Introduction
Inflammation is a primary process in which the
immune system reacts against infectious agents, irritations or
other injuries. It is characterized by several events involving
increased blood flow, vascular permeability and migration of cells
and cytokines to the site of the injury (1). Pain is a sign of inflammation, and it
serves as a protective mechanism induced by the unpleasant sensory
and emotional experiences associated with tissue damage (2). Current pharmacological therapies for
pain relief primarily revolve around anti-inflammatory drugs, and
their undesirable effects, such as gastric discomfort, induction of
an allergic response and impairment of renal function, highlight
the need for increased efforts for the identification of novel
therapeutic agents that act safely and efficiently (3,4).
Isatin-thiosemicarbazones compounds have garnered
significant interest, due to their versatility of obtainment and
variability of therapeutic activities. Isatins [1H-indol-2,3-dione]
are indole heterocyclic compounds that can regulate the growth,
differentiation and death of cells. Isatin derivatives have shown a
wide range of biological activities including cytotoxic,
antimicrobial, antifungal, tuberculostatic, antiviral,
anticonvulsive, antioxidant and anticancer properties (5,6).
Previous studies have shown that
isatin-thiosemicarbazone compounds exhibit antiviral properties,
particularly against smallpox, Human Immunodeficiency Virus and
Encephalitis japonica viruses (6,7).
Additionally, these compounds exhibit antitumor, antifungal,
antibacterial, antimalarial, antileishmanial and larvicide
activities (7-10).
Thus, considering the myriad of functional activities exhibited,
the aim of the present study was to investigate the in vivo
effects of the isatin-thiosemicarbazone compound
(Z)-2-(5-nitro-2-oxoindolin-3-ylidene)-N-phenylhydrazinecarbothioamide
(PA-Int5; Fig. 1) on inflammation
and nociception in mice.
Materials and methods
Animals
All experimental procedures were approved by the
Ethics Committees for Animal Use of the Federal University of Rio
Grande do Norte (Brazil) (approval no. 088.007/2018) in compliance
with Brazilian law (no. 11.794/2008). Male and female Swiss mice
(Mus musculus), aged 6-8 weeks, weighing 30±2 g, were bred
at the animal facility at the Health Sciences Center (Federal
University of Rio Grande do Norte, Brazil) under standard
conditions (23±2˚C; 12 h light-dark cycle, lights on at 6:00 am)
with ad libitum access to food and water in plastic cages.
Mice were fasted of food for 2 h prior to beginning the tests. A
total of 76 male and 71 female Swiss mice were used. After assays,
the animals were euthanized by heart exsanguination, after
anesthesia with thiopental 100 mg/kg (Tiopentax®,
Cristália Prod. Químicos Farmacêuticos) and lidocaine 2% w/v
(EMS/SA; Hotolândia/SP) intraperitoneally (ip) in accordance with
regulations described by the National Council for Control of Animal
Experimentation from 2016(11).
Drugs and treatments
PA-Int5
((Z)-2-(5-nitro-2-oxoindolin-3-ilideno)-N-phenil-hydroazinecarbothioamide)
was obtained from the Laboratory of Planning in Medicinal Chemistry
from the Federal University of Pernambuco, Brazil. This derivative
was synthesized from an isatin and a thiosemicarbazide, using a
method similar to the one described by Moraes Gomes (2016)
(12), and it was chemically
characterized by Nuclear Magnetic Resonance, infrared and elemental
analysis (unpublished data).
In all assays, drug treatments were administered in
a volume of 0.5 ml, solubilized in 1% DMSO. All animals were orally
pre-treated or post-treated, depending on the assay, according to
the specified group: Control, vehicle or PBS (pH=7.2);
dexamethasone (2.0 mg/kg); indomethacin (25 mg/kg); codeine (7.5
mg/kg) or PA-Int5 (1, 2.5 or 5 mg/kg).
The drugs used as standards (codeine and
indomethacin) were used at doses based on tests that demonstrated
both efficacy for the model and safety for animals (13-18).
The doses of PA-In5 were determined according to the protocols of
the Organization for Economic Co-operation and Development
(protocol nos. 420, 423 and 407) to perform the toxicity tests
(19-21).
The control group was treated with the control agent
via the same route and with an equivalent volume as the treated
groups. All drugs used as standards in the tests were commercially
purchased from Sigma-Aldrich (Merck KGaA).
Evaluation of central nervous system
performance
The same group of mice were treated with PA-Int5 [5
mg/kg, oral gavage (vo)] or 1% DMSO and after 1 h, 24 h or 7 days,
they underwent the open field and rotarod tests.
Open field test. The spontaneous locomotor
activity of the mouse was measured using the open field test as
described previously by Seibenhener and Wooten (22). The apparatus, made of wood covered
with impermeable formica, had a black floor, 40x40 cm and black
walls 40 cm high. The test room had controlled illumination
(dimly-lit condition; <10 lux in the center of the open field).
Each mouse was placed in the center of the apparatus, and the
following parameters were automatically registered by a video
tracking system (Anymaze, Stoelting Co.) for 30 min: Distance moved
(in meters) and time spent immobile (in sec). After each
evaluation, the area was cleaned with 5% ethanol solution.
Rotarod test. The rotarod apparatus (AVS
Projetos, Riberão Preto) was composed of a 5-lane rod, which has a
3 cm diameter and was elevated 22 cm from the platform. Before the
test, the animals were trained for 2 consecutive sessions of 120
sec, each one on an automated rotarod unit at 10 rpm. During the
test session, animals were placed on the rod and sequentially
tested at 10 rpm for a maximum of 120 sec with a 2-min rest time
between trials. The total time spent on the bar during a 120 sec
session was recorded using a stopwatch, and the number of falls
during sessions was also recorded (23).
