Bilateral iliac
artery branches | 7 | CD | Laparoscopic
anterior resection | N/A | N/A | (29) |
| Williams et
al, 1998 | 9 | 31 | Male | N/A | Erector spinae
muscle | Right fifth lumber
artery and right internal iliac artery | 1 | Biopsy: CD | Erector spinae
muscle resection | Little blood
loss | No recurrence in 24
months | (41) |
| Amano et al,
2013 | 10 | 30 | Female | N/A | Subcarinal
azygoesophageal recess | Right bronchial
artery | 1 | Paraganglioma and
CD | VATS, complete
resection | 400 | No recurrence in 12
months | (42) |
| Present case | 11 | 44 | Male | 5 | Under the
bifurcation of aorta in pelvic retroperitoneal | Middle sacral
artery | 1 | CD | Complete
resection | 160 | No recurrence in 21
months | Present case
report |
In the present case, the patient had previously been
shown to possess a pelvic calcification in an abdominal x-ray. It
has been reported that calcifications are seen in 31% of patients
with abdominal or pelvic CD (23).
Pelvic calcifications are usually indicative of neurogenic tumors,
teratomas, uterine fibroids and intravesical stones, amongst other
potential conditions (24,25). However, it is important to consider
the possibility of pelvic CD in the differential diagnosis of a
pelvic calcification in an abdominal X-ray.
In conclusion, CD is a rare lymphoproliferation
disorder of uncertain etiology. Pelvic CD is extremely rare, so it
is important to consider CD as a differential diagnosis when a
pelvic lesion is found. Although the clinical course of complete
surgical resection for unicentric CD is good, surgical resection
may be difficult due to attachment with the surrounding tissues or
high hypervascularity. Preoperative angiography and embolization of
the arteries feeding the tumor can prevent or limit intraoperative
bleeding.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated and/or analyzed in the present
study are included in this published article.
Authors' contributions
MK, NM, SF, TO, HT, MU, TM, YD and HE contributed to
the diagnosis at the preoperative conference, NM and SF performed
the resection, and contributed to the follow-up. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of this case report and the
accompanying images.
Competing interests
The author declare that they have no competing
interests.
References
|
1
|
Ren N, Ding L, Jia E and Xue J: Recurrence
in unicentric Castleman's disease postoperatively: A case report
and literature review. BMC Surg. 18(1)2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Castleman B, Iverson L and Menendez VP:
Localized mediastinal lymphnode hyperplasia resembling thymoma.
Cancer. 9:822–830. 1956.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Li XF, Liu JZ, Zhang CJ and Miao Q:
Unicentric Castleman's disease with Cardiovascular Involvement.
Chin Med Sci J. 32:198–200. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Gopi P, Potty VS, Kaurav RS and Govindan
K: Unicentric Castleman's disease as a localized retroperitoneal
mass: A case report and review of literature. Int J Appl Basic Med
Res. 8:259–262. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Soumerai JD, Sohani AR and Abramson JS:
Diagnosis and management of Castleman disease. Cancer Control.
21:266–278. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ko SF, Ng SH, Hsieh MJ, Lin JW, Huang CC,
Lee TY and Chen WJ: Castleman disease of the pleura: Experience
with eight surgically proven cases. Ann Thorac Surg. 76:219–224.
2003.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Madan R, Chen J-H, Trotman-Dickenson B,
Jacobson F and Hunsaker A: The spectrum of Castleman's disease:
Mimics, radiologic pathologic correlation and role of imaging in
patient management. Eu J Radiol. 81:123–131. 2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Tampakis A, Tampaki EC, Daikeler T and
Lardinois D: Intrathoracic tumor of the chest wall: A case of
Castleman's disease mimicking myositis of the lower extremities.
Gen Thorac Cardiovasc Surg. 65:664–666. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Svensson JF, Marshall-Heyman M, Lindgren F
and Ghaffarpour N: Minimal access surgery in Castleman disease in a
child, a case report. J Pediatr Surg Case Rep. 3:289–291. 2015.
|
|
10
|
Bracale U, Pacelli F, Milone M, Bracale
UM, Sodo M, Merola G, Troiani T and Di Salvo E: Laparoscopic
treatment of abdominal unicentric Castleman's disease: A case
report and literature review. BMC Surg. 17(38)2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Xu J, Zhou BO, Cao HL, Wang BO, Yan S and
Zheng SS: Surgical management of isolated retroperitoneal
Castleman's disease: A case report. Oncol Lett. 11:2123–2126.
2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Bucher P, Chassot G, Zufferey G, Ris F,
Huber O and Morel P: Surgical management of abdominal and
retroperitoneal Castleman's disease. World J Surg Oncol.
3(33)2005.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Vassos N, Raptis D, Lell M, Klein P,
Perrakis A, Köhler J, Croner RS, Hohenberger W and Agaimy A:
Intra-abdominal localized hyaline-vascular Castleman disease:
Imaging characteristics and management of a rare condition. Arch
Med Sci. 12:227–232. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Jiang Y, Hou G, Zhu Z, Huo L, Li F and
Cheng W: The value of multiparameter 18F-FDG PET/CT
imaging in differentiating retroperitoneal paragangliomas from
unicentric Castleman disease. Sci Rep. 10(12887)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wong RSM: Unicentric Castleman disease.
Hematol Oncol Clin North Am. 32:65–73. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Talat N, Belgaumkar AP and Schulte KM:
Surgery in Castleman's disease: A systematic review of 404
published cases. Ann Surg. 255:677–684. 2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Masoum SHF, Nooghabi AJ and Rezaei R:
Castleman's disease: Report of four cases and review of the
literature. Acta Medica Iranica. 56:132–136. 2018.
|
|
18
|
Goetze O, Banasch M, Junker K, Schmidt WE
and Szymanski C: Unicentric Castleman's disease of the pancreas
with massive central calcification. World J Gastroenterol.