Evaluation of anti-nociceptive
activity Acetic acid-induced abdominal writhing test
First, the mice were allowed to habituate for 20 min
in an individual cage, then they were treated orally with vehicle
(1% DMSO), indomethacin (25 mg/kg) and PA-Int5 (1, 2.5 or 5 mg/kg).
After 1 h, acetic acid (0.6% v/v) was injected intraplantarly
(intraperitonealy; 10 ml/kg body weight), and the frequency of
writhing reflexes (as a measure of visceral pain) was counted for
20 min. Writhing reflexes (characterized by the presence of
contractions of the abdominal muscles) consist of inward
outstretching of the hind limbs, hind paw reflexes and extension of
the whole body (24). The percentage
of reduction in the number of abdominal contractions was calculated
as follows: [(C-T)/C]x100, in which C and T are the number of
abdominal contractions in the Control and Treatment groups,
respectively.
Formalin test. The procedure was performed as
previously described by Hunskaar and Hole (25). Mice were individually placed in a
glass cone (20 cm in diameter) for 20 min. Following the
acclimatization period, formalin (2.5% solubilized in 0.9% NaCl)
was injected (ipl) in a volume of 20 µl into the right hind paw of
the mice. Immediately after the formalin injection, animals were
placed back into the glass cone and were observed for 30 min. A
mirror was placed behind the glass cone to allow an unobstructed
view of the formalin-injected paw. The ipl formalin injection
induces a biphasic nociceptive response: i) An acute phase of short
duration followed by ii) a longer-lasting tonic phase (25). Hence, the evaluation of the
nociceptive behavior is divided into two phases. The first 5 min
after formalin injection, is considered the first phase followed by
a quiescent period of ~10 min, and then the second phase occurs
from 15-30 min after injection A total of 1 h before the formalin
injection, the mice were orally treated with vehicle (DMSO 1%),
codeine (7.5 mg/kg), indomethacin (25.0 mg/kg) or PA-In5 (1, 2.5 or
5 mg/kg), and the time (in sec) that animals spent licking, shaking
and retracting the injected paw was measured with a chronometer,
and was considered to be an indication of ongoing nociception. The
percent reduction of paw pain time was calculated using the formula
[(C-T)/C]x100, where C is the paw pain time in the control group
and T is the paw pain time in the treatment group (tests and
standard).
Evaluation of anti-inflammatory
activity Carrageenan-induced paw edema model
In vivo anti-inflammatory activity was
determined using the carrageenan-induced paw edema method in mice
as described previously (26) with
some modifications. Animals were injected ipl with 50 µl 1%
λ-carrageenan (Sigma-Aldrich; Merck KGaA) or PBS (pH 7.4). A total
of 50 µl is recommended for the formation of edema in the paw,
being a more standardized way of evaluating edema in this
experimental model, as used in previous studies (27-30).
A total of 30 min later, following the inflammatory
challenge, mice were treated by vo with PBS, dexamethasone (2
mg/kg), or PA-In5 (1, 2.5 or 5 mg/kg). The paw edema was measured
after 0 (immediately after treatment), 1, 2, 3 and 4 h (after
carrageenan administration) with a digital caliper (Digemess
100.174BL; Digemess Instruments, Ltd.). Paw edema was measured in
mm and calculated as the percentage of edema. The following
equation was used to obtain the percentage of the respective
experimental groups: Percentage edema = [average paw thickness
(after 1, 2, 3 or 4 h) - average paw thickness (0 h)]/average paw
thickness (0 h).
The area under the time-course curve was also
determined using the trapezoidal rule (26,28).
Zymosan-induced air-pouch model.
Anti-inflammatory activity was evaluated using the zymosan-induced
air pouch model with some modifications (31). Briefly, the Swiss mice received 5 ml
sterile air subcutaneously injected into their back. After 3 days,
2.5 ml of sterile air was injected into the cavity, and 6 days
after the initial air injection, the animals were administered a
zymosan solution (1 mg/ml) into the air pouch simultaneously with
oral administration of PBS or dexamethasone (2 mg/kg), or PA-Int5
(1, 2.5 or 5 mg/kg). A total of 6 h after the treatments, the
animals were euthanized and the exudates were harvested from each
air pouch with 2 ml PBS. The samples were centrifuged at 200 x g
for 10 min at 4˚C, the cell pellet was resuspended in 1 ml PBS, and
diluted in Turk's solution (Sigma-Aldrich, Merck KGaA) (1:10 v/v).
The total number of leukocytes were determined using a Neubauer
chamber with the aid of a Nikon ECLIPSE E200® microscope
(Nikon Corporation) at x40 magnification. The results are expressed
as the number of leukocytes per ml. The supernatants were collected
for the determination of total proteins.
Determination of total protein concentration.
The supernatants were utilized for the determination of total
protein concentration using a Bradford assay. A total of 10 µl of
each sample was added to 96-well plates, followed by the addition
of 200 µl Bradford reagent. Results were obtained using a
microplate reader (BioTek Instruments, Inc.) at 595 nm and
expressed as µg/ml (32).
Statistical analysis
Data are expressed as the mean ± standard deviation.
Statistical analyses were performed using a one-way ANOVA with a
Tukey's post hoc test in GraphPad Prism version 5 (GraphPad
Software, Inc.). P<0.05 was considered to indicate a
statistically significant difference.
Results
Effects of PA-Int5 on the central
nervous system
Due to the absence of in vivo information of
the effects of PA-Int5, the animals were first assessed with regard
to their motor performance, before the evaluation its
anti-nociceptive and anti-inflammatory activity.
To exclude any motor impairment induced by the
treatment with PA-Int5, the effects of this compound were tested in
the open field and rotarod tests. PA-Int5 (5 mg/kg) did not affect
the total distance travelled and the time spent immobile in male
and female mice in the open field test when they were assessed for
1 h, 24 h and 7 days after treatment (Fig. 2; P>0.05).
The oral administration of PA-Int5 (5 mg/kg) did not
impair the motor performance of male and female mice evaluated in
the rotarod test, since significant alterations were not observed
in the number of falls and in the time spent on the rotating bar
when evaluated for 1 h, 24 h and 7 days after treatment (Fig. 3; P>0.05).
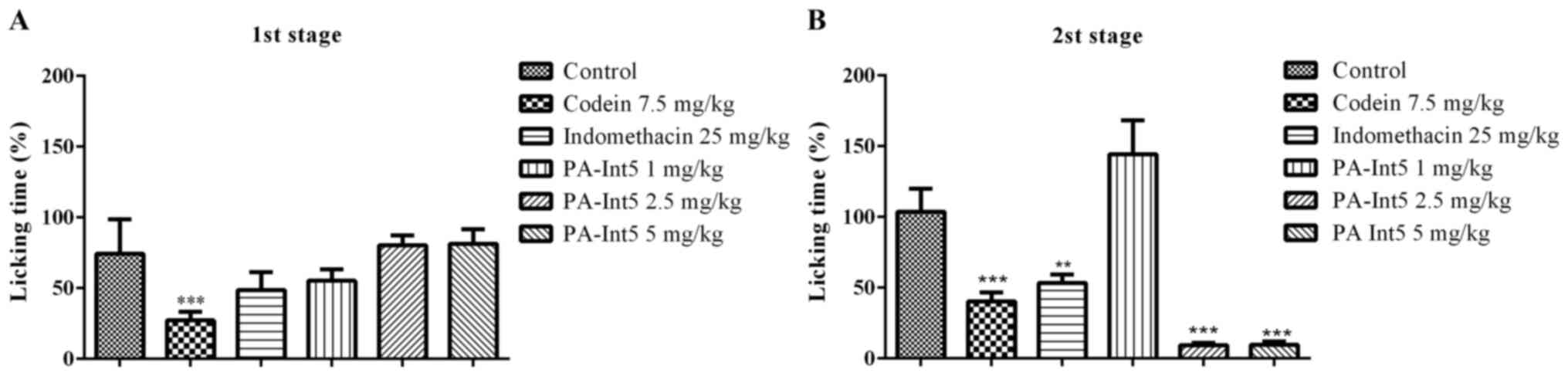
Anti-nociceptive effects
As shown in Fig. 4,
treatment with indomethacin reduced the acetic acid-induced
abdominal contractions by 52.5%. Similar to the positive control,
treatment with PA-Int5 reduced the acetic acid-induced abdominal
contractions in a dose-dependent manner, displaying a significant
reduction in nociceptive behaviors at 2.5 (31%) and 5 mg/kg (34%)
compared with the vehicle [F-value (4.18)=31.64; P<0.05].
Fig. 5 illustrates
the effects of the standard analgesic drugs, indomethacin and
codeine, on ongoing nociception induced by formalin. Animals that
received the injection with formalin displayed a typical biphasic
nociceptive response. Pre-treatment with codeine (7.5 mg/kg)
significantly attenuated the amount of time mice exhibited
nociceptive behavior for in both phases of the formalin test. The
inhibitory effect exerted by the opioid in the nociceptive
behaviors amounted to 75 and 65% of the controls for phases 1 and
2, respectively (Fig. 5). Moreover,
animals treated with indomethacin (25 mg/kg) displayed a
statistically significant reduction in nociceptive behavior only
during phase 2. The inhibitory effect of indomethacin was 55% that
of the control (Fig. 5). In the
first phase of the formalin test, treatment with PA-Int5 did not
evoke any anti-nociceptive effects (Fig.
5A; P>0.05). However, during the second phase, oral
administration of 2.5 and 5 mg/kg PA-Int5 significantly reduced the
time spent exhibiting nociceptive behaviors (92.7 and 93.2%
compared with the control; Fig. 5B;
F-value (5.18)=74.90; P<0.05).
Anti-inflammatory effects
Fig. 6 shows the
effects of treatment with PA-Int5 and dexamethasone in the
carrageenan-induced paw edema in mice. The injection of carrageenan
in the paw significantly increased the edema compared with the
control group during the observation period. The administration of
dexamethasone significantly reduced paw edema compared with the
carrageenan group. The oral administration of PA-Int5, at a dose of
1 mg/kg significantly inhibited the formation of edema from 2 h
after treatment when compared with the carrageenan group; 2.5 mg/kg
showed significant inhibition only after 4 h; and the most
effective dose was 5 mg/kg, which inhibited edema after only 1 h
(Fig. 6A). When evaluated, the
progression of the edema after the 4 h of observation, calculated
through the area under the curve of edema kinetics using the
trapezoidal rule (26,28), showed a significant decrease in all
doses of PA-Int5 tested when compared with the carrageenan group
[Fig. 6B; F-value (5.21)=43.17;
P<0.05]. Doses of 1, 2.5 and 5 mg/kg PA-Int5 inhibited the
formation of edema by 36, 20.2 and 47.3%, respectively.