11:6725–6727. 2005.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Miyoshi H, Mimura S, Nomura T, Tani J,
Morishita A, Kobara H, Mori H, Yoneyama H, Deguchi A, Himoto T, et
al: A rare case of hyaline-type Castleman disease in the liver.
World J Hepatol. 5:404–408. 2005.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Jang SM, Han H, Jang KS, Jun YJ, Lee TY
and Paik SS: Castleman's disease of the renal sinus presenting as a
urothelial malignancy: A brief case report. Korean J Pathol.
46:503–506. 2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Husainy MA, Sayyed F and McPherson SJ:
Castleman's disease: A rare indication for endovascular therapy for
hemoptysis. Indian J Radiol Imaging. 27:33–35. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Swee W, Housseini AM, Angle JF, Jones DR,
Daniel TM, Turba UC, Abdel-Gawad EA and Hagspiel KD: Preoperative
embolization of Castleman's disease using microspheres. Ann Thorac
Surg. 88:1999–2001. 2009.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Robert JH, Sgourdos G, Kritikos N, Didier
D and Terraz S: Preoperative embolization of hypervascular
Castleman's disease of the mediastinum. Cardiovasc Interven Radiol.
31:186–188. 2008.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Meador TL and McLarney JK: CT features of
Castleman disease of the abdomen and pelvis. Am J Roentgenol.
175:115–118. 2000.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sadamoto Y, Abe Y, Higuchi K, Kato K,
Matsumoto S, Arima N and Nawata H: Retroperitoneal Castleman's
disease of the hyaline vascular type presenting arborizing
calcificatio. Intern Med. 37:691–693. 1998.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Menenakos C, Braumann C, Hartmann J and
Jacobi CA: Retroperitoneal Castleman's tumor and paraneoplastic
pemphigus: Report of a case and review of the literature. World J
Surg Oncol. 5(45)2007.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Sato A: Castleman's disease in the pelvic
retroperitoneum: A case report and review of the Japanese
literature. Int J Surg Case Rep. 4:19–22. 2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Al-Natour S, Sawalhi S, Al-Muhtady D and
Hijazi E: Mesenteric Castleman's disease: Case report and
literature review. Asian J Surg. 33:150–153. 2010.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yu G, Cao F, Gong H, Liu P, Sun G and
Zhang W: Embolization of blood-supply artery followed by surgery
for treatment of mesorectal Castleman's disease: Case report and
literature review. Gastroenterol Rep. 7:141–145. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Benjamin B, Zaltzman R, Shpitz B, Gordon
CR and Avital S: Presacral mass discovered during pregnancy
followed by myasthenia gravis. Isr Med Assoc J. 17:318–320.
2015.PubMed/NCBI
|
|
31
|
Hwang MR, Chang HJ, Kim MJ, Seo GJ, Yoo
SB, Park JW, Choi HS and Oh JH: Castleman's disease of the
mesorectum: Report of a case. Surg Today. 41:271–275.
2011.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Watson G, Keane A and Chawdhery Z: Pelvic
Castleman's disease shown by angiography and MRI. Eur Radiol.
10:1837–1839. 2000.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zhang KR and Jia HM: Mesenteric Castleman
disease. J Pediatr Surg. 43:1398–1400. 2008.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Schelble AP and Merritt DF: Pelvic
Castleman's disease presenting as an adnexal mass in an adolescent.
J Pediatr Adolesc Gynecol. 32:86–89. 2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Guthrie PJ, Thomas JV, Peker D, Turkbey B
and Rais-Bahrami S: Perivesical unicentric Castleman disease
initially suspected to be metastatic prostate cancer. Urol Annals.
8:245–248. 2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Nagano S, Yokouchi M, Yamamoto T, Kaieda
H, Setoguchi T, Hiraki T, Tashiro Y, Yonezawa S and Komiya S:
Castleman's disease in the retroperitoneal space mimicking a
paraspinal schwannoma: A case report. World J Surg Oncol.
11(108)2013.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Gorospe L, Valdebenito-Montecino AP and
Munoz-Molina GM: Preoperative embolization of mediastinal
Castleman's disease presenting with stroke. Asian Cardiovasc Thorac
Ann. 25:158–159. 2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Sanchez-Ros-Sanchez A, Infante-Cossio P,
Gonzalez-Garcia A and Borrero-Martin JJ: Preoperative embolization
for the treatment of cervical Castleman disease. J Craniofac Surg.
23:e257–e259. 2012.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Aydemir B, Okay T, Imamoglu O, Sahin S and
Dogusoy I: Preoperative embolization in mediastinal Castleman's
disease. Thorac Cardiovasc Surg. 58:496–498. 2010.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Safford SD, Lagoo AS and Mahaffey SA:
Preoperative embolization as an adjunct to the operative management
of mediastinal Castleman disease. J Pediatr Surg. 38:E21–E23.
2003.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Williams HR, Millner PA and Coral A:
Castleman's disease of the erector spinae muscle. Skel Radiol.
27:637–640. 1998.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Amano Y, Takai D, Ohishi N,
Shinozaki-Ushiku A, Fukayama M, Akahane M, Nakajima J and Nagase T:
Successful treatment of mediastinal unicentric Castleman's disease
using video-assisted thoracoscopic surgery with preoperative
embolization. Case Rep Med. 2013(354507)2013.PubMed/NCBI View Article : Google Scholar
|