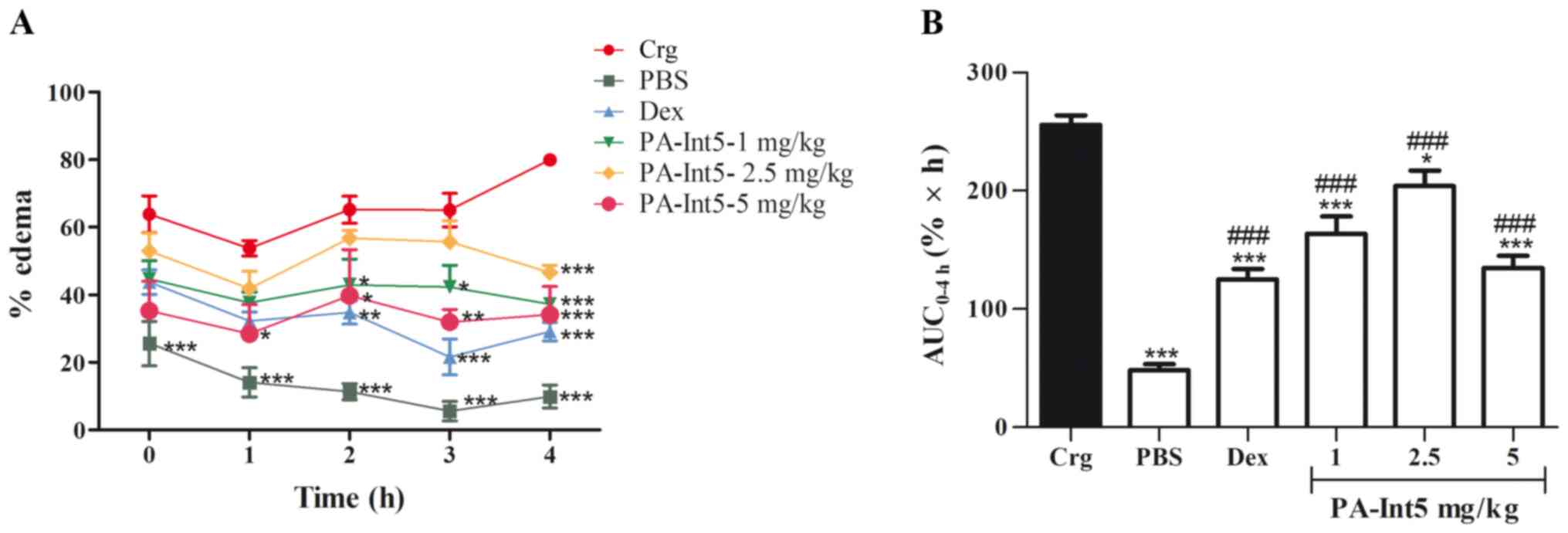 | Figure 6Anti-edematogenic effects of Dex (2
mg/kg, vo) and PA-Int5 (1, 2.5 and 5 mg/kg, vo) in the Crg-induced
paw edema in mice. (A) Percentage of paw edema measured after 0, 1,
2, 3 and 4 h following intraplantar injection of Crg. (B) Area
under the curve for each drug treatment. Data are presented as mean
± standard deviation of 5 mice/group. ###P<0.001 vs.
PBS group; *P<0.05, **P<0.01,
***P<0.001 vs. carrageenan group. Dex, dexamethasone;
vo, oral gavage; Crg, carrageenan. |
In the zymosan-induced air-pouch model, treatment
with zymosan significantly increased leukocyte migration compared
with the PBS group. PA-Int5, at all doses tested, significantly
inhibited the migration of leukocytes into the dorsal cavity, when
compared with the zymosan group, as shown in Fig. 7A and Table
I. Concomitantly, it was shown that there was a reduction in
protein concentration at this site (Fig.
7B). Treatment with dexamethasone reduced cell migration by 65%
compared with zymosan. The oral administration of PA-Int5 (1, 2.5
and 5 mg/kg) significantly inhibited leukocyte migration to the
dorsal cavity by 64, 62 and 66%, respectively [Fig. 7A; F-value (5.19)=22.41; P<0.05].
Concerning the amount of protein measured in the exudates of the
dorsal cavity, it was observed that there was a significant
increase in this parameter when treated with zymosan. By contrast,
there was a significant reduction in protein extravasation to the
inflammation site when treated with dexamethasone and all doses of
PA-Int5 [Fig. 7B; F-value
(5.17)=177.6; P<0.05]. PA-Int5 (1, 2.5 or 5 mg/kg) treatment
reduced protein exudation by 72, 68 and 67%, respectively, compared
with the zymosan-treated mice.
 | Table IAnti-inflammatory activity of the
PA-Int5 in the zymosan-induced air pouch model. |
Table I
Anti-inflammatory activity of the
PA-Int5 in the zymosan-induced air pouch model.
| Group | Dose, mg/kg | Cell migration,
1x106/ml | Inhibition, % |
|---|
| Zymosan 1
mg/ml | - | 56.750±5.732 | - |
| Dexamethasone | 2 |
18.375±4.695a | 65 |
| PA-Int5 | 1 |
20.000±3.048a | 64 |
| PA-Int5 | 2.5 |
21.250±2.618a | 62 |
| PA-Int5 | 5 |
24.500±3.060a | 66 |
Discussion
The present study is the first to show that an
isatin-thiosemicarbazone compound exhibited potential
anti-inflammatory and anti-nociceptive effects in mice. Toxicity
studies performed in our research group have shown that PA-Int5 is
safe at the doses tested (unpublished data). Considering these
promising biological properties reported here for PA-Int5,
systematic studies for assessing its safety and efficacy are
recommended as the next steps.
Pain is produced by several mechanisms, and it can
be classified as physiological, inflammatory or neuropathic
(33). In this sense, different
animal models of nociception were used to mimic the different
causes of pain: Chemical (formalin test and acetic-acid induced
writhing reflexes) and inflammatory (carrageenan-induced paw edema
and zymosan-induced air-pouch). The results showed that PA-Int5
exhibited potent anti-nociceptive and anti-inflammatory effects at
the doses tested in all models of nociception and inflammation in
mice.
The anti-nociceptive activity was assessed based on
acetic acid-induced abdominal contortion is a sensitive assay used
for the evaluation of peripheral-acting analgesics clinically used
in the relief of inflammatory and visceral pain (34). Acetic acid induces nociception due to
the local inflammatory response resulting from the release of
arachidonic acid from resident peritoneal cells, in addition to the
release of TNF-α and prostaglandin E2 that sensitizes nociceptive
fibers (34). Non-steroidal
anti-inflammatory drugs, such as indomethacin, are effective in
inhibiting prostaglandin synthesis (35); this drug was used as positive control
here. Similar to indomethacin, treatment with the
isatin-thiosemicarbazone derivative compound significantly reduced
abdominal contortions induced by acetic acid, suggesting that
Pa-Int5 exhibited analgesic activity.
The formalin test is one of the most widely used
methods to investigate anti-nociceptive activity (25,36-38).
Formalin sensitizes the peripheral nerve endings, inducing
nociceptive effects (3). The
formalin test was divided into two phases. The initial phase, which
is termed the neurogenic phase, begins immediately after formalin
injection and persists for up to 5 min. During the early stage,
substance P and bradykinin mediate the elicitation of pain
(25). The second phase, termed the
inflammatory phase, begins 15-20 min after formalin injection and
persists for up to 30 min. During this stage, histamine, serotonin,
prostaglandins and bradykinin are involved in the induction of the
inflammation and the elicitation of pain (25). Opioids are effective in both phases
of the test, whereas non-steroidal anti-inflammatory drugs and
steroids are only effective in the second phase (3,16,25).
When designing the experimental conditions, two positive-control
drugs were used: Codeine (7.5 mg/kg), an analgesic opioid, and
indomethacin (25 mg/kg), a non-selective COX inhibitor. The opioid
agonist reduced formalin-induced nociceptive behaviors during the
first and second phases, whereas indomethacin was effective only
during the second phase. Similar to the COX inhibitor
positive-control, PA-Int5, at the higher doses, promoted pain
relief only during the second phase in the formalin test. Such
anti-nociceptive effects suggest inhibition of synthesis or release
of arachidonic acid and its metabolites, including prostaglandins
and leukotrienes (3,39,40).
The effect of PA-Int5 on open-field and rotarod
tests were investigated to exclude non-specific behaviors, such as
sedation or psychomotor agitation, which may influence the
interpretation of anti-nociceptive actions (41). Open-field testing is one of the most
popular types of behavioral tests used to evaluate spontaneous
locomotor activity in rodents (41,42).
Locomotion is the most universal and conserved form of movement,
which involves mechanisms controlled by the CNS (41). The rotarod test is a widely used
method in preclinical research, as it evaluates motor coordination
and it can detect physical deficiencies caused by pharmacological
agents, such as muscle relaxants and CNS depressants (41). Motor coordination is a complex
behavior that reflects muscle strength, balance and standard gait,
and is influenced by the effects of benzodiazepines or barbiturates
(41,42). The results of the present study
showed that the administration of PA-Int5 (5 mg/kg, vo) did not
affect locomotion or motor coordination. Thus, this compound did
not promote alterations that compromise the interpretation of
biological activities that are based on motor performance.
The effects of treatment with drugs on inflammatory
components in the present study were evaluated using a carrageenan
and zymosan-induced air-pouch model. These tests induce acute and
not immune inflammation, that is well-validated, and widely
reproduced in numerous studies (43,44).
Signs of inflammation (edema, hyperalgesia, erythema) become
visible shortly after subcutaneous injection of such stimuli
through the activation of pro-inflammatory agents (43).
Carrageenan-mediated inflammation is mediated by
three mechanisms in the production of edema: The primary mechanism
is mediated by histamine, the secondary mechanism is
kinin-mediated, and the tertiary mechanism is characterized by the
local synthesis of prostaglandins and their metabolites, and it is
the tertiary mechanism where the majority of anti-inflammatory
drugs exert their effects (43,45). In
the present study, the glucocorticoid dexamethasone was used to
attenuate vasodilation, arachidonic acid metabolism synthesis and
leukocyte migration (40,43,46).
The results of the present study indicated that
PA-Int5, at the highest dose, exhibited anti-edematogenic activity
with reduction in paw edema to an equivalent degree of that
observed with dexamethasone, acting on the first signs of
inflammation. It was possible to observe biases inherent to animals
in the evaluation of these activities, such as the differentiated
absorption of the sample by the individuals of each group, and the
different anti-inflammatory responses produced by different
individuals from the same experimental group, reflecting on the
anti-edematogenic activity, over 4 h, in the other doses. Even so,
the data were consistent with regard to the activity of the
compound.
The zymosan-induced air-pouch model employs zymosan
(glucan) as an anti-inflammatory agent, which is a cell wall
extract of yeast (43). Its
mechanism of action involves the activation of the inflammatory
process via the complement system, thus inducing lysosomal enzymes,
prostaglandins and leukotriene release (47). The primary findings showed that a
significant decrease in leukocyte migration and protein levels
measured at the site of the inflammation were observed in animals
treated with dexamethasone and PA-Int5. These results suggest that
PA-Int5 exerts its anti-inflammatory action using a mechanism
similar to that of glucocorticoids (48), which would contribute to the
inhibition of polymorphonuclear cell migration as well as reduction
of proteins and other inflammatory components at the site of the
inflammation.
Structural changes of compounds has been extensively
used and studied in attempts to improve their biological
activities. Thus, several studies on the structure-activity
relationship can provide security in the inferences produced in
this work. The structural changes in the molecules can alter the
binding sites and pharmacophoric groups, favoring improved activity
and lower toxicity, via the substitution with electron donor groups
that have improved anti-inflammatory activity, as observed in this
work (5,49-51).
Data from the literature suggest that the
anti-inflammatory action of PA-Int5 can be attributed to the
presence of electron donor groups in its structure at the para
position of the isatin ring; such action may increase the
anti-inflammatory and antioxidant activities (51). Additionally, as reported by Jarapula
et al (51), the presence of
a halogen atom in the isatin ring serves a key role in
anti-inflammatory activity. It has also been reported that the
substitution at the C5 and C7 positions of the isatin ring results
in higher anti-inflammatory activity than other chain substitutions
(51). When combined, the potent
in vivo anti-inflammatory activity of PA-Int5 is, at least
in part, justified by its chemical structure. Furthermore,
comparing PA-Int5 with other isatin-thiosemicarbazone derivatives,
the presence of a nitro group (NO2) serves an important
role in the increase in biological activities (5,12).
Additionally, the presence of different substituents at the C5
position of the isatin structure and the phenyl ring at N4 in the
thiosemicarbazone showed a reduction or elimination of the toxic
potential (5,7,12), thus
increasing the interest in the study of this compound.
Another important point related to the chemical
characteristics of these substituents is the hydrophilic radicals
that favor lower toxicity of the compounds (5,44-46).
However, further in vivo studies are required to investigate
the specific underlying mechanisms.
The present study has some limitations. One of these
is related to the mechanisms of action of the compound that are not
yet molecularly defined. Another limitation is the lack of
histological analysis of the paws from animals submitted to the paw
edema model. However, to minimize this limitation, other
methodologies were used for the evaluation of the anti-inflammatory
parameters of PA-Int5, and through them, the promising effects of
the studied compound were inferred.
In summary, the present study revealed potent in
vivo anti-inflammatory and anti-nociceptive actions of PA-Int5,
an isatin-thiosemicarbazone compound. The anti-nociceptive activity
was observed in visceral inflammatory pain and in moderate
inflammatory pain models induced by formalin, whereas the
anti-inflammatory activity was shown by its anti-edematogenic
actions and reduction of leukocyte migration and protein levels to
the site of the inflammation. Further studies are required to
investigate the mechanisms by which PA-int5 induces these
biological activities as well as for assessing the safety and
efficacy of the compound, and are currently being performed in our
lab.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
TMAML, AGDF, MDFFP and ECG conceived and designed
the study. AGDF, LLDSFRD, JPR, MPDCAF, PATDMG, VGDMS and AAF
performed the experiments. AGDF, MJBDMR, MGDRP, ACLL, MDFFP, ECG
and TMAML wrote and edited the manuscript. TMAML, AGDF, LLDSFRD,
MDFFP, MJBDMR, MGDRP, ACLL and ECG analyzed and interpreted the
data. LLDSFRD, JPR and TMAML confirm the authenticity of all the
raw data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All experimental procedures were approved by the
Ethics Committees for Animal Use of the Federal University of Rio
Grande do Norte (Brazil) (approval no. 088.007/2018) in compliance
with Brazilian law (no. 11.794/2008).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Serhan CN, Chiang N and Dalli J: The
resolution code of acute inflammation: Novel pro-resolving lipid
mediators in resolution. Semin Immunol. 27:200–215. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Raja SN, Carr DB, Cohen M, Finnerup NB,
Flor H, Gibson S, Keefe FJ, Mogil JS, Ringkamp M, Sluka KA, et al:
The revised International Association for the Study of Pain
definition of pain: Concepts, challenges, and compromises. Pain.
161:1976–1982. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Giorno TBS, Silva BVD, Pinto ADC and
Fernandes PD: Antinociceptive effect and mechanism of action of
isatin, N-methyl isatin and oxopropyl isatin in mice. Life Sci.
151:189–198. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
de Oliveira JF, Nonato FR, Zafred RRT,
Leite NMS, Ruiz ALTG, de Carvalho JE, da Silva AL, de Moura RO and
Alves de Lima MDC: Evaluation of anti-inflammatory effect of
derivative
(E)-N-(4-bromophenyl)-2-(thiophen-2-ylmethylene)-thiosemicarbazone.
Biomed Pharmacother. 80:388–392. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Pakravan P, Kashanian S, Khodaei MM and
Harding FJ: Biochemical and pharmacological characterization of
isatin and its derivatives: From structure to activity. Pharmacol
Rep. 65:313–335. 2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Rane RA, Karunanidhi S, Jain K, Shaikh M,
Hampannavar G and Karpoormath R: A recent perspective on discovery
and development of diverse therapeutic agents inspired from isatin
alkaloids. Curr Top Med Chem. 16:1262–1289. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Tenório RP, Góes AJS, Lima JG, Faria AR,
Alves AJ and Aquino TM: Thiosemicarbazones: preparation methods,
synthetic applications and biological importance. Quim Nova.
28:1030–1037. 2005.
|
|
8
|
Graser-Loescher G, Schoenhuber A, Ciglenec
C, Eberl S, Krupitza G, Mader RM, Jadav SS, Jayaprakash V,
Fritzer-Szekeres M, Szekeres T, et al: Thiosemicarbazone
derivatives, thiazolyl hydrazones, effectively inhibit leukemic
tumor cell growth: Down-regulation of ribonucleotide reductase
activity and synergism with arabinofuranosylcytosine. Food Chem
Toxicol. 108 (Pt A):53–62. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Opletalová V, Kalinowski DS, Vejsová M,
Kunes J, Pour M, Jampílek J, Buchta V and Richardson DR:
Identification and characterization of thiosemicarbazones with
antifungal and antitumor effects: Cellular iron chelation mediating
cytotoxic activity. Chem Res Toxicol. 21:1878–1889. 2008.PubMed/NCBI View Article : Google Scholar
|
|
10
|
da Silva JB, Navarro DMAF, da Silva AG,
Santos GKN, Dutra KA, Moreira DR, Ramos MN, Espíndola JW, de
Oliveira AD, Brondani DJ, et al: Thiosemicarbazones as Aedes
aegypti larvicidal. Eur J Med Chem. 100:162–175. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Universidade Federal de Goiás (UFG):
Diretrizes de Integridade e de Boas Práticas para Produção,
Manutenção ou Utilização de Animais em Atividades de Ensino ou
Pesquisa Científica. Publicada no D.O.U. de 08.09.2016, Seção I,
Pág. 5.
|
|
12
|
de Moraes Gomes PAT, de Oliveira Barbosa
M, Farias Santiago E, de Oliveira Cardoso MV, Capistrano Costa NT,
Hernandes MZ, Moreira DRM, da Silva AC, Dos Santos TAR, Pereira
VRA, et al: New 1,3-thiazole derivatives and their biological and
ultrastructural effects on Trypanosoma cruzi. Eur J Med Chem.
121:387–398. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Silva GL, Luft C, Lunardelli A, Amaral RH,
Melo DA, Donadio MV, Nunes FB, de Azambuja MS, Santana JC, Moraes
CM, et al: Antioxidant, analgesic and anti-inflammatory effects of
lavender essential oil. An Acad Bras Cienc. 87:1397–1408.
2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Chen YF, Tsai HY and Wu TS:
Anti-inflammatory and analgesic activities from roots of Angelica
pubescens. Planta Med. 61:2–8. 1995.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Lo TN, Almeida AP and Beaven MA: Dextran
and carrageenan evoke different inflammatory responses in rat with
respect to composition of infiltrates and effect of indomethacin. J
Pharmacol Exp Ther. 221:261–267. 1982.PubMed/NCBI
|
|
16
|
Patil KR, Mahajan UB, Unger BS, Goyal SN,
Belemkar S, Surana SJ, Ojha S and Patil CR: Animal Models of
Inflammation for Screening of Anti-inflammatory Drugs: Implications
for the Discovery and Development of Phytopharmaceuticals. Int J
Mol Sci. 20(4367)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Muthukumar VA, George S and Vaidhyalingam
V: Synthesis and pharmacological evaluation of
1-(1-((substituted)methyl)-5-methyl-2-oxoindolin-3-ylidene)-4-(substituted
pyridin-2-yl)thiosemicarbazide. Biol Pharm Bull. 31:1461–1464.
2008.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yasukawa K, Shigemi R, Kanbe T, Mutsumoto
Y, Oda F, Ichikawa K, Yamada KI, Tun X and Utsumi H: In Vivo
Imaging of the Intra- and Extracellular Redox Status in Rat Stomach
with Indomethacin-Induced Gastric Ulcers Using Overhauser-Enhanced
Magnetic Resonance Imaging. Antioxid Redox Signal. 30:1147–1161.
2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
OECD: Test No. 407: Repeated Dose 28-day
Oral Toxicity Study in Rodents. OECD Publishing, Paris, 2008.
https://doi.org/10.1787/9789264070684-.
|
|
20
|
OECD: Test No. 420: Acute Oral
Toxicity-Fixed Dose Procedure. OECD Publishing, Paris, 2002.
https://doi.org/10.1787/9789264070943-.
|
|
21
|
OECD: Test No. 423: Acute Oral
toxicity-Acute Toxic Class Method. OECD Publishing, Paris, 2002.
https://doi.org/10.1787/9789264071001-.
|
|
22
|
Seibenhener ML and Wooten MC: Use of the
Open Field Maze to measure locomotor and anxiety-like behavior in
mice. J Vis Exp. 96(e52434)2015.PubMed/NCBI View
Article : Google Scholar
|
|
23
|
Rozas G, Guerra MJ and Labandeira-García
JL: An automated rotarod method for quantitative drug-free
evaluation of overall motor deficits in rat models of parkinsonism.
Brain Res Brain Res Protoc. 2:75–84. 1997.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Whittle BA: The use of changes in
capillary permeability in mice to distinguish between narcotic and
non narcotic analgesics. Br J Pharmacol Chemother. 22:246–253.
1964.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Hunskaar S and Hole K: The formalin test
in mice: Dissociation between inflammatory and non-inflammatory
pain. Pain. 30:103–114. 1987.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Winter CA, Risley EA and Nuss GW:
Carrageenin-induced edema in hind paw of the rat as an assay for
antiiflammatory drugs. Proc Soc Exp Biol Med. 111:544–547.
1962.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zouari Bouassida K, Makni S, Tounsi A,
Jlaiel L, Trigui M and Tounsi S: Effects of Juniperus
phoenicea Hydroalcoholic Extract on Inflammatory Mediators and
Oxidative Stress Markers in Carrageenan-Induced Paw Oedema in Mice.
BioMed Res Int. 2018(3785487)2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Dantas-Medeiros R, Furtado AA, Zanatta AC,
Torres-Rêgo M, Guimarães Lourenço EM, Ferreira Alves JS, Galinari
É, Alexandre de Oliveira Rocha H, Bernardo Guerra GC, Vilegas W, et
al: Mass spectrometry characterization of Commiphora leptophloeos
leaf extract and preclinical evaluation of toxicity and
anti-inflammatory potential effect. J Ethnopharmacol.
264(113229)2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kataoka T, Teraoka J, Sakoda A, Nishiyama
Y, Yamato K, Monden M, Ishimori Y, Nomura T, Taguchi T and Yamaoka
K: Protective effects of radon inhalation on carrageenan-induced
inflammatory paw edema in mice. Inflammation. 35:713–722.
2012.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Marques JI, Alves JSF, Torres-Rêgo M,
Furtado AA, Siqueira EMDS, Galinari E, Araújo DFS, Guerra GCB,
Azevedo EP, Fernandes-Pedrosa MF, et al: Phytochemical analysis by
HPLC–HRESI-MS and anti-inflammatory activity of Tabernaemontana
catharinensis. Int J Mol Sci. 19(636)2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Torres-Rêgo M, Furtado AA, Bitencourt MA,
Lima MC, Andrade RC, Azevedo EP, Soares TC, Tomaz JC, Lopes NP, da
Silva-Júnior AA, et al: Anti-inflammatory activity of aqueous
extract and bioactive compounds identified from the fruits of
Hancornia speciosa Gomes (Apocynaceae). BMC Complement
Altern Med. 16(275)2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Campbell JN and Meyer RA: Mechanisms of
neuropathic pain. Neuron. 52:77–92. 2006.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Pandurangan K, Krishnappan V, Subramanian
V and Subramanyan R: Antinociceptive effect of certain dimethoxy
flavones in mice. Eur J Pharmacol. 727:148–157. 2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Ribeiro RA, Vale ML, Thomazzi SM,
Paschoalato ABP, Poole S, Ferreira SH and Cunha FQ: Involvement of
resident macrophages and mast cells in the writhing nociceptive
response induced by zymosan and acetic acid in mice. Eur J
Pharmacol. 387:111–118. 2000.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Lenardão EJ, Savegnago L, Jacob RG,
Victoria FN and Martinez DM: Antinociceptive Effect of Essential
Oils and Their Constituents: An Update Review. J Braz Chem Soc.
27:435–474. 2016.
|
|
37
|
Erami E, Azhdari-Zarmehri H, Imoto K and
Furue H: Characterization of nociceptive behaviors induced by
formalin in the glabrous and hairy skin of rats. Basic Clin
Neurosci. 8:37–42. 2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Lugo-Lugo DE, Pozos-Guillén AJ,
Zapata-Morales JR, Rodríguez-Chong A, Rangel-López AJ,
Saavedra-Leos MZ, et al: Antinociceptive local activity of
4-allyl-1-hydroxy-2-methoxybenzene (eugenol) by the formalin test:
an anti-inflammatory effect. Braz J Pharm Sci. 55(e18022)2019.
|
|
39
|
Jamison RN and Mao J: Opioid Analgesics.
Mayo Clin Proc. 90:957–968. 2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Sousa FSS, Anversa RG, Birmann PT, de
Souza MN, Balaguez R, Alves D, Luchese C, Wilhelm EA and Savegnago
L: Contribution of dopaminergic and noradrenergic systems in the
antinociceptive effect of α-(phenylalanyl) acetophenone. Pharmacol
Rep. 69:871–877. 2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Ferreira-Pinto MJ, Ruder L, Capelli P and
Arber S: Connecting Circuits for Supraspinal Control of Locomotion.
Neuron. 100:361–374. 2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Gupta BD, Dandiya PC and Gupta ML: A
psycho-pharmacological analysis of behaviour in rats. Jpn J
Pharmacol. 21:293–298. 1971.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Morris CJ: Carrageenan-induced paw edema
in the rat and mouse. Methods Mol Biol. 225:115–121.
2003.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Almeida Junior S: In vivo methods for the
evaluation of anti-inflammatory and antinoceptive potential. BrJP.
2:386–389. 2019.
|
|
45
|
Strehl C and Buttgereit F: Long-term
glucocorticoid therapy: Is there a safe dosage? Internist (Berl).
57:934–939. 2016.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
46
|
Calhoun W, Chang J and Carlson RP: Effect
of selected antiinflammatory agents and other drugs on zymosan,
arachidonic acid, PAF and carrageenan induced paw edema in the
mouse. Agents Actions. 21, 3-4. 306–9. 1987.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Konno S and Tsurufuji S: Induction of
zymosan-air-pouch inflammation in rats and its characterization
with reference to the effects of anticomplementary and
anti-inflammatory agents. Br J Pharmacol. 80:269–277.
1983.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Ramamoorthy S and Cidlowski JA:
Corticosteróides: Mecanismos de Ação na Saúde e na Doença. Rheum
Dis Clin North Am. 42:15–vii. 2016.
|
|
49
|
Prakash CR, Raja S, Panneer Selvam T,
Saravanan G, Karthick V and Dinesh Kumar P: Synthesis and
antimicrobial activities of some novel Schiff bases of 5-
substituted isatin derivatives. Rasayan J Chem. 2:960–968.
2009.
|
|
50
|
Sharma K, Biyala MK, Swami M, Fahmi N and
Singh RV: Coordination chemistry of palladium (II) and platinum
(II) complexes with bioactive Schiff bases: Synthetic, spectral,
and biocidal aspects. Russ J Coord Chem. 35:142–148. 2009.
|
|
51
|
Jarapula R, Gangarapu K, Manda S and
Rekulapally S: Synthesis, In Vivo Anti-Inflammatory Activity, and
Molecular Docking Studies of New Isatin Derivatives. Int J Med
Chem. 2016(2181027)2016.PubMed/NCBI View Article : Google Scholar
